Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the MAPL
2.2. Data Analysis
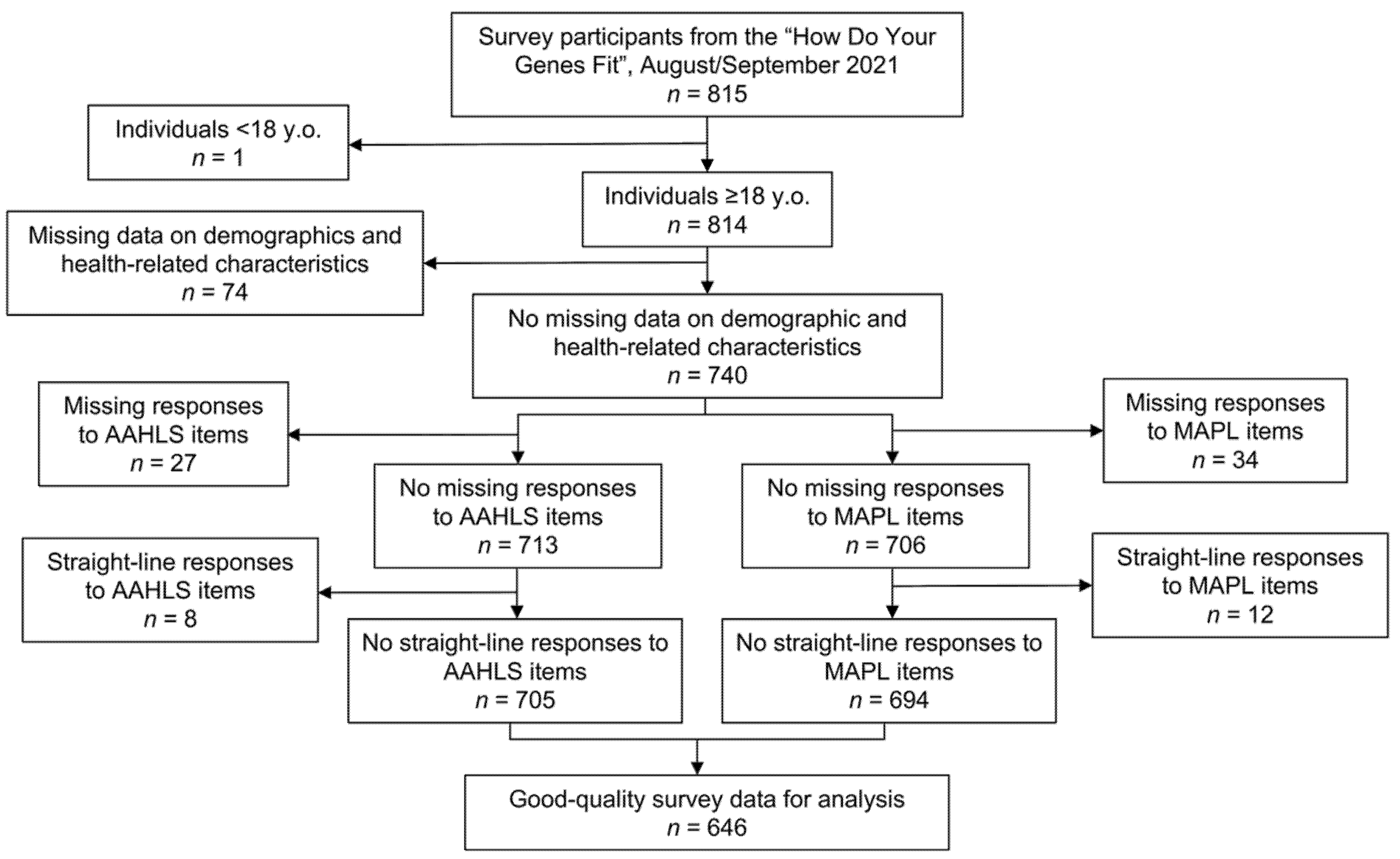
2.2.1. Data Cleaning
2.2.2. Statistical Analysis
2.2.3. Assessment of Internal Reliability and Construct Validity
3. Results
3.1. MAPL Development
3.2. MAPL Validation and Performance Characteristics
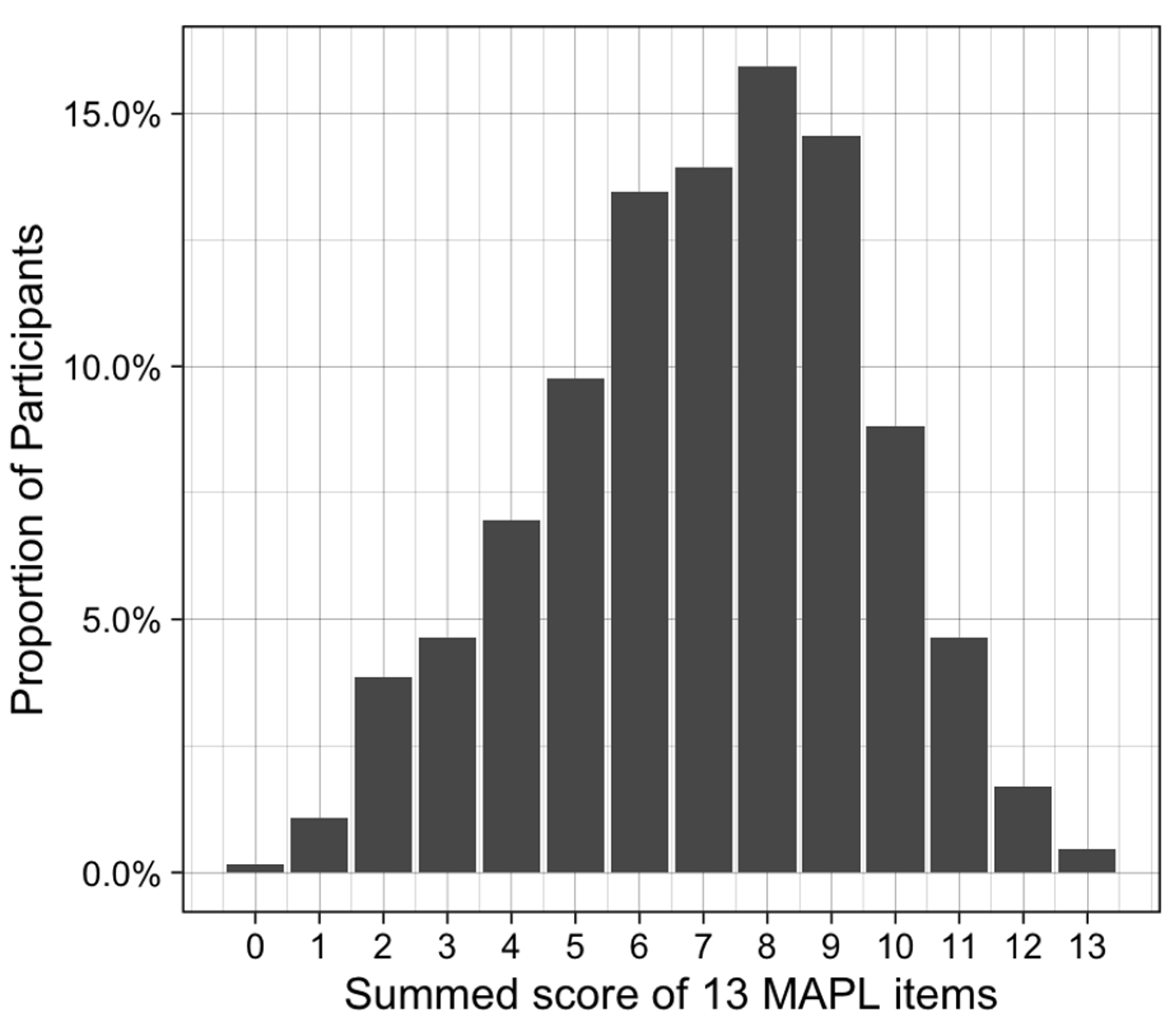
3.2.1. Descriptive Statistics
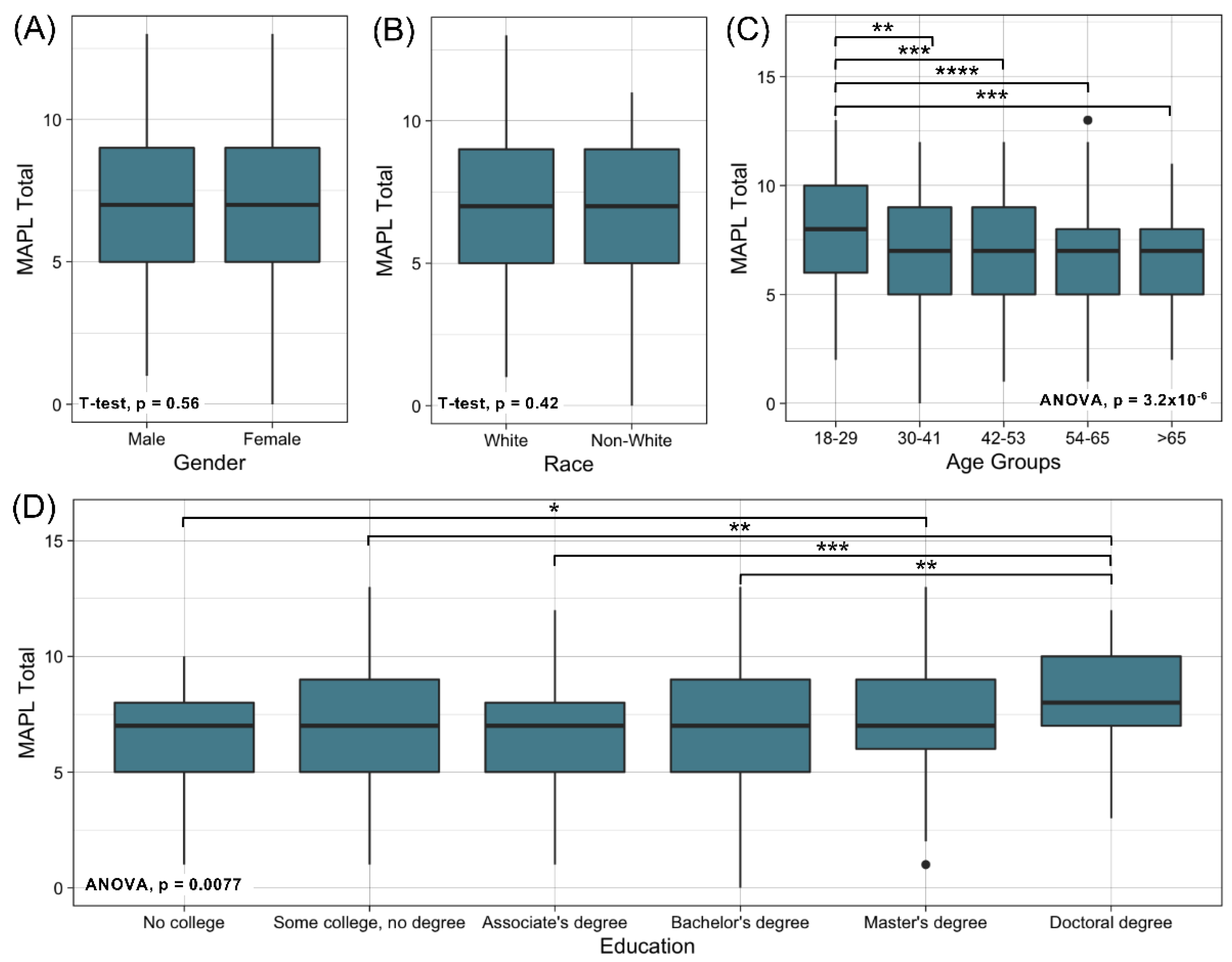
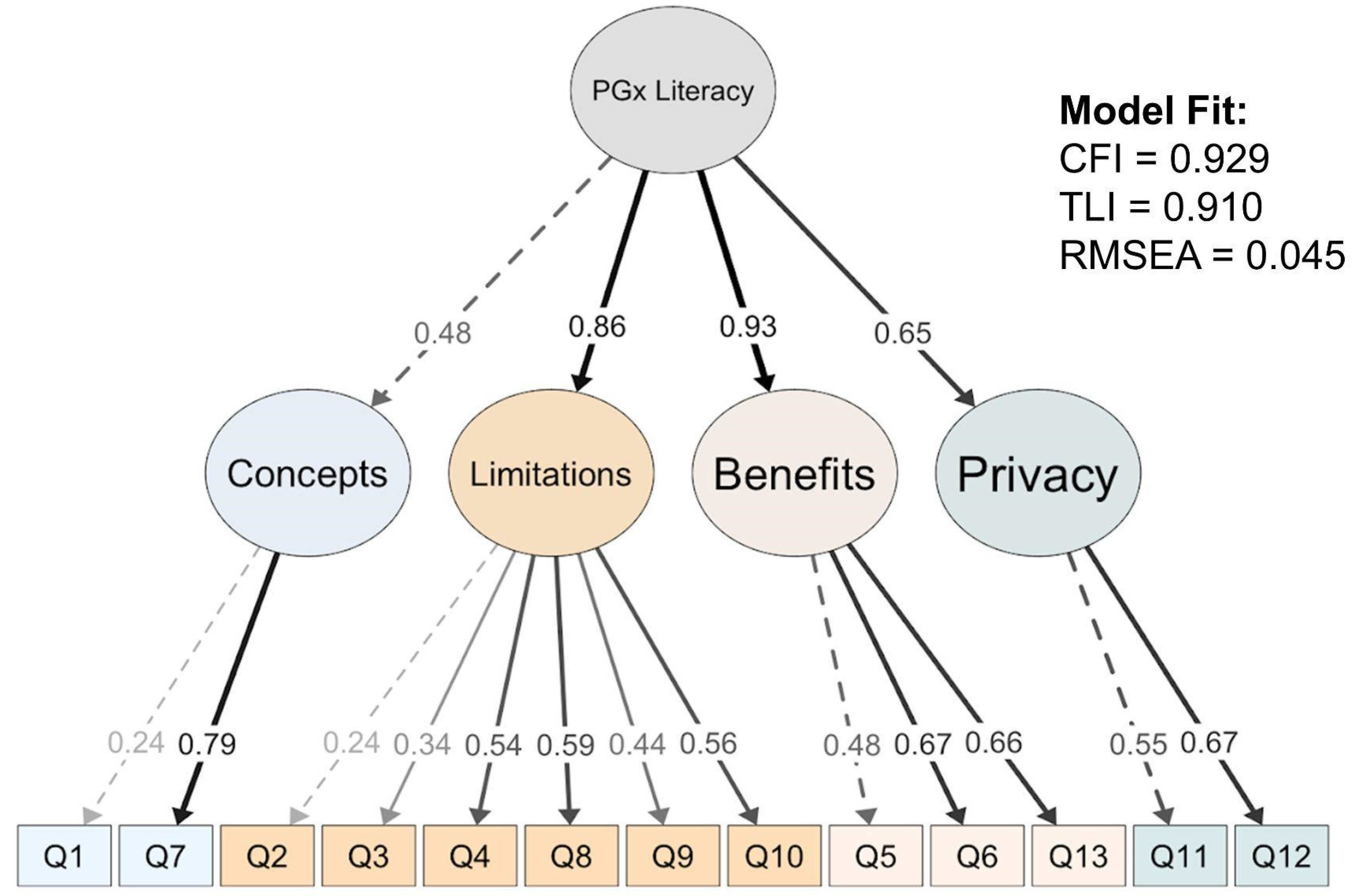
3.2.2. Assessment of Internal Reliability and Construct Validity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haga, S.B.; O’Daniel, J.M.; Tindall, G.M.; Lipkus, I.R.; Agans, R. Survey of US Public Attitudes toward Pharmacogenetic Testing. Pharm. J. 2012, 12, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.T.; Bell, G.C.; Gregornik, D.B.; Ho, T.T.; Dunnenberger, H.M. Synthesis of Major Pharmacogenomics Pretest Counseling Themes: A Multisite Comparison. Pharmacogenomics 2021, 22, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Pittenger, A.L.; Bishop, J.R. A Scoping Review of Attitudes and Experiences with Pharmacogenomic Testing among Patients and the General Public: Implications for Patient Counseling. J. Pers. Med. 2022, 12, 425. [Google Scholar] [CrossRef]
- Lautenbach, D.M.; Christensen, K.D.; Sparks, J.A.; Green, R.C. Communicating Genetic Risk Information for Common Disorders in the Era of Genomic Medicine. Annu. Rev. Genom. Hum. Genet. 2013, 14, 491–513. [Google Scholar] [CrossRef] [PubMed]
- The Honorable Kathleen Sebelius Secretary of Health and Human Services. Secretary’s Advisory Committee on Genetics Health and Society Genetics Education and Training; Secretary’s Advisory Committee on Genetics, Health, and Society: Bethesda, MD, USA, 2011.
- Furr, L.A.; Kelly, S.E. The Genetic Knowledge Index: Developing a Standard Measure of Genetic Knowledge. Genet. Test. 1999, 3, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Erby, L.H.; Roter, D.; Larson, S.; Cho, J. The Rapid Estimate of Adult Literacy in Genetics (REAL-G): A Means to Assess Literacy Deficits in the Context of Genetics. Am. J. Med. Genetics. Part A 2008, 146, 174–181. [Google Scholar] [CrossRef]
- Fitzgerald-Butt, S.M.; Bodine, A.; Fry, K.M.; Ash, J.; Zaidi, A.N.; Garg, V.; Gerhardt, C.A.; McBride, K.L. Measuring Genetic Knowledge: A Brief Survey Instrument for Adolescents and Adults. Clin. Genet. 2016, 89, 235–243. [Google Scholar] [CrossRef]
- Langer, M.M.; Roche, M.I.; Brewer, N.T.; Berg, J.S.; Khan, C.M.; Leos, C.; Moore, E.; Brown, M.; Rini, C. Development and Validation of a Genomic Knowledge Scale to Advance Informed Decision Making Research in Genomic Sequencing. MDM Policy Pract. 2016, 2, 2381468317692582. [Google Scholar] [CrossRef]
- Yehya, A.; Matalgah, L. Toward Interprofessional Education of Pharmacogenomics: An Interdisciplinary Assessment. Pharmacology 2021, 106, 534–541. [Google Scholar] [CrossRef]
- Agrawal, M.; Kirtania, L.; Jha, A.; Hishikar, R. Students’ Knowledge and Views on Pharmacogenomic Education in the Medical Curriculum. Indian J. Pharmacol. 2021, 53, 19–24. [Google Scholar] [CrossRef]
- Dodson, C. Oncology Nurses’ Knowledge of Pharmacogenomics Before and After Implementation of an Education Module. Oncol. Nurs. Forum 2018, 45, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Hui, A.C.; Walker, A.D.; Peshkin, B.N.; Swain, S.M.; Smith, D.M. Evaluation of a Longitudinal Pharmacogenomics Education on Pharmacist Knowledge in a Multicampus Healthcare System. Pharmacogenomics 2022, 23, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Anderson, K.B.; Coons, J.C.; Smith, R.B.; Meyer, S.M.; Parker, L.S.; Empey, P.E. Advancing Pharmacogenomics Education in the Core PharmD Curriculum through Student Personal Genomic Testing. Am. J. Pharm. Educ. 2016, 80, 3. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.M.; Lee, K.C.; Ma, J.D. Implementation and Outcomes of a Live Continuing Education Program on Pharmacogenomics. Pharmacogenomics 2013, 14, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Assem, M.; Broeckel, U.; MacKinnon, G.E. Personal DNA Testing Increases Pharmacy Students’ Confidence and Competence in Pharmacogenomics. Am. J. Pharm. Educ. 2021, 85, 8249. [Google Scholar] [CrossRef]
- Formea, C.M.; Nicholson, W.T.; McCullough, K.B.; Berg, K.D.; Berg, M.L.; Cunningham, J.L.; Merten, J.A.; Ou, N.N.; Stollings, J.L. Development and Evaluation of a Pharmacogenomics Educational Program for Pharmacists. Am. J. Pharm. Educ. 2013, 77, 10. [Google Scholar] [CrossRef]
- Springer, J.A.; Iannotti, N.V.; Kane, M.D.; Haynes, K.; Sprague, J.E. Pharmacogenomics Training Using an Instructional Software System. Am. J. Pharm. Educ. 2011, 75, 32. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Bray, B.S.; Wright, S.K.; Ashmore, J.; Kabasenche, W.; Wang, S.; Lazarus, P.; Daoud, S.S. Design, Implementation, and Assessment Approaches within a Pharmacogenomics Course. Am. Pharm. Educ. 2017, 81, 11. [Google Scholar] [CrossRef]
- Creswell, J.W. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Added Bytes Ltd Readable.Com. Available online: https://readable.com/ (accessed on 22 April 2022).
- Demerath, E. Research on a Stick: Recruitment and Data Collection Experiences at the Minnesota State Fair. In Proceedings of the Society of Clinical Research Associates Annual Conference, Virtual, 23–26 September 2020. [Google Scholar]
- Chinn, D.; McCarthy, C. All Aspects of Health Literacy Scale (AAHLS): Developing a Tool to Measure Functional, Communicative and Critical Health Literacy in Primary Healthcare Settings. Patient Educ. Couns. 2013, 90, 247–253. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making Sense of Cronbach’s Alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Tucker, L.R.; Lewis, C. A Reliability Coefficient for Maximum Likelihood Factor Analysis. Psychometrika 1973, 38, 1–10. [Google Scholar] [CrossRef]
- Steiger, J.H. Structural Model Evaluation and Modification: An Interval Estimation Approach. Multivar. Behav. Res. 1990, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rahma, A.T.; Elbarazi, I.; Ali, B.R.; Patrinos, G.P.; Ahmed, L.A.; Elsheik, M.; Al-Maskari, F. Development of the Pharmacogenomics and Genomics Literacy Framework for Pharmacists. Hum. Genom. 2021, 15, 62. [Google Scholar] [CrossRef]
- Basyouni, D.; Shatnawi, A. Pharmacogenomics Instruction Depth, Extent, and Perception in US Medical Curricula. J. Med. Educ. Curric. Dev. 2020, 7, 2382120520930772. [Google Scholar] [CrossRef]
- Grace, C.; Larriva, M.M.; Steiner, H.E.; Marupuru, S.; Campbell, P.J.; Patterson, H.; Cropp, C.D.; Quinn, D.; Klimecki, W.; Nix, D.E.; et al. Efficacy of Personal Pharmacogenomic Testing as an Educational Tool in the Pharmacy Curriculum: A Nonblinded, Randomized Controlled Trial. Clin. Transl. Sci. 2021, 14, 2532–2543. [Google Scholar] [CrossRef]
- Rahma, A.T.; Ahmed, L.A.; Elsheik, M.; Elbarazi, I.; Ali, B.R.; Patrinos, G.P.; Al-Maskari, F. Mapping the Educational Environment of Genomics and Pharmacogenomics in the United Arab Emirates: A Mixed-Methods Triangulated Design. OMICS 2021, 25, 285–293. [Google Scholar] [CrossRef]
- Just, K.S.; Steffens, M.; Swen, J.J.; Patrinos, G.P.; Guchelaar, H.-J.; Stingl, J.C. Medical Education in Pharmacogenomics—Results from a Survey on Pharmacogenetic Knowledge in Healthcare Professionals within the European Pharmacogenomics Clinical Implementation Project Ubiquitous Pharmacogenomics (U-PGx). Eur. J. Clin. Pharmacol. 2017, 73, 1247–1252. [Google Scholar] [CrossRef]
- Pisanu, C.; Tsermpini, E.-E.; Mavroidi, E.; Katsila, T.; Patrinos, G.P.; Squassina, A. Assessment of the Pharmacogenomics Educational Environment in Southeast Europe. Public Health Genom. 2014, 17, 272–279. [Google Scholar] [CrossRef]
- Drelles, K.; Pilarski, R.; Manickam, K.; Shoben, A.B.; Toland, A.E. Impact of Previous Genetic Counseling and Objective Numeracy on Accurate Interpretation of a Pharmacogenetics Test Report. Public Health Genom. 2021, 24, 26–32. [Google Scholar] [CrossRef]
- Truong, T.M.; Lipschultz, E.; Danahey, K.; Schierer, E.; Ratain, M.J.; O’Donnell, P.H. Assessment of Patient Knowledge and Perceptions of Pharmacogenomics before and after Using a Mock Results Patient Web Portal. Clin. Transl. Sci. 2020, 13, 78–87. [Google Scholar] [CrossRef]
- Meagher, K.M.; Stuttgen Finn, K.; Curtis, S.H.; Borucki, J.; Beck, A.T.; Cheema, A.W.; Sharp, R.R. Lay Understandings of Drug-Gene Interactions: The Right Medication, the Right Dose, at the Right Time, but What Are the Right Words? Clin. Transl. Sci. 2022, 15, 721–731. [Google Scholar] [CrossRef]
- Meagher, K.M.; Curtis, S.H.; Borucki, S.; Beck, A.; Srinivasan, T.; Cheema, A.; Sharp, R.R. Communicating Unexpected Pharmacogenomic Results to Biobank Contributors: A Focus Group Study. Patient Educ. Couns. 2021, 104, 242–249. [Google Scholar] [CrossRef]
- Culhane-Pera, K.A.; Straka, R.J.; Moua, M.; Roman, Y.; Vue, P.; Xiaaj, K.; Lo, M.X.; Lor, M. Engaging Hmong Adults in Genomic and Pharmacogenomic Research: Toward Reducing Health Disparities in Genomic Knowledge Using a Community-Based Participatory Research Approach. J. Community Genet. 2017, 8, 117–125. [Google Scholar] [CrossRef]
- Jones, L.K.; Kulchak Rahm, A.; Gionfriddo, M.R.; Williams, J.L.; Fan, A.L.; Pulk, R.A.; Wright, E.A.; Williams, M.S. Developing Pharmacogenomic Reports: Insights from Patients and Clinicians. Clin. Transl. Sci. 2018, 11, 289–295. [Google Scholar] [CrossRef]
- Truong, T.M.; Lipschultz, E.; Schierer, E.; Danahey, K.; Ratain, M.J.; O’Donnell, P.H. Patient Insights on Features of an Effective Pharmacogenomics Patient Portal. Pharm. Genom. 2020, 30, 191–200. [Google Scholar] [CrossRef]
- Qureshi, S.; Latif, A.; Condon, L.; Akyea, R.K.; Kai, J.; Qureshi, N. Understanding the Barriers and Enablers of Pharmacogenomic Testing in Primary Care: A Qualitative Systematic Review with Meta-Aggregation Synthesis. Pharmacogenomics 2022, 23, 135–154. [Google Scholar] [CrossRef]
- Klein, M.E.; Parvez, M.M.; Shin, J.-G. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J. Pharm. Sci. 2017, 106, 2368–2379. [Google Scholar] [CrossRef]
- Giri, J.; Curry, T.B.; Formea, C.M.; Nicholson, W.T.; Rohrer Vitek, C.R. Education and Knowledge in Pharmacogenomics: Still a Challenge? Clin. Pharmacol. Ther. 2018, 103, 752–755. [Google Scholar] [CrossRef]
- Carroll, J.C.; Allanson, J.; Morrison, S.; Miller, F.A.; Wilson, B.J.; Permaul, J.A.; Telner, D. Informing Integration of Genomic Medicine into Primary Care: An Assessment of Current Practice, Attitudes, and Desired Resources. Front. Genet. 2019, 10, 1189. [Google Scholar] [CrossRef] [Green Version]
- Vest, B.M.; Wray, L.O.; Brady, L.A.; Thase, M.E.; Beehler, G.P.; Chapman, S.R.; Hull, L.E.; Oslin, D.W. Primary Care and Mental Health Providers’ Perceptions of Implementation of Pharmacogenetics Testing for Depression Prescribing. BMC Psychiatry 2020, 20, 518. [Google Scholar] [CrossRef]
- Tuteja, S.; Salloum, R.G.; Elchynski, A.L.; Smith, D.M.; Rowe, E.; Blake, K.V.; Limdi, N.A.; Aquilante, C.L.; Bates, J.; Beitelshees, A.L.; et al. Multisite Evaluation of Institutional Processes and Implementation Determinants for Pharmacogenetic Testing to Guide Antidepressant Therapy. Clin. Transl. Sci. 2022, 15, 371–383. [Google Scholar] [CrossRef]
- Mai, Y.; Koromila, T.; Sagia, A.; Cooper, D.N.; Vlachopoulos, G.; Lagoumintzis, G.; Kollia, P.; Poulas, K.; Stathakopoulos, V.; Patrinos, G.P. A Critical View of the General Public’s Awareness and Physicians’ Opinion of the Trends and Potential Pitfalls of Genetic Testing in Greece. Per. Med. 2011, 8, 551–561. [Google Scholar] [CrossRef]
- Haga, S.B.; Mills, R.; Moaddeb, J.; Allen Lapointe, N.; Cho, A.; Ginsburg, G.S. Patient Experiences with Pharmacogenetic Testing in a Primary Care Setting. Pharmacogenomics 2016, 17, 1629–1636. [Google Scholar] [CrossRef]
- Olson, J.E.; Rohrer Vitek, C.R.; Bell, E.J.; McGree, M.E.; Jacobson, D.J.; St Sauver, J.L.; Caraballo, P.J.; Griffin, J.M.; Roger, V.L.; Bielinski, S.J. Participant-Perceived Understanding and Perspectives on Pharmacogenomics: The Mayo Clinic RIGHT Protocol (Right Drug, Right Dose, Right Time). Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 819–825. [Google Scholar] [CrossRef]
- Kastrinos, A.; Campbell-Salome, G.; Shelton, S.; Peterson, E.B.; Bylund, C.L. PGx in Psychiatry: Patients’ Knowledge, Interest, and Uncertainty Management Preferences in the Context of Pharmacogenomic Testing. Patient Educ. Couns. 2021, 104, 732–738. [Google Scholar] [CrossRef]
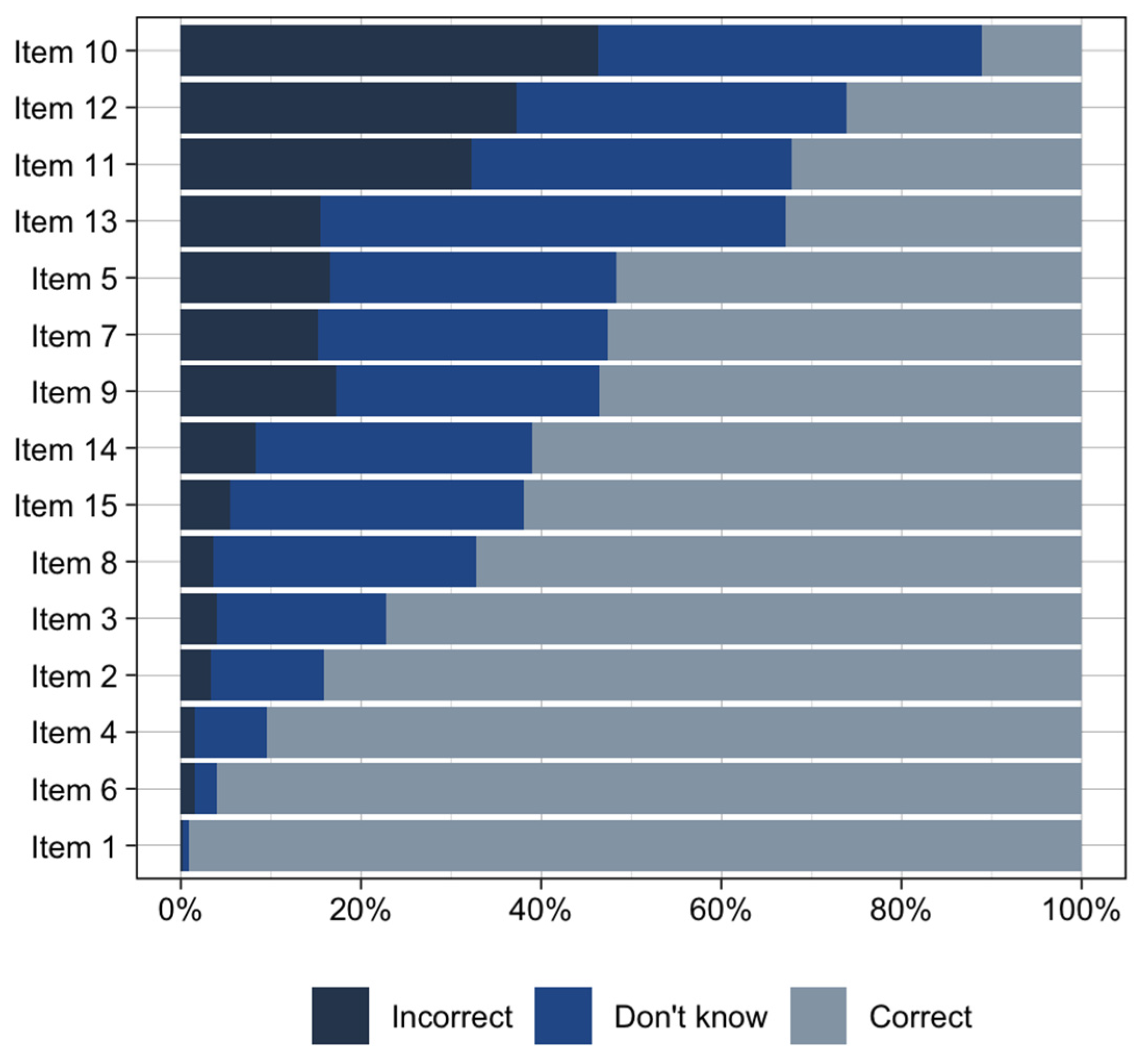
| Item Number | Question | Correct Answer | Domain |
|---|---|---|---|
| 1 * | Different people will respond to medications differently. | True | Underlying Concepts |
| 2 | Genes are made of DNA. | True | Underlying Concepts |
| 3 | If a medication works for your family member, it will work for you too. | False | Limitations |
| 4 | Genes are one of many different things that can affect how you respond to a medication. | True | Limitations |
| 5 | Pharmacogenomic test results will tell you how you will respond to every medication. | False | Limitations |
| 6 * | Your genes are inherited from your parents. | True | Underlying Concepts |
| 7 | Genes can affect how much medication is in your body after you take a pill. | True | Benefits |
| 8 | Pharmacogenomic test results may tell you that a medication is likely to cause side effects. | True | Benefits |
| 9 | Your body breaks down medications to get rid of them. | True | Underlying Concepts |
| 10 | Pharmacogenomic testing will tell you the best medication to treat your condition. | False | Limitations |
| 11 | When deciding what medication is best for you, your genetic makeup is more important than age, weight, or other medications you are taking. | False | Limitations |
| 12 | Pharmacogenomic testing will help determine your diagnosis. | False | Limitations |
| 13 | Health insurance companies can use your pharmacogenomic test results to deny coverage. | False | Privacy |
| 14 | Pharmacogenomic testing companies have the right to use your data however they want without your consent. | False | Privacy |
| 15 | Pharmacogenomic testing can tell you that you may need a different dose of a medication. | True | Benefits |
| N (n = 646) | % | |
|---|---|---|
| Gender | ||
| Men | 235 | 36.4% |
| Women | 408 | 63.2% |
| Other | 3 | 0.4% |
| Age | ||
| 18–29 | 182 | 28.2% |
| 30–41 | 93 | 14.4% |
| 42–53 | 125 | 19.3% |
| 54–65 | 167 | 25.9% |
| 66–77 | 74 | 11.5% |
| 78+ | 5 | 0.8% |
| Race | ||
| American Indian or Alaska Native | 4 | 0.6% |
| Asian | 46 | 7.1% |
| Black or African American | 6 | 0.9% |
| Native Hawaiian or Pacific Islanders | 2 | 0.3% |
| White | 545 | 84.4% |
| Multiracial | 15 | 2.3% |
| Other | 13 | 2.0% |
| Unknown/Prefer not to answer | 15 | 2.3% |
| Hispanic, Latino, or Spanish origin | ||
| Yes | 36 | 5.6% |
| No | 601 | 93.0% |
| Unknown/Prefer not to answer | 9 | 1.4% |
| Education attainment | ||
| Less than high school | 3 | 0.5% |
| High school graduate/GED/equivalent | 47 | 7.3% |
| Some college, no degree | 119 | 18.4% |
| Associate degree | 91 | 14.1% |
| Bachelor’s degree | 224 | 37.8% |
| Master’s degree | 92 | 14.2% |
| Doctoral degree | 50 | 7.7% |
| n (Mean) (n = 646) | % (SD) | |
|---|---|---|
| Having primary care provider | ||
| Yes | 541 | 83.7% |
| No | 105 | 16.3% |
| Having health insurance | ||
| Yes | 614 | 95.0% |
| No | 32 | 5.0% |
| The number of prescription medications in the last 30 days | ||
| 0 | 228 | 35.3% |
| 1 | 259 | 40.1% |
| 2 | 113 | 17.5% |
| 3+ | 46 | 7.1% |
| The number of non-prescription medications in the last 30 days | ||
| 0 | 116 | 18.0% |
| 1 | 338 | 52.3% |
| 2 | 128 | 19.8% |
| 3+ | 64 | 9.9% |
| All Aspects of Health Literacy Scale (AAHLS) total score | 16.4 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, J.D.; Zhang, L.; Johnson, A.N.K.; Jacobson, P.A.; McCarty, C.A.; Pittenger, A.L.; Bishop, J.R. Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL). J. Pers. Med. 2022, 12, 1398. https://doi.org/10.3390/jpm12091398
Allen JD, Zhang L, Johnson ANK, Jacobson PA, McCarty CA, Pittenger AL, Bishop JR. Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL). Journal of Personalized Medicine. 2022; 12(9):1398. https://doi.org/10.3390/jpm12091398
Chicago/Turabian StyleAllen, Josiah D., Lusi Zhang, Alyssa N. K. Johnson, Pamala A. Jacobson, Catherine A. McCarty, Amy L. Pittenger, and Jeffrey R. Bishop. 2022. "Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL)" Journal of Personalized Medicine 12, no. 9: 1398. https://doi.org/10.3390/jpm12091398
APA StyleAllen, J. D., Zhang, L., Johnson, A. N. K., Jacobson, P. A., McCarty, C. A., Pittenger, A. L., & Bishop, J. R. (2022). Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL). Journal of Personalized Medicine, 12(9), 1398. https://doi.org/10.3390/jpm12091398






