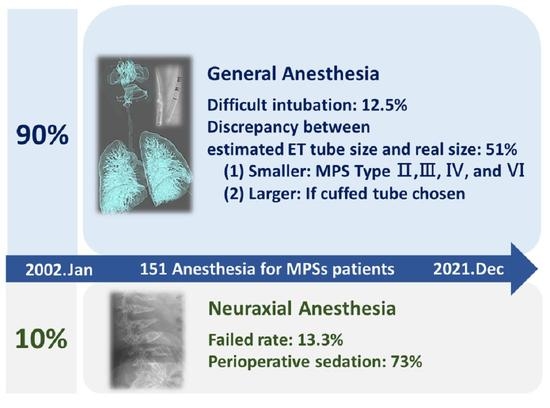
The Anesthetic Strategy for Patients with Mucopolysaccharidoses: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Ethics Statement
2.2. Data Acquisition
2.3. Anesthesia Method
2.4. Outcome Measures
2.5. Specification of Endotracheal Tube
2.6. Statistical Analysis
3. Results
3.1. Anesthesia Methods and Intervention Procedures
3.2. General Anesthesia
3.2.1. Distribution of Subtypes of MPS Patients Underwent General Anesthesia
3.2.2. Airway Management
3.2.3. Difficulties of Airway Management
3.2.4. Respiratory and Cardiovascular Adverse Events
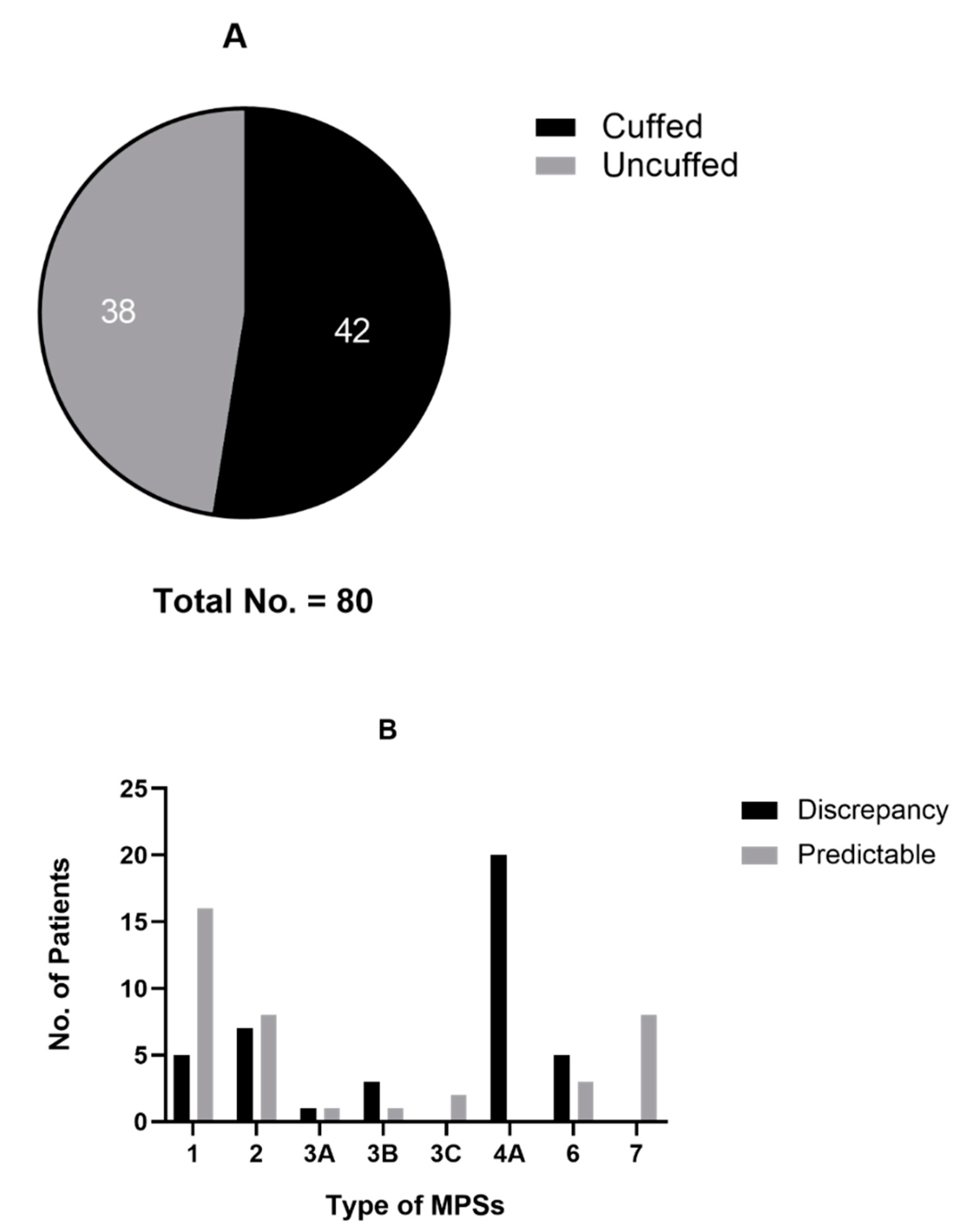
3.2.5. Specification of Endotracheal Tube
3.3. Neuraxial Anesthesia
3.3.1. Predominance of Adolescents and Adults
3.3.2. Cardiovascular Events: Two Cases of Hypotension
3.3.3. Necessity of Premedication and Perioperative Sedation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fecarotta, S.; Tarallo, A.; Damiano, C.; Minopoli, N.; Parenti, G. Pathogenesis of Mucopolysaccharidoses, an Update. Int. J. Mol. Sci. 2020, 21, 2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.K.; Lee, C.L.; Tu, R.Y.; Lo, Y.T.; Sisca, F.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; et al. Nationwide Newborn Screening Program for Mucopolysaccharidoses in Taiwan and an Update of the “Gold Standard” Criteria Required to Make a Confirmatory Diagnosis. Diagnostics 2021, 11, 1583. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lee, C.L.; Chang, C.Y.; Chiu, P.C.; Chien, Y.H.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; Lin, S.J.; Lin, J.L.; et al. Survival and diagnostic age of 175 Taiwanese patients with mucopolysaccharidoses (1985–2019). Orphanet J. Rare Dis. 2020, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.M.; Sprung, J.; Weingarten, T.N.; Warner, M.E. Anesthesia for patients with mucopolysaccharidoses: Comprehensive review of the literature with emphasis on airway management. Bosn. J. Basic Med. Sci. 2018, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-L.; Lee, K.-S.; Chuang, C.-K.; Su, C.-H.; Chiu, H.-C.; Tu, R.-Y.; Lo, Y.-T.; Chang, Y.-H.; Lin, H.-Y.; Lin, S.-P. Otorhinolaryngological management in Taiwanese patients with mucopolysaccharidoses. Int. J. Med. Sci. 2021, 18, 3373–3379. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Lin, S.-P.; Man, T.-T.; Cheng, C.-R. Clinical experience in anesthetic management for children with mucopolysaccharidoses: Report of ten cases. Acta Paediatr. Taiwanica 2001, 42, 306–308. [Google Scholar]
- Sun, L.; Zhang, J.; Zhao, X. Successful spinal anesthesia in a patient with mucopolysaccharidosis type I under femoral fracture reduction and external fixation. Pediatr. Investig. 2019, 3, 55–57. [Google Scholar] [CrossRef]
- Kumar, K.R.; Kumar, H.; Baidya, D.K.; Arora, M.K. Successful use of spinal anesthesia for inguinal hernia repair in a child with Hunter syndrome with difficult airway. J. Clin. Anesth. 2016, 30, 99–100. [Google Scholar] [CrossRef]
- Yalcin, S.; Aydogan, H.; Yuce, H.H.; Kucuk, A.; Boleken, M.E. Caudal anesthesia in Hurler’s syndrome. Pediatr. Anesth. 2011, 21, 1270–1272. [Google Scholar] [CrossRef]
- Ingrosso, M.; Picilli, M.M.; Capasso, A.; Cecere, F.; Cirillo, V.; Merolla, V. Anaestethic problems in Sanfilippo syndrome: A rare case of adult patient. Minerva Anestesiol. 2003, 69, 641–643, 644–645. [Google Scholar]
- Tobias, J.D. Anesthetic care for the child with Morquio syndrome: General versus regional anesthesia. J. Clin. Anesth. 1999, 11, 242–246. [Google Scholar] [CrossRef]
- Sethna, N.F.; Berde, C.B. Continuous subarachnoid analgesia in two adolescents with severe scoliosis and impaired pulmonary function. Reg. Anesth. 1991, 16, 333–336. [Google Scholar] [PubMed]
- Sjøgren, P.; Pedersen, T. Anaesthetic problems in Hurler-Scheie syndrome. Report of two cases. Acta Anaesthesiol. Scand. 1986, 30, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Kodani, Y.; Matsuura, T.; Mori, T.; Nishikawa, K.; Asada, A. [Anesthesia for three patients with Hunter syndrome]. Masui. 2011, 60, 846–849. [Google Scholar] [PubMed]
- Vas, L.; Naregal, F. Failed epidural anaesthesia in a patient with Hurler’s disease. Pediatr. Anesth. 2000, 10, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.C.; Krane, E.J.; Tomatsu, S.; Theroux, M.C.; Lee, R.R. Paraplegia after epidural-general anesthesia in a Morquio patient with moderate thoracic spinal stenosis. Can. J. Anesth. 2015, 62, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Cormack, R.S.; Lehane, J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984, 39, 1105–1111. [Google Scholar] [CrossRef]
- Cole, F. Pediatric formulas for the anesthesiologist. AMA J. Dis. Child. 1957, 94, 672–673. [Google Scholar] [CrossRef]
- Dohrmann, T.; Muschol, N.M.; Sehner, S.; Punke, M.A.; Haas, S.A.; Roeher, K.; Breyer, S.; Koehn, A.F.; Ullrich, K.; Zöllner, C.; et al. Airway management and perioperative adverse events in children with mucopolysaccharidoses and mucolipidoses: A retrospective cohort study. Pediatr. Anesth. 2020, 30, 181–190. [Google Scholar] [CrossRef]
- Clark, B.M.; Sprung, J.; Weingarten, T.N.; Warner, M.E. Airway management changes in patients with mucopolysaccharidoses: The role of video laryngoscopy. Can. J. Anesth. 2017, 64, 981–982. [Google Scholar] [CrossRef] [Green Version]
- Tzanova, I.; Schwarz, M.; Jantzen, J.P. Securing the airway in children with the Morquio-Brailsford syndrome. Anaesthesist 1993, 42, 477–481. [Google Scholar] [PubMed]
- Shiroyama, K.; Izumi, H.; Kubo, T. [Estimation of size of the uncuffed endotracheal tube for pediatric cardiac anesthesia—Cole’s formula cannot estimate the appropriate tube size]. Masui 2001, 50, 284–286. [Google Scholar] [PubMed]
- Cohen, M.A.; Stuart, G.M. Delivery of anesthesia for children with mucopolysaccharidosis type III (Sanfilippo syndrome): A review of 86 anesthetics. Pediatr. Anesth. 2017, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Eisleben, L.S.; Kessler, J.; Jensen, K.; Plaschke, K. Role of ultrasound measuring position and ventilation pressure in determining correct tube size in children. Pediatr. Anesth. 2017, 27, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Nakajima, Y.; Ishii, S.; Shimizu, F.; Shime, N.; Sessler, D.I. Prediction of pediatric endotracheal tube size by ultrasonography. Anesthesiology 2010, 113, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Ahn, J.; Yoon, S.U.; Choo, K.S.; Kim, H.J.; Chung, M.; Kim, H.Y. Prediction of endotracheal tube size using a printed three-dimensional airway model in pediatric patients with congenital heart disease: A prospective, single-center, single-group study. Korean J. Anesthesiol. 2021, 74, 333–341. [Google Scholar] [CrossRef]
- De Orange, F.A.; Andrade, R.G.; Lemos, A.; Borges, P.S.; Figueiroa, J.N.; Kovatsis, P.G. Cuffed versus uncuffed endotracheal tubes for general anaesthesia in children aged eight years and under. Cochrane Database Syst. Rev. 2017, 11, CD011954. [Google Scholar] [CrossRef]
- Figueirêdo, B.B.; Reinaux, C.; Fuzari, H.; Sarmento, A.; Fernandes, J.; Andrade, A.D.d. Chest wall volumes, diaphragmatic mobility, and functional capacity in patients with mucopolysaccharidoses. Disabil. Rehabil. 2022, 1–10. [Google Scholar] [CrossRef]


| Anesthesia (No./%) | Duration of Anesthesia 1 (min) | Type of Procedures | No. of Procedures Total N = 163 |
|---|---|---|---|
| General anesthesia (136/100%) | 148 | ||
| Bag-Mask (15/11.0%) | 137.7 (60–210) | MRI 2 | 12 |
| ENT 3 | 2 | ||
| Orthopedic surgery | 1 | ||
| Supraglottic device (15/11.7%) | 103.7 (55–325) | ENT | 7 |
| General or visceral surgery | 7 | ||
| Orthopedic surgery | 1 | ||
| Neurosurgery | 1 | ||
| Endotracheal intubation (80/58.8%) | 176.2 (60–540) | ENT | 55 |
| General or visceral surgery | 19 | ||
| Orthopedic surgery | 7 | ||
| Dental surgery | 4 | ||
| Neurosurgery | 3 | ||
| Cardiac surgery | 3 | ||
| Pre-existing airway (26/19.1%) | 193.5 (80–440) | ENT | 18 |
| General or visceral surgery | 6 | ||
| Orthopedic surgery | 1 | ||
| Neurosurgery | 1 | ||
| MRI | 1 | ||
| Neuraxial anesthesia (15/100%) | 105.7 (45–190) | General or visceral surgery | 14 |
| Orthopedic surgery | 1 |
| Classification | Total Patient | Type I | Type II | Type IIIA | Type IIIB | Type IIIC | Type IVA | Type VI | Type VII |
|---|---|---|---|---|---|---|---|---|---|
| Episodes of anesthesia | 136 | 26 | 33 | 2 | 15 | 2 | 35 | 12 | 11 |
| Gender (M/F) | 97/39 | 9/17 | 33/0 | 2/0 | 13/2 | 2/0 | 29/6 | 9/3 | 0/11 |
| Age (y/o) 1 | 13.7 (0–38) | 15.2 (0–35) | 12.8 (2–25) | 9 | 5.7 (2–14) | 26 | 20.2 (6–33) | 12.6 (6–26) | 3.4 (1–6) |
| Body weight (kg) | 27.4 (4.4–84) | 38.4 (4.4–84) | 29.1 (15–53) | 25 | 21.6 (15.7–36) | 42 | 26.8 (13–39.3) | 19.9 (13–33) | 11.6 (8–16.3) |
| Body height (cm) 2 | 112.1 (58–167;94) | 128.1 (58–167;21) | 125.9 (98–158;17) | n.a. 4 | 108.1 (99–140;11) | 160 | 99.6 (84–129;30) | 94.6 (86–110;7) | 91.4 (86–99;6) |
| Body mass index (kg/m2) | 23.4 (11.8–49.4) | 22.2 (11.8–31.6) | 21.1 (12.8–27.9) | n.a. | 18.1 (14.4–20.0) | 16.4 | 29.7 (13.2–49.4) | 20.7 (17.6–27.3) | 17.4 (16.6–19.1) |
| Bag-Mask | 15 | 1 | 2 | 0 | 9 | 0 | 1 | 1 | 1 |
| Supraglottic device | 15 | 2 | 6 | 0 | 1 | 0 | 2 | 2 | 2 |
| Pre-existing airway | 26 | 2 | 10 | 0 | 1 | 0 | 12 | 1 | 0 |
| Endotracheal intubation | 80 | 21 | 15 | 2 | 4 | 2 | 20 | 8 | 8 |
| Direct laryngoscopy | 34 | 9 | 3 | 0 | 3 | 0 | 8 | 3 | 8 |
| Video-assisted | 18 | 3 | 4 | 0 | 1 | 2 | 8 | 0 | 0 |
| Fiberoptic or bronchoscope | 28 | 9 | 8 | 2 | 0 | 0 | 4 | 5 | 0 |
| (C/L) III° or IV° documented 3 | 28 | 16 | 5 | 0 | 0 | 0 | 4 | 3 | 0 |
| Difficult airway management | 12 | 4 | 3 | 0 | 0 | 0 | 2 | 3 | 0 |
| Respiratory adverse event | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Cardiovascular adverse event | 5 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| Postoperative ICU 5 care | 54 | 10 | 17 | 1 | 5 | 0 | 13 | 4 | 4 |
| Classification | Type I | Type II | Type III | Type IV | Total Patient |
|---|---|---|---|---|---|
| Subtype: number | I(S):1 | 11 | IIIB:1 IIIC:1 | IV(A):1 | 15 |
| Fender (M/F) | 1/0 | 11/0 | 1/1 | 1/0 | 14/1 |
| Age (y/o) 1 | 25 | 15.8 (7–23) | 21 (17–25) | 28 | 17.9 |
| Body weight (kg) | 74 | 37.8 (24–57) | 43 (39–47) | 26.6 | 40.2 |
| Body height (cm) | 161 | 133.5 (110.5–156) 2 | 152 (144–160) | h104 | 136.4 |
| Dose of intrathecal 5% bupivacaine (mg) | 13 | 8.4 (5–11) | 10 | 7.5 | 8.9 |
| Combine with epidural anesthesia | 0 | 1 | 0 | 0 | 1 |
| Premedication for analgesia or sedation | 0 | 6 | 1 | 0 | 7 |
| Intraoperative opioids | 0 | 5 | 1 | 1 | 7 |
| Intraoperative midazolam or ketamine | 0 | 7 | 1 | 1 | 9 |
| Intraoperative sevoflurane or propofol | 0 | 2 | 1 | 0 | 3 |
| Intraoperative local anesthesia | 0 | 3 | 0 | 1 | 4 |
| Duration of surgery (min) | 170 | 100.5 (55–190) | 77.5 (45–110) | 155 | 105.7 |
| Hypotensive adverse event | 0 | 1 | 1 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, H.-C.; Lin, Y.-C.; Liang, M.-L.; Yang, Y.-W.; Huang, Y.-H.; Chan, Y.-L.; Hsu, Y.-W.; Lin, S.-P.; Chuang, C.-K.; Cheng, J.-K.; et al. The Anesthetic Strategy for Patients with Mucopolysaccharidoses: A Retrospective Cohort Study. J. Pers. Med. 2022, 12, 1343. https://doi.org/10.3390/jpm12081343
Lao H-C, Lin Y-C, Liang M-L, Yang Y-W, Huang Y-H, Chan Y-L, Hsu Y-W, Lin S-P, Chuang C-K, Cheng J-K, et al. The Anesthetic Strategy for Patients with Mucopolysaccharidoses: A Retrospective Cohort Study. Journal of Personalized Medicine. 2022; 12(8):1343. https://doi.org/10.3390/jpm12081343
Chicago/Turabian StyleLao, Hsuan-Chih, Ying-Chun Lin, Muh-Lii Liang, Ying-Wei Yang, Ya-Hsien Huang, Ying-Lun Chan, Yung-Wei Hsu, Shuan-Pei Lin, Chih-Kuang Chuang, Jen-Kun Cheng, and et al. 2022. "The Anesthetic Strategy for Patients with Mucopolysaccharidoses: A Retrospective Cohort Study" Journal of Personalized Medicine 12, no. 8: 1343. https://doi.org/10.3390/jpm12081343
APA StyleLao, H.-C., Lin, Y.-C., Liang, M.-L., Yang, Y.-W., Huang, Y.-H., Chan, Y.-L., Hsu, Y.-W., Lin, S.-P., Chuang, C.-K., Cheng, J.-K., & Lin, H.-Y. (2022). The Anesthetic Strategy for Patients with Mucopolysaccharidoses: A Retrospective Cohort Study. Journal of Personalized Medicine, 12(8), 1343. https://doi.org/10.3390/jpm12081343