Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence
Abstract
:1. Introduction
2. Intradermal Vaccination
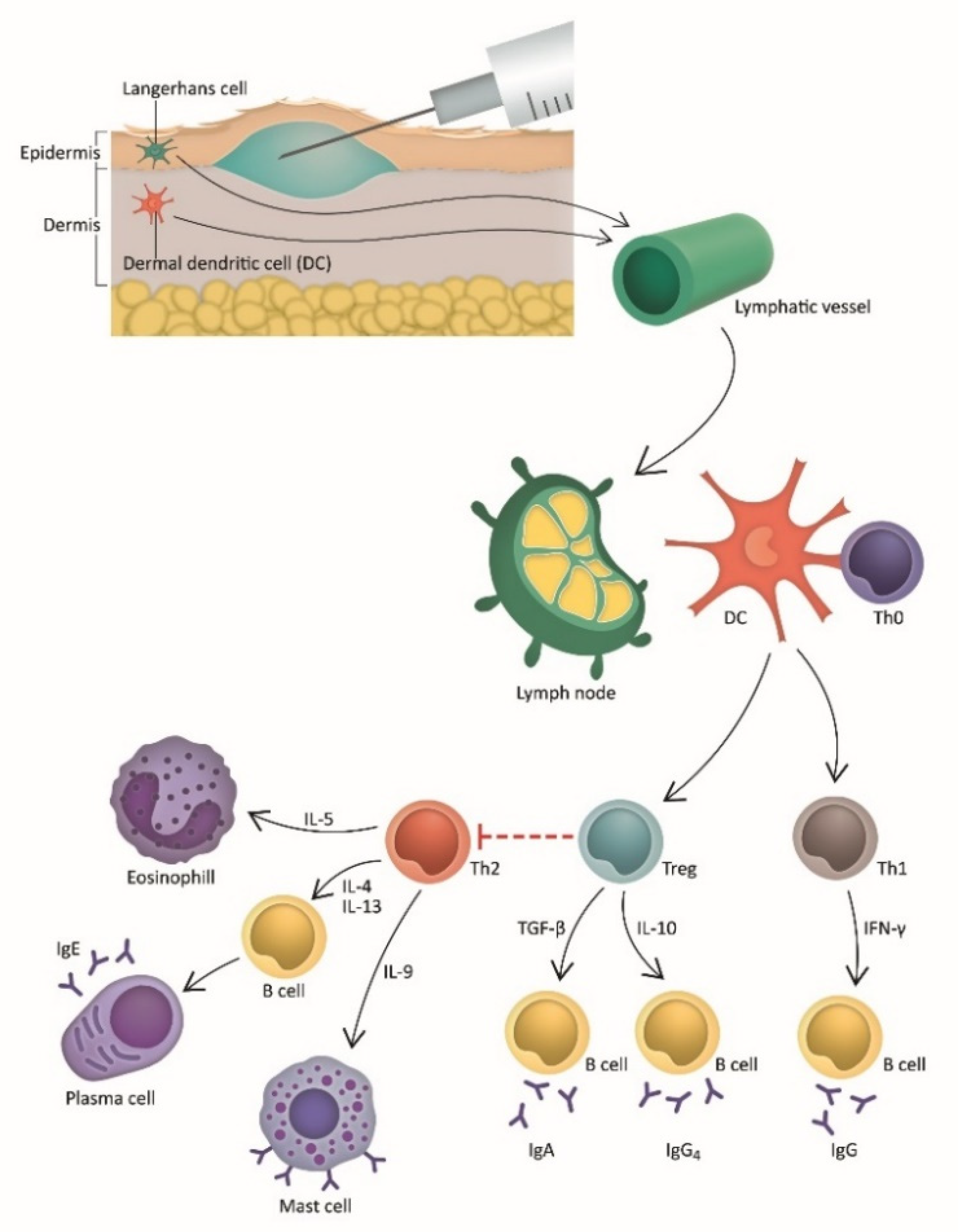
3. Intradermal Immunotherapy (IDIT): Pathophysiology and Mechanism
4. Clinical Studies of Intradermal Immunotherapy
5. Modified Formulations of Intradermal Immunotherapy
5.1. Allergoids
5.2. Peptide Immunotherapy
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wise, S.K.; Lin, S.Y.; Toskala, E.; Orlandi, R.R.; Akdis, C.A.; Alt, J.A.; Azar, A.; Baroody, F.M.; Bachert, C.; Canonica, G.W.; et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int. Forum Allergy Rhinol. 2018, 8, 108–352. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152, S1–S43. [Google Scholar] [CrossRef] [PubMed]
- Lundbäck, B. Epidemiology of rhinitis and asthma. Clinical and experimental allergy. J. Br. Soc. Allergy Clin. Immunol. 1998, 28, 3–10. [Google Scholar]
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. N. Am. 2016, 36, 235–248. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Halken, S.; Agache, I.; Angier, E.; Fernandez-Rivas, M.; van Wijk, R.G.; Jutel, M.; Lau, S.; Pajno, G.; et al. EAACI guidelines on allergen immunotherapy: Executive statement. Allergy 2018, 73, 739–743. [Google Scholar] [CrossRef]
- Bousquet, J.; Pfaar, O.; Togias, A.; Schünemann, H.J.; Ansotegui, I.; Papadopoulos, N.G.; Tsiligianni, I.; Agache, I.; Anto, J.M.; Bachert, C.; et al. 2019 ARIA Care pathways for allergen immunotherapy. Allergy 2019, 74, 2087–2102. [Google Scholar] [CrossRef]
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631. [Google Scholar] [CrossRef] [Green Version]
- Calderon, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst. Rev. 2007, 2007, Cd001936. [Google Scholar] [CrossRef]
- Cox, L.; Calderón, M.; Pfaar, O. Subcutaneous allergen immunotherapy for allergic disease: Examining efficacy, safety and cost-effectiveness of current and novel formulations. Immunotherapy 2012, 4, 601–616. [Google Scholar] [CrossRef]
- Noon, L. Prophylactic inoculation against hay fever. Historical document. Ann. Allergy 1960, 18, 287–291. [Google Scholar]
- Cohen, S.G.; Richard, E., 3rd. Allergen immunotherapy in historical perspective. In Allergens and Allergen Immunotherapy, 4th ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 21, pp. 1–30. [Google Scholar]
- Radulovic, S.; Wilson, D.; Calderon, M.; Durham, S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy 2011, 66, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, D.; Bilancia, M.; Albanesi, M.; Caiaffa, M.F.; Macchia, L. Cost-effectiveness of grass pollen allergen immunotherapy in adults. Allergy 2020, 75, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.A.; Röder, E.; Gerth van Wijk, R.; Al, M.J.; Hop, W.C.; Rutten-van Mölken, M.P. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2013, 132, 353–360.e352. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; van Wijk, R.G.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.C.; Jarrahian, C.; Zehrung, D.; Mitragotri, S.; Prausnitz, M.R. Delivery systems for intradermal vaccination. In Intradermal Immunization; Teunissen, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 351, pp. 77–112. [Google Scholar] [CrossRef]
- Migliore, A.; Gigliucci, G.; di Marzo, R.; Russo, D.; Mammucari, M. Intradermal Vaccination: A Potential Tool in the Battle Against the COVID-19 Pandemic? Risk Manag. Healthc. Policy 2021, 14, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M. Intradermal covid-19 vaccination could solve supply problems. BMJ 2021, 374, n1980. [Google Scholar] [CrossRef]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef]
- Nicolas, J.F.; Guy, B. Intradermal, epidermal and transcutaneous vaccination: From immunology to clinical practice. Expert Rev. Vaccines 2008, 7, 1201–1214. [Google Scholar] [CrossRef]
- Schnyder, J.L.; de Pijper, C.A.; Garrido, H.M.G.; Daams, J.G.; Goorhuis, A.; Stijnis, C.; Schaumburg, F.; Grobusch, M.P. Fractional dose of intradermal compared to intramuscular and subcutaneous vaccination—A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 37, 101868. [Google Scholar] [CrossRef]
- Senti, G.; von Moos, S.; Kündig, T.M. Epicutaneous allergen administration: Is this the future of allergen-specific immunotherapy? Allergy 2011, 66, 798–809. [Google Scholar] [CrossRef]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Veen, W.; Akdis, M. Tolerance mechanisms of allergen immunotherapy. Allergy 2020, 75, 1017–1018. [Google Scholar] [CrossRef] [Green Version]
- Sözener, Z.C.; Mungan, D.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C. Tolerance mechanisms in allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2011, 127, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, T.; Ura, T.; Taniguchi, M.; Yoshida, H. Intradermal Delivery of Antigens Enhances Specific IgG and Diminishes IgE Production: Potential Use for Vaccination and Allergy Immunotherapy. PLoS ONE 2016, 11, e0167952. [Google Scholar] [CrossRef]
- Phillips, E.W. Relief of hay-fever by intradermal injections of pollen extract. JAMA 1926, 86, 182–184. [Google Scholar] [CrossRef]
- Phillips, E.W. Intradermal pollen therapy during the attack. J. Allergy 1933, 5, 29–36. [Google Scholar] [CrossRef]
- Rotiroti, G.; Shamji, M.; Durham, S.R.; Till, S.J. Repeated low-dose intradermal allergen injection suppresses allergen-induced cutaneous late responses. J. Allergy Clin. Immunol. 2012, 130, 918–924.e911. [Google Scholar] [CrossRef]
- Vieira-Hernandez, A.; Capriles-Hulett, A.; Sanchez-Borges, M.; Fabiano, F.; Albarran-Barrios, C. Intradermal immunotherapy with low-dose house dust mite allergens in patients with allergic rhinitis: A proof-of-concept study. Revista Alergia México 2018, 65, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondon, C.; Sánchez-Borges, M.; Cupello, E.R.; Fabiano, F.; Capriles-Hulett, A. Aqueous intradermal low-dose house dust mite immunotherapy in tropical settings: A valid cost-effective approach for developing nations? Allergol. Immunopathol. 2021, 49, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Slovick, A.; Douiri, A.; Muir, R.; Guerra, A.; Tsioulos, K.; Hay, E.; Lam, E.P.S.; Kelly, J.; Peacock, J.L.; Ying, S.; et al. Intradermal grass pollen immunotherapy increases T(H)2 and IgE responses and worsens respiratory allergic symptoms. J. Allergy Clin. Immunol. 2017, 139, 1830–1839.e1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, C.E. The use of Allergoids and Adjuvants in Allergen Immunotherapy. Arch. Asthma Allergy Immunol. 2017, 1, 40–60. [Google Scholar] [CrossRef]
- Martínez, F.J.S.; BJiménez, R.M.B.; García, C.M.; Sánchez, C.S.; Guerra, C.B.; Fernández-Rivas, M.; Castro, A.V.; González, I.D.; Martínez, A.C.; Bravo, C.P.; et al. Intradermal Phleum pratense allergoid immunotherapy. Double-blind, randomized, placebo-controlled trial. Clin. Exp. Allergy 2020, 50, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C.; Krishna, M.T. Peptide allergen-specific immunotherapy for allergic airway diseases-State of the art. Clin. Exp. Allergy 2021, 51, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.S.; Ohman, J.L., Jr.; Long, A.A.; Creticos, P.S.; Gefter, M.A.; Shaked, Z.; Wood, R.A.; Eggleston, P.A.; Hafner, K.B.; Rao, P.; et al. Treatment of cat allergy with T-cell reactive peptides. Am. J. Respir. Crit. Care Med. 1996, 154, 1623–1628. [Google Scholar] [CrossRef]
- Simons, F.E.; Imada, M.; Li, Y.; Watson, W.T.; HayGlass, K.T. Fel d 1 peptides: Effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int. Immunol. 1996, 8, 1937–1945. [Google Scholar] [CrossRef] [Green Version]
- Ellis, A.K.; Frankish, C.W.; O’Hehir, R.E.; Armstrong, K.; Steacy, L.; Larché, M.; Hafner, R.P. Treatment with grass allergen peptides improves symptoms of grass pollen-induced allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2017, 140, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Ellis, A.K.; Frankish, C.W.; Armstrong, K.; Steacy, L.; Tenn, M.W.; Pawsey, S.; Hafner, R.P. Persistence of the clinical effect of grass allergen peptide immunotherapy after the second and third grass pollen seasons. J. Allergy Clin. Immunol. 2020, 145, 610–618.e619. [Google Scholar] [CrossRef] [Green Version]
- Worm, M.; Lee, H.H.; Kleine-Tebbe, J.; Hafner, R.P.; Laidler, P.; Healey, D.; Buhot, C.; Verhoef, A.; Maillère, B.; Kay, A.B.; et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011, 127, 89–97e14. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Couroux, P.; Hickey, P.; Salapatek, A.M.; Laidler, P.; Larché, M.; Hafner, R.P. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: A randomized, placebo-controlled study. J. Allergy Clin. Immunol. 2013, 131, 103–109.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couroux, P.; Patel, D.; Armstrong, K.; Larché, M.; Hafner, R.P. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin. Exp. Allergy 2015, 45, 974–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, L.; Nelson, H.; Lockey, R.; Calabria, C.; Chacko, T.; Finegold, I.; Nelson, M.; Weber, R.; Bernstein, D.I.; Blessing-Moore, J.; et al. Allergen immunotherapy: A practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, S1–S55. [Google Scholar] [CrossRef]
- Schaumburg, F.; de Pijper, C.A.; Grobusch, M.P. Intradermal travel vaccinations-when less means more. Travel Med. Infect. Dis. 2019, 28, 3–5. [Google Scholar] [CrossRef]
| Study | Study Design | Allergen | Study Population | Dose | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|
| Phillips et al. (1926) [30] | Cohort | Local pollens | NR (n = 29) | -Injection with increasing dose with 3 or 6 doses/week adjusted by patient’s size of local sensitization. -Interval was increased after the relief of symptoms. | NR | -Relief of symptoms -Safety | -Complete or near relief occurred in all 29 cases. -Symptom relief was shown to be associated with the size of local reaction. |
| Phillips et al. (1933) [31] | Cohort | Local pollens | Children and adults (n = 322) | -Injection with increasing dose daily, adjusted according to local reaction. -Interval was increased after the relief of symptoms. | NR | -Relief of symptoms -Safety | -91.6% of the patients expressed satisfactory relief. -Prompt relief was usually before 7 days. -12 general reactions occurred in nine patients (1: 625 doses). -There was no fatal reaction. |
| Rotiroti et al. (2012) [32] | RCT | Timothy grass (Phleum pratense) and/or silver birch (Betula verrucosa) pollen | Adults (10/10/9) (n = 29) | Injection of 0.1, 1.0, 10 BU of grass and/or birch pollens each visit; 2-week interval/visit Group A: Grass and birch pollen extracts at visits 1 and 6, grass pollen extract at visits 2–5; Group B: Grass and birch pollen extracts at visits 1 and 6; Group C: Grass and birch pollen extracts at visit 6 only | 10 weeks | -Cutaneous response -Specific IgG, IgG1, IgG4, IgE-FAB | -Cutaneous response at 24 h was significantly suppressed in group A compared to groups B and C, although early responses were equivalent among groups. -Grass pollen-specific IgG was increased in group A at both week 6 and week 10. -Absolute level of IgG1 and IgG4 were not statistically increased; however, 2.4-fold increase of IgG1 was observed in group A. -Increase in inhibition of IgE allergen complex binding to B cells in group A between week 6 and week 10. |
| Slovick et al. (2016) [35] | RCT | Timothy grass (Phleum pratense) pollen | Adults (47/46) (n = 93) | Injection of 10 BU of grass pollen or histamine pre-seasonally for 7 visits at 2-week intervals Group A: grass pollen Group B: histamine | 12 weeks | -CSMS -Symptoms score -Medication score -VAS -Mini-RQLQ -EQ-5D-5L -Medication/symptom-free day -AEs -Skin biopsy -Cutaneous response -Serum-specific IgE, IgG -Basophil activation test | -CSMS was similar between two groups over the entire pollen season. -Nasal symptom score and VAS nasal score were 44% and 28% higher, respectively, in the treatment group. -Mini-RQLQ scores, EQ-5D-5L scores, and the numbers of symptom-free or medication-free days were not different between both comparisons. -There were no serious AEs. -There was no difference in AEs between both comparisons. -Skin surface markers demonstrated higher TH2 marker CRTH2 expression and lower TH1 cell marker CXCR3 in treatment group. -Suppression of late-phase cutaneous response was shown at 4 and 7 months but not at 10 and 13 months. -Serum-specific IgE reduction was lower in the treatment group. -Serum-specific IgG4 was similar between both comparisons. -There was no significant effect of treatment on basophil activation markers. |
| Vieira-Hernández et al. (2018) [33] | Cohort ; A pilot study of Rondon et al. (2021) [34] | Mixed dust mite (Dermatophagoides pteronyssinus/Dermatophagoides farinae and Blomia tropicalis) -5 ng of HDM major allergens and 2.5 DBU of Blomia tropicalis allergens per 0.05 mL. | Children (n = 8) | Injection of allergen with a mix of 5 ng of Dermatophagoides pteronyssinus/Dermatophagoides farinae and 2.5 DBU of Blomia tropicalis at a 1-week interval x 3 months | 12 weeks | -TNSS -fVAS -Serum-specific IgG4 | -TNSS and fVAS were decreased. -Specific IgG4 was significantly increased for Blomia tropicalis, and a trend toward increased specific IgG4 was observed for Dermatophagoides pteronyssinus/Dermatophagoides farinae. |
| Rondon et al. (2021) [34] | Cohort | Mixed dust mite (Dermatophagoides pteronyssinus/Dermatophagoides farinae and Blomia tropicalis) | Children (n = 17) | Injection of allergen with a mix of 50 ng of Dermatophagoides pteronyssinus/Dermatophagoides farinae and 120 ng of Blomia tropicalis at a 1-week interval x 3 months, followed by a 2-week interval x 3 months, followed by a 3 week interval x 3 months, followed by a 4-week interval x 3 months | 1 year | -TNSS -fVAS -Serum-specific IgE, IgG4, IL 10 | -TNSS and fVAS were decreased after 42 and 49 days, and remained so until 1 year. -Specific IgG4 and IL-10 were increased after treatment. -Only minor local reactions were observed. |
| Study | Study Design | Allergen | Study Population | Dose | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|
| Martínez et al. (2020) [37] | RCT | Timothy grass (Phleum pratense) allergoid | Children and adults (53/42/53) (n = 148) | Injection of allergoid 0.03 or 0.06 μg protein/dose or placebo pre-seasonally for 2 consecutive years at 1-week intervals for 6 weeks each year Group A: low dose Group B: high dose Group C: placebo | 2 years | -CSMS -Symptom score -Medication score -Medication/symptom-free day -Conjunctival provocation test -Serum-specific IgE, IgG4 -AEs | -High-dose group had lower CSMS than the low-dose or placebo groups. -Increase in protein concentrations was needed to induce the conjunctival provocation test in all active groups. -P. pratense IgE level after 2 years in high-dose group was lower than baseline. -Specific IgG4 level did not increase in any group. -There were no differences in AEs between the treatment and placebo group. |
| Study | Study Design | Allergen | Study Population | Dose | Duration | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|
| Ellis et al. (2017) [41] | RCT | Mixed grass allergen peptides (derived from Cyn d 1, Lol p 5, Dac g 5, Hol l 5, and Phl p 5) | Adults (71/70/71/70) (n = 282) | Injection of allergen peptide at different intervals pre-seasonally Group A: 6 nmol pep-tide at 2-week intervals for 8 doses Group B: 12 nmol peptide at 4-week intervals for 4 doses Group C: 12 nmol peptide at 2-week intervals for 8 doses Group D: placebo | 14 weeks | -TRSS (4 days of EEU challenge at 25 weeks post-treatment initiation) -Serum-specific IgA, IgE, and IgG4 -AEs | -The mean TRSS was significantly improved only in group A compared to the placebo. -Group B showed a reduction in TRSS at all but one time point after the EEU challenge compared to placebo. -TRSS in group C was not different from that of the placebo. -There were no significant changes in specific IgA, IgE, and IgG4 levels from baseline in all groups. -There was a similar rate of AEs between the treatment and placebo group. |
| Ellis et al. (2020) [42] | RCT; Follow-up study of Ellis et al. (2017) | Mixed grass allergen peptides (derived from Cyn d 1, Lol p 5, Dac g 5, Hol l 5, and Phl p 5) | Adults (1-year, n= 122; 2 year, n = 85) | Injection of allergen peptide in different interval pre-seasonally Group A: 6 nmol peptide at 2-week intervals for 8 doses Group B: 12 nmol peptide at 4-week intervals for 4 doses Group C: 12 nmol peptide at 2-week intervals for 8 doses Group D: placebo | 14 weeks | -TRSS (4 days of EEU challenge at 1 year and 2 years post-treatment initiation) -Serum-specific IgA, IgE, and IgG4 -AEs | -Group A and B regimens demonstrated a trend toward a reduction in the mean TRSS after the EEU challenge compared to the placebo at 1-year and 2-year follow ups. However, no statistical significance was shown. -Group C showed effects comparable to that of the placebo. -There were no changes in specific IgA, IgE, and IgG4 levels from the baseline in all groups. |
| Worm et al. (2011) [43] | RCT | Cat allergen peptide (derived from Fel d 1) | Adults (40/48) (n = 88) | Single injection of allergen peptide at different concentrations or placebo Group A: IDIT (dose level of 0.03, 0.3, 1, 3, 12 nmol, placebo) Group B: SCIT (dose level of 0.03, 0.3, 1, 3, 12, 20 nmol, placebo) | Single injection | -Cutaneous response -AEs | -IDIT showed a trend toward a reduction in cutaneous response at 8-h, 21 days after injection with a 3 nmol dose compared to the placebo; however, no statistical significance was shown. -There was no severe AE reported. |
| Patel et al. (2013) [44] | RCT | Cat allergen peptide (derived from Fel d 1) | Adults (67/66/69) (n = 202) | Injection of allergen peptide at different intervals Group A: 3 nmol at 2-week intervals for 8 doses Group B: 6 nmol at 4-week intervals for 4 doses Group C: placebo | 12–14 weeks | -TRSS (4 days of EEU challenge at 18–22 and 50–54 weeks post-treatment initiation) -Serum-specific IgE -AEs | -Group B had significantly better TRSS reduction compared to group A and the placebo at the 1-year follow up. -There were no changes in specific IgE levels compared to the baseline in any group. -No severe AE was reported in any group. |
| Couroux et al. (2015) [45] | RCT; Follow-up study of Patel et al. (2013) [44] | Cat allergen peptide (derived from Fel d 1) | Adults (n = 51) | Injection of allergen peptide at different intervals Group A: 3 nmol at 2-week intervals for 8 doses Group B: 6 nmol at 4-week intervals for 4 doses Group C: placebo | 12–14 weeks | -TRSS (4 days of EEU challenge at 2 years post-treatment initiation) -AEs | -Group B demonstrated a trend toward reduction in mean TRSS compared to the placebo at 2 years after the EEU challenge; however, no statistical significance was shown. -Group A showed effects comparable to that of the placebo. -No severe AE was reported in any group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atipas, K.; Kanjanawasee, D.; Tantilipikorn, P. Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence. J. Pers. Med. 2022, 12, 1341. https://doi.org/10.3390/jpm12081341
Atipas K, Kanjanawasee D, Tantilipikorn P. Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence. Journal of Personalized Medicine. 2022; 12(8):1341. https://doi.org/10.3390/jpm12081341
Chicago/Turabian StyleAtipas, Kawita, Dichapong Kanjanawasee, and Pongsakorn Tantilipikorn. 2022. "Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence" Journal of Personalized Medicine 12, no. 8: 1341. https://doi.org/10.3390/jpm12081341
APA StyleAtipas, K., Kanjanawasee, D., & Tantilipikorn, P. (2022). Intradermal Allergen Immunotherapy for Allergic Rhinitis: Current Evidence. Journal of Personalized Medicine, 12(8), 1341. https://doi.org/10.3390/jpm12081341







