Echocardiographic and Cardiac MRI Comparison of Longitudinal Strain and Strain Rate in Cancer Patients Treated with Immune Checkpoint Inhibitors
Abstract
:1. Introduction
2. Methods
2.1. Definitions and Outcomes of Interest
2.2. Covariates
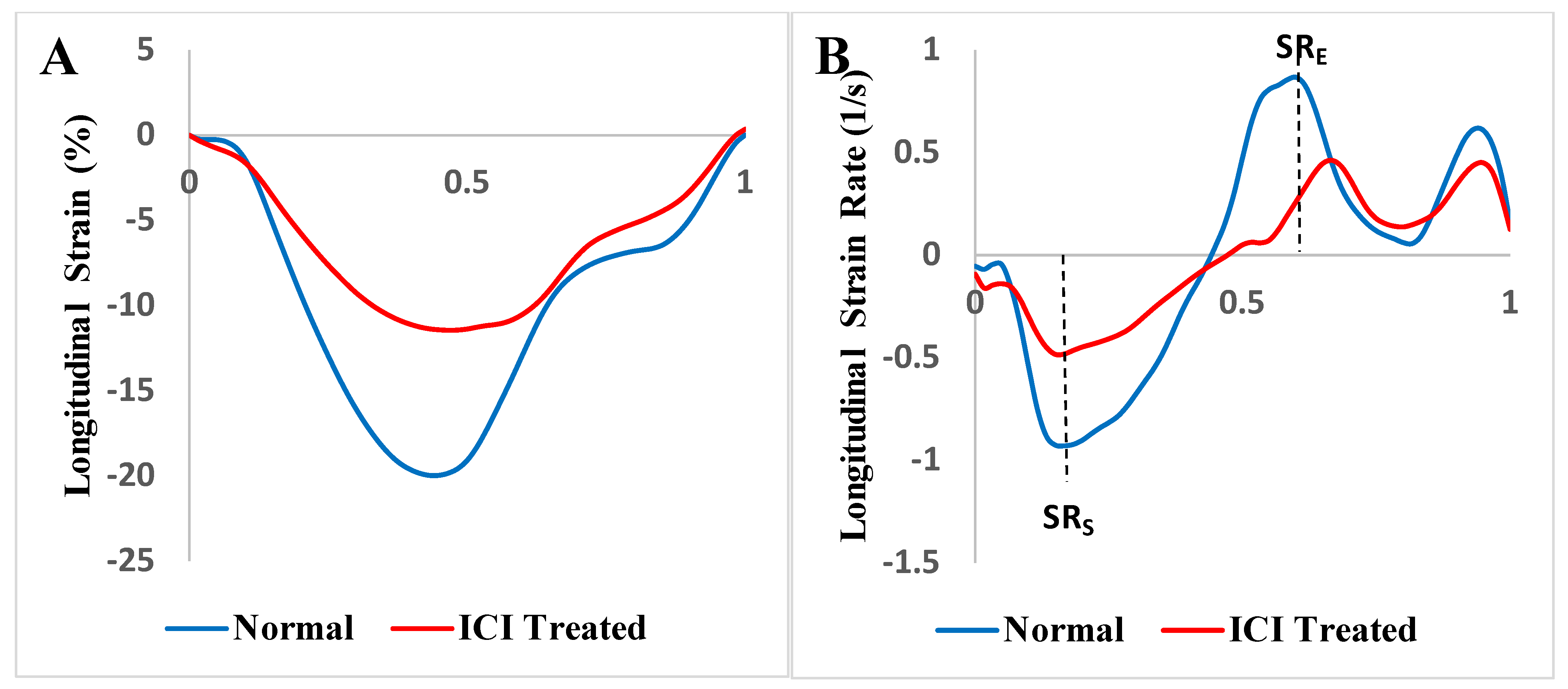
2.3. Strain and Strain Rate Analysis
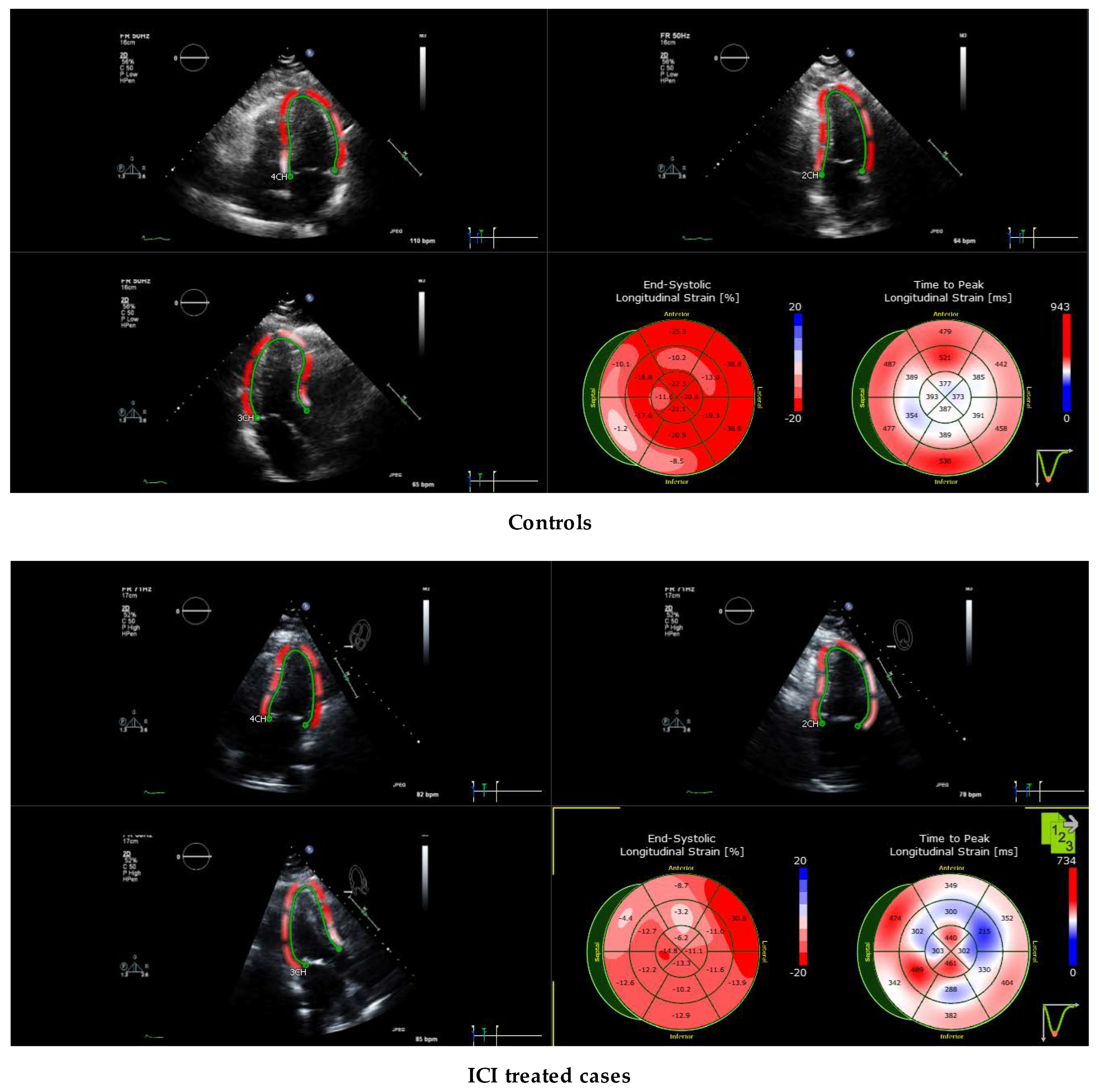
2.4. Echocardiography Protocol for Strain Evaluation
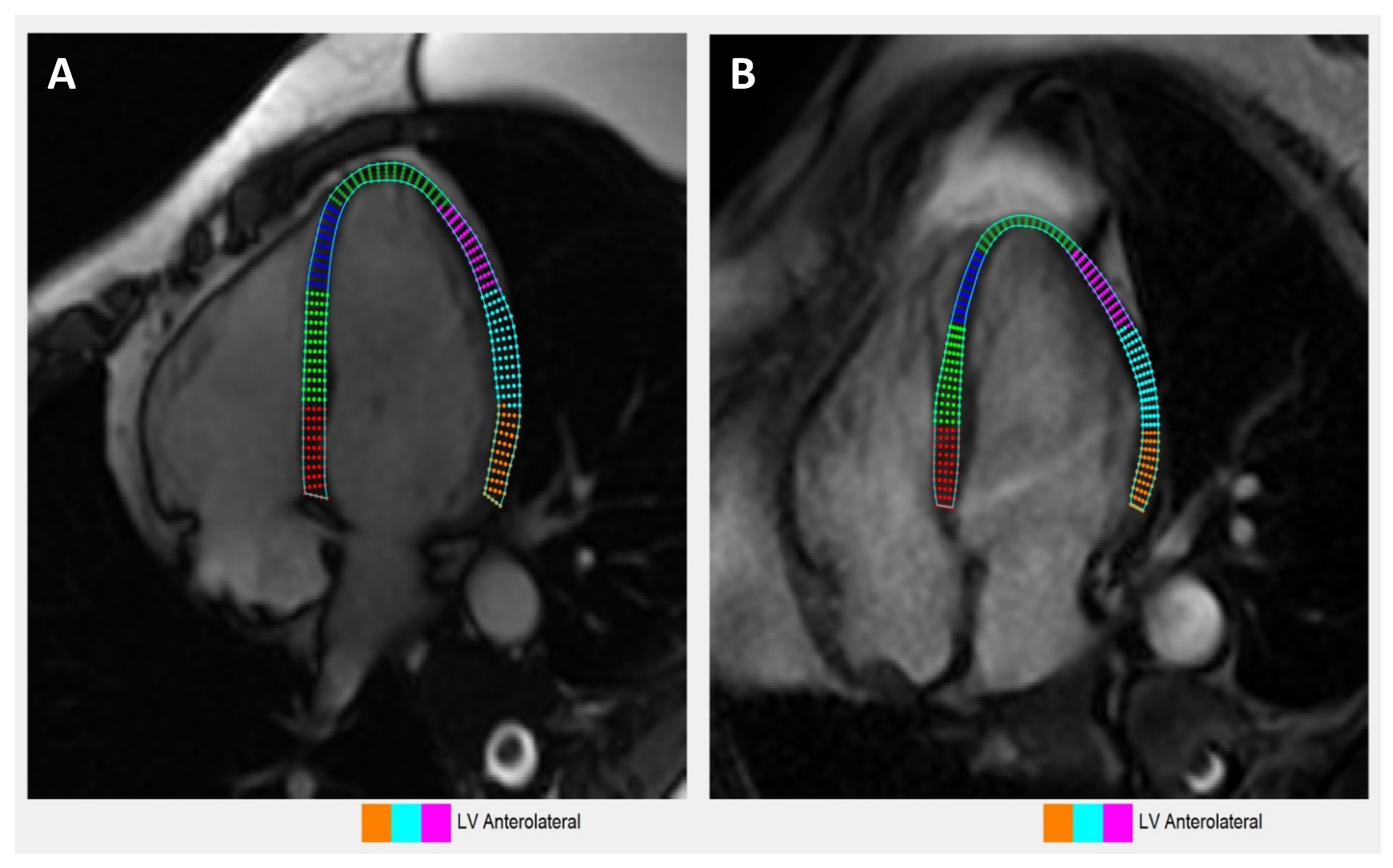
2.5. Contrast-Enhanced CMR Protocol
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Cancer Type and ICI Treatment Characteristics
3.3. Cardiac Biomarkers
3.4. Myocarditis Onset, Grading, Treatment and Outcomes
3.5. Conduction Abnormalities in Electrocardiogram
3.6. Echocardiographic Characteristics
3.7. Cardiac MRI Characteristics
3.8. Comparison of Echocardiography vs. Cardiac Magnetic Resonance Imaging-Based Strains
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICI | Immune Checkpoint Inhibitors |
| GLS | Global Longitudinal Strain |
| CMR | Cardiac MRI |
| LGE | Late Gadolinium Enhancement |
| CTCAE | Common Toxicity Criteria for Adverse Events |
References
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Yau, M.; Mezey, N.; Joarder, I.; Micieli, J.A. Neuro-ophthalmic Complications of Immune Checkpoint Inhibitors: A Systematic Review. Eye Brain. 2020, 12, 139–167. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-Related Thyroiditis with Immune Checkpoint Inhibitors. Thyroid 2018, 28, 1243–1251. [Google Scholar] [CrossRef]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’Ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Cottrell, T.R.; Lipson, E.J.; Forde, P.M.; Illei, P.B.; Yarmus, L.B.; Voong, K.R.; Feller-Kopman, D.; Lee, H.; Riemer, J.; et al. Chronic immune checkpoint inhibitor pneumonitis. J. Immunother. Cancer 2020, 8, e000840. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Rahma, O.E.; Hashemi, N.; Lim, R.M. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: Algorithms for Management. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 13–19. [Google Scholar] [CrossRef]
- Braaten, T.J.; Brahmer, J.R.; Forde, P.M.; Le, D.; Lipson, E.J.; Naidoo, J.; Schollenberger, M.; Zheng, L.; Bingham, C.O., 3rd; Shah, A.A.; et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann. Rheum. Dis. 2020, 79, 332. [Google Scholar] [CrossRef]
- Pourhassan, H.Z.; Tryon, D.; Schaeffer, B.; Mirshahidi, H.; Wong, J. Autoimmune rhabdomyolysis and a multiorgan display of PD-1 inhibitor induced immune related adverse events during treatment of metastatic melanoma. Exp. Hematol. Oncol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Chen, X.; Haggiagi, A.; Tzatha, E.; DeAngelis, L.M.; Santomasso, B. Electrophysiological findings in immune checkpoint inhibitor-related peripheral neuropathy. Clin. Neurophysiol. 2019, 130, 1440–1445. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Boland, P.; Heath, J.; Sandigursky, S. Immune checkpoint inhibitors and vasculitis. Curr. Opin. Rheumatol. 2020, 32, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, D.V.; Donisan, T.; Palaskas, N.L.; Iliescu, C. Emerging Concerns in Cardio-Oncology: Immune Checkpoint Inhibitor Cardiotoxicity: American College of Cardiology. 2019. Available online: https://www.acc.org/latest-in-cardiology/articles/2019/06/21/08/45/emerging-concerns-in-cardio-oncology (accessed on 1 July 2022).
- Xu, S.; Sharma, U.C.; Tuttle, C.; Pokharel, S. Immune Checkpoint Inhibitors: Cardiotoxicity in Pre-clinical Models and Clinical Studies. Front Cardiovasc. Med. 2021, 8, 619650. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Subramanian, P.; Yatsynovich, Y.V.; Jacobs, D.M.; Chilbert, M.R.; Sharma, U.C.; Ito, F.; Feuerstein, S.G.; Stefanovic, F.; Switzer, B.; et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J. Immunother. Cancer. 2021, 9, e002553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-W.; Zhu, Y.-J.; Wang, M.-N.; Xie, Y.; Chen, C.-Y.; Zhang, T.; Xia, F.; Ding, Z.-Y.; Liu, J.-Y. Immune Checkpoint Inhibitor-Associated Cardiotoxicity: Current Understanding on Its Mechanism, Diagnosis and Management. Front. Pharmacol. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar]
- Reddy, N.; Moudgil, R.; Lopez-Mattei, J.; Karimzad, K.; Mouhayar, E.N.; Somaiah, N.; Conley, A.P.; Patel, S.; Giza, D.E.; Iliescu, C. Progressive and Reversible Conduction Disease with Checkpoint Inhibitors. Can. J. Cardiol. 2017, 33, 1335.e13–1335.e15. [Google Scholar] [CrossRef]
- Giancaterino, S.; Abushamat, F.; Duran, J.; Lupercio, F.; DeMaria, A.; Hsu, J.C. Complete heart block and subsequent sudden cardiac death from immune checkpoint inhibitor-associated myocarditis. HeartRhythm Case Rep. 2020, 6, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Zlotoff, D.A.; Hassan, M.Z.O.; Zafar, A.; Alvi, R.M.; Awadalla, M.; Mahmood, S.S.; Zhang, L.; Chen, C.L.; Ederhy, S.; Barac, A.; et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J. Immunother. Cancer 2021, 9, e002007. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; Arbune, A.; Soufer, A.; Ragheb, E.; Kwan, J.M.; Lamy, J.; Henry, M.; Cuomo, J.R.; Charifa, A.; Gallegos, C.; et al. Left ventricular myocardial strain and tissue characterization by cardiac magnetic resonance imaging in immune checkpoint inhibitor associated cardiotoxicity. PLoS ONE 2021, 16, e0246764. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Sonkawade, S.D.; Pokharel, S.; Preda, M.; Schweser, F.; Zivadinov, R.; Kim, M.; Sharma, U.C. Tagged cine magnetic resonance imaging to quantify regional mechanical changes after acute myocardial infarction. Magn. Reson. Imaging 2020, 66, 208–218. [Google Scholar] [CrossRef]
- Reddy, A.; Singh, V.; Karthikeyan, B.; Jiang, L.; Kristo, S.; Kattel, S.; Amuthan, R.; Pokharel, S.; Sharma, U. Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy. Cardiogenetics 2021, 11, 98–110. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef]
- Morais, P.; Marchi, A.; Bogaert, J.A.; Dresselaers, T.; Heyde, B.; D’Hooge, J.; Bogaert, J. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: Assessment of variability in a real-life clinical setting. J. Cardiovasc. Magn. Reson. 2017, 19, 24. [Google Scholar] [CrossRef]
- Morais, P.; Heyde, B.; Barbosa, D.; Queirós, S.; Claus, P.; D’hooge, J. (Eds.) Cardiac Motion and Deformation Estimation from Tagged MRI Sequences Using a Temporal Coherent Image Registration Framework. In Functional Imaging and Modeling of the Heart; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Heyde, B.; Jasaityte, R.; Barbosa, D.; Robesyn, V.; Bouchez, S.; Wouters, P.; Maes, F.; Claus, P.; D’Hooge, J. Elastic image registration versus speckle tracking for 2-D myocardial motion estimation: A direct comparison In Vivo. IEEE Trans. Med. Imaging 2013, 32, 449–459. [Google Scholar] [CrossRef]
- Heiberg, E.; Sjögren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and validation of Segment—Freely available software for cardiovascular image analysis. BMC Med. Imaging 2010, 10, 1. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, H.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation 2020, 141, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Chryssos, E.D.; Parker, H. Magnetic resonance imaging: A wealth of cardiovascular information. Ochsner. J. 2009, 9, 266–277. [Google Scholar]
- Aquaro, G.D.; Ghebru Habtemicael, Y.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lancillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients with Acute Myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef]

| Baseline Characteristics | |
|---|---|
| Age, years | 69.1 (54–80) |
| Female, % | 37.5 |
| Male, % | 62.5 |
| Risk factors and past medical history | |
| Atrial fibrillation, % | 37.5 |
| Hypertension, % | 87.5 |
| Hyperlipidemia, % | 12.5 |
| Hypothyroidism, % | 50.0 |
| Diabetes mellitus, % | 62.5 |
| Dementia, % | 12.5 |
| Pulmonary embolism, % | 12.5 |
| Cancer | |
| Metastatic melanoma, % | 50% |
| Non-small cell lung carcinoma, % | 25% |
| Small cell lung carcinomatosis, % | 12.5% |
| Peritoneal carcinomatosis, % | 12.5% |
| Immune checkpoint inhibitor | |
| Pembrolizumab, % | 37.5% |
| Nivolumab, % | 37.5% |
| Avelumab, % | 12.5% |
| Ipilimumab, % | 25% |
| Durvalumab, % | 12.5% |
| Combination Ipilimumab and Nivolumab, % | 12.5% |
| Average days from first dose and onset of toxicity from ICI initiation | |
| Mean, days | 46 |
| Median, days | 35 |
| Mode, days | 21 |
| Cardiac biomarker data | |
| Mean Troponin at onset of symptoms, ng/mL | 1.79 (Range 0.24–6.18) |
| Mean Troponin Peak, ng/mL | 2.52 (Range 0.28–6.18) |
| Mean Maximum BNP, ng/mL | 136 (Range 34–318 pg/mL) |
| Mean Maximum CK-MB, ng/mL | 960 (Range 160–2494) |
| Mean Maximum Myoglobin, mg/dL | 1220 (39–3612) |
| Signs and Symptoms | |
|---|---|
| Cerebral vascular event | 1 case(s) |
| Chest pain | 3 case(s) |
| Dizziness | 1 case(s) |
| Dyspnea on exertion | 4 case(s) |
| Incidental troponin elevation | 1 case(s) |
| Lightheadedness | 1 case(s) |
| Orthopnea | 1 case(s) |
| Paroxysmal nocturnal dyspnea | 1 case(s) |
| Palpitations | 1 case(s) |
| Pruritis | 1 case(s) |
| Side effects beyond myocarditis | |
| Hepatitis | 2 case(s) |
| None | 4 case(s) |
| Thyroiditis | 1 case(s) |
| Vision changes | 1 case(s) |
| CTCAE grading for myocarditis | |
| Grade 3 | 7 case(s) |
| Grade 4 | 1 case(s) |
| Steroid start time and onset of symptoms (days) | |
| 0 | 4 case(s) |
| 1 | 1 case(s) |
| 3 | 1 case(s) |
| 21 | 1 case(s) |
| Treatment | |
| Aspirin 81 mg | 2 case(s) |
| Colchicine | 1 case(s) |
| Rosuvastatin | 1 case(s) |
| Ibuprofen | 1 case(s) |
| Infliximab | 1 case(s) |
| Methyl prednisone | 5 case(s) |
| Prednisone | 1 case(s) |
| Treatment outcome | |
| Myocarditis resolved | 7 case(s) |
| Death from other cause | 1 case(s) |
| Cause of death | |
| Cerebral vascular accident | 1 case(s) |
| Multi-organ failure | 1 case(s) |
| Myocardial infarction and cardiogenic shock | 1 case(s) |
| Electrocardiography on Presentation | |
|---|---|
| Sinus bradycardia | 2 case(s) |
| Sinus bradycardia with first degree AV block | 1 case(s) |
| Normal sinus rhythm with right bundle branch block | 1 case(s) |
| Sinus tachycardia with right bundle branch block | 1 case(s) |
| Normal sinus rhythm with right bundle branch block | 1 case(s) |
| Normal sinus rhythm | 1 case(s) |
| Sinus tachycardia | 1 case(s) |
| Atrial tachycardia with worsening ST depressions in inferior and precordial leads compared to previous electrocardiography | 1 case(s) |
| Electrocardiography changes from baseline | 1 case(s) |
| Echocardiography on presentation | |
| Ejection fraction ≥ 60% | 4 case(s) |
| Ejection fraction ≥ 50% | 2 case(s) |
| Pericardial effusion | 1 case(s) |
| Systolic dysfunction | 0 case(s) |
| Diastolic dysfunction | 1 case(s) |
| Controls | Mean Longitudinal Systolic Strain (%) | Mean Longitudinal Peak Systolic Strain Rate (s−1) | Mean Longitudinal Early Diastolic Strain Rate (s−1) |
|---|---|---|---|
| 1 | −23.179 | −1.192 | 1.430 |
| 2 | −18.125 | −0.773 | 0.687 |
| 3 | −18.274 | −0.887 | 1.035 |
| 4 | −18.671 | −0.873 | 0.873 |
| 5 | −22.272 | −1.054 | 1.249 |
| 6 | −18.510 | −0.852 | 1.027 |
| 7 | −19.221 | −0.918 | 1.099 |
| 8 | −19.836 | −1.031 | 1.183 |
| Average | −19.761 | −0.947 | 1.073 |
| Standard Deviation | 1.925 | 0.135 | 0.228 |
| Cases | Mean Longitudinal Systolic Strain (%) | Mean Longitudinal Peak Systolic Strain Rate (s−1) | Mean Longitudinal Early Diastolic Strain Rate (s−1) |
| 1 | −17.303 | −0.973 | 0.757 |
| 2 | −11.340 | −0.642 | 0.429 |
| 3 | −11.856 | −0.463 | 0.244 |
| 4 | −10.884 | −0.449 | 0.508 |
| 5 | −18.406 | −0.790 | 1.191 |
| 6 | −12.553 | −0.657 | 0.434 |
| 7 | −11.864 | −0.519 | 0.561 |
| 8 | −4.846 | −0.279 | 0.375 |
| Average | −12.381 | −0.597 | 0.562 |
| Standard Deviation | 4.161 | 0.218 | 0.295 |
| p value (Controls vs. Cases) | <0.001 | 0.002 | 0.002 |
| Parameters | Controls (n = 8) | ICI-Treated Patients (n = 8) | p Value | Hedges’s g for Effect Size |
|---|---|---|---|---|
| LV Systolic Longitudinal Strain (%) | ||||
| Basal | −22.602 ± 5.792 | −13.763 ± 2.530 | 0.001 (*) | 1.870 |
| Midventricular | −16.630 ± 2.292 | −9.800 ± 4.126 | 0.001 (*) | 1.935 |
| Apical | −20.196 ± 2.877 | −14.180 ± 7.564 | 0.054 | 0.994 |
| Global | −19.761 ± 1.925 | −12.381 ± 4.161 | <0.001 (*) | 2.152 |
| LV Peak Systolic Longitudinal Strain Rate (s−1) | ||||
| Basal | −1.146 ± 0.345 | −0.630 ± 0.172 | 0.002 (*) | 1.787 |
| Midventricular | −0.786 ± 0.135 | −0.473 ± 0.134 | <0.001 (*) | 2.202 |
| Apical | −0.891 ± 0.142 | −0.732 ± 0.559 | 0.446 | 0.370 |
| Global | −0.947 ± 0.135 | −0.597 ± 0.218 | 0.002 (*) | 1.830 |
| LV Early Diastolic Longitudinal Strain Rate (s−1) | ||||
| Basal | 1.333 ± 0.446 | 0.627 ± 0.210 | 0.001 (*) | 1.913 |
| Midventricular | 0.811 ± 0.182 | 0.385 ± 0.144 | <0.001 (*) | 2.460 |
| Apical | 1.076 ± 0.412 | 0.731 ± 0.798 | 0.295 | 0.514 |
| Global | 1.073 ± 0.228 | 0.562 ± 0.295 | 0.002 (*) | 1.834 |
| Controls | Mean Longitudinal Systolic Strain (%) | Mean Longitudinal Peak Systolic Strain Rate (s−1) | Mean Longitudinal Early Diastolic Strain Rate (s−1) |
| 1 | −16.960 | −0.828 | 0.565 |
| 2 | −15.825 | −0.492 | 0.661 |
| 3 | −13.026 | −0.619 | 0.432 |
| 4 | −17.419 | −0.825 | 0.666 |
| 5 | −13.159 | −0.623 | 0.352 |
| 6 | −16.746 | −0.748 | 0.516 |
| 7 | −13.299 | −0.569 | 0.435 |
| 8 | −13.941 | −0.605 | 0.412 |
| Average | −15.047 | −0.664 | 0.505 |
| Standard Deviation | 1.879 | 0.123 | 0.117 |
| Cases | Mean Longitudinal Systolic Strain (%) | Mean Longitudinal Peak Systolic Strain Rate(s−1) | Mean Longitudinal Early Diastolic Strain Rate(s−1) |
| 1 | −18.438 | −0.683 | 0.602 |
| 2 | −10.935 | −0.435 | 0.253 |
| 3 | −14.392 | −0.633 | 0.485 |
| 4 | −11.553 | −0.421 | 0.328 |
| 5 | −13.515 | −0.530 | 0.338 |
| 6 | −14.332 | −0.656 | 0.428 |
| 7 | −11.938 | −0.406 | 0.293 |
| 8 | −6.908 | −0.232 | 0.094 |
| Average | −12.751 | −0.499 | 0.353 |
| Standard Deviation | 3.331 | 0.155 | 0.154 |
| p value (Controls vs. Cases) | 0.112 | 0.034 | 0.043 |
| Parameters | Controls (n = 8) | ICI-Treated Patients (n = 8) | p Value | Hedges’s g for Effect Size |
|---|---|---|---|---|
| LV Systolic Longitudinal Strain (%) | ||||
| Basal | −18.359 ± 2.179 | −15.725 ± 4.035 | 0.127 | 0.768 |
| Midventricular | −14.880 ± 2.752 | −12.024 ± 4.278 | 0.135 | 0.751 |
| Apical | −10.330 ± 2.846 | −9.382 ± 3.540 | 0.565 | 0.279 |
| Global | −15.047 ± 1.879 | −12.751 ± 3.331 | 0.112 | 0.803 |
| LV Peak Systolic Longitudinal Strain Rate (s−1) | ||||
| Basal | −0.792 ± 0.147 | −0.605 ± 0.212 | 0.060 | 0.967 |
| Midventricular | −0.652 ± 0.141 | −0.491 ± 0.191 | 0.075 | 0.909 |
| Apical | −0.490 ± 0.169 | −0.353 ± 0.138 | 0.097 | 0.840 |
| Global | −0.664 ± 0.123 | −0.499 ± 0.155 | 0.034 (*) | 1.113 |
| LV Early Diastolic Longitudinal Strain Rate (s−1) | ||||
| Basal | 0.653 ± 0.162 | 0.455 ± 0.251 | 0.082 | 0.886 |
| Midventricular | 0.478 ± 0.159 | 0.264 ± 0.118 | 0.008 (*) | 1.449 |
| Apical | 0.323 ± 0.216 | 0.333 ± 0.177 | 0.926 | 0.045 |
| Global | 0.505 ± 0.117 | 0.353 ± 0.154 | 0.043 (*) | 1.051 |
| Parameters | Controls (n = 8) | ICI-Treated Patients (n = 8) | p Value | Hedges’s g for Effect Size |
|---|---|---|---|---|
| RV Systolic Longitudinal Strain (%) | ||||
| Free Wall | −19.965 ± 5.617 | −13.143 ± 5.168 | 0.024 (*) | 1.195 |
| Septal | −13.794 ± 3.398 | −10.186 ± 2.171 | 0.024 (*) | 1.196 |
| Global | −16.879 ± 4.026 | −11.665 ± 3.457 | 0.015 (*) | 1.314 |
| RV Peak Systolic Longitudinal Strain Rate (s−1) | ||||
| Free Wall | −0.861 ± 0.219 | −0.636 ± 0.305 | 0.112 | 0.801 |
| Septal | −0.573 ± 0.172 | −0.401 ± 0.138 | 0.044 (*) | 1.043 |
| Global | −0.717 ± 0.173 | −0.518 ± 0.218 | 0.063 | 0.954 |
| RV Early Diastolic Longitudinal Strain Rate (s−1) | ||||
| Free Wall | 0.689 ± 0.311 | 0.381 ± 0.189 | 0.031 (*) | 1.133 |
| Septal | 0.357 ± 0.172 | 0.290 ± 0.095 | 0.357 | 0.451 |
| Global | 0.523 ± 0.199 | 0.336 ± 0.108 | 0.035 (*) | 1.105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirza, J.; Sunder, S.S.; Karthikeyan, B.; Kattel, S.; Pokharel, S.; Quigley, B.; Sharma, U.C. Echocardiographic and Cardiac MRI Comparison of Longitudinal Strain and Strain Rate in Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Pers. Med. 2022, 12, 1332. https://doi.org/10.3390/jpm12081332
Mirza J, Sunder SS, Karthikeyan B, Kattel S, Pokharel S, Quigley B, Sharma UC. Echocardiographic and Cardiac MRI Comparison of Longitudinal Strain and Strain Rate in Cancer Patients Treated with Immune Checkpoint Inhibitors. Journal of Personalized Medicine. 2022; 12(8):1332. https://doi.org/10.3390/jpm12081332
Chicago/Turabian StyleMirza, Jibran, Sunitha Shyam Sunder, Badri Karthikeyan, Sharma Kattel, Saraswati Pokharel, Brian Quigley, and Umesh C. Sharma. 2022. "Echocardiographic and Cardiac MRI Comparison of Longitudinal Strain and Strain Rate in Cancer Patients Treated with Immune Checkpoint Inhibitors" Journal of Personalized Medicine 12, no. 8: 1332. https://doi.org/10.3390/jpm12081332
APA StyleMirza, J., Sunder, S. S., Karthikeyan, B., Kattel, S., Pokharel, S., Quigley, B., & Sharma, U. C. (2022). Echocardiographic and Cardiac MRI Comparison of Longitudinal Strain and Strain Rate in Cancer Patients Treated with Immune Checkpoint Inhibitors. Journal of Personalized Medicine, 12(8), 1332. https://doi.org/10.3390/jpm12081332






