Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Statistical Analysis
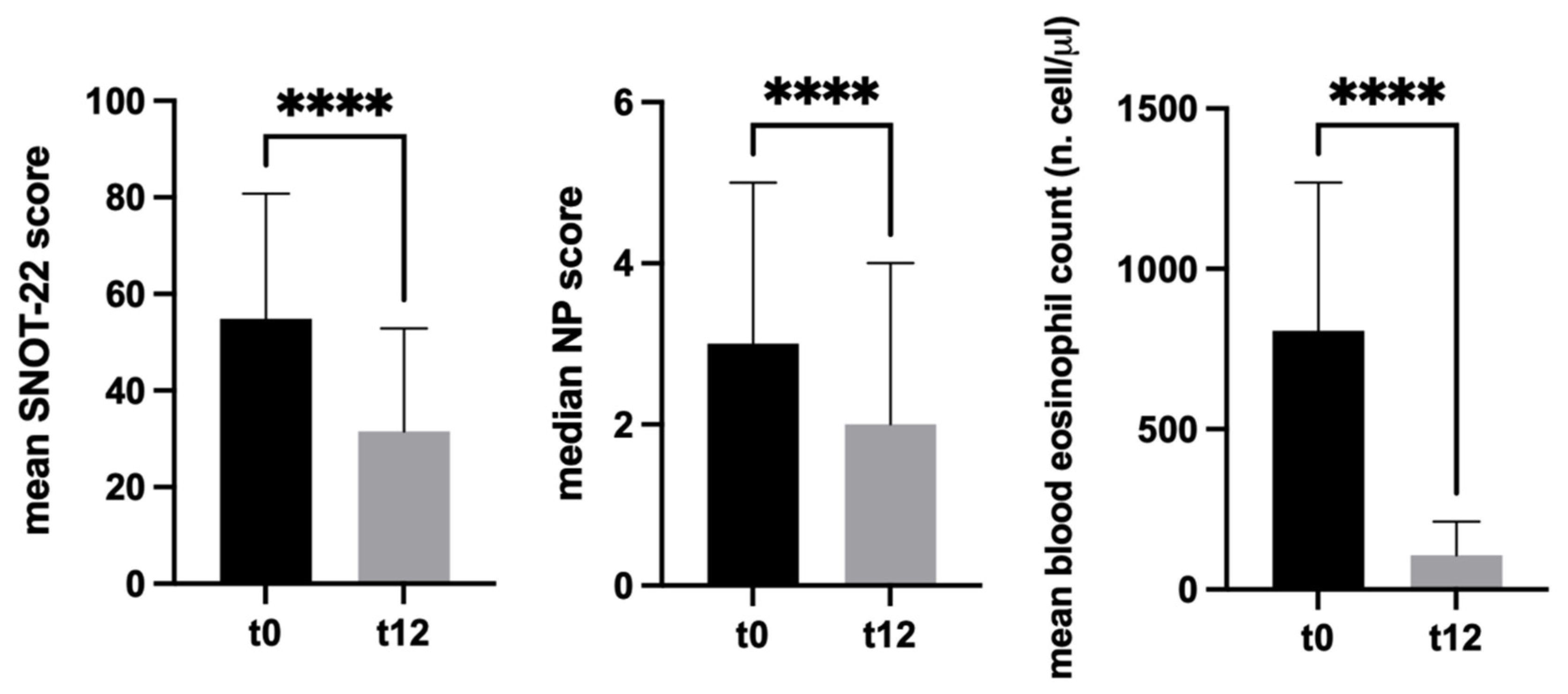
3. Results
3.1. Baseline Characteristics of the Population
3.2. Effects of Mepolizumab on Clinical Parameters
3.3. Quantification of Mepolizumab Effects
3.4. Tolerability Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelaia, C.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Terracciano, R.; Savino, R.; Pelaia, G. Severe eosinophilic asthma: From the patho-genic role of interleukin-5 to the therapeutic action of mepolizumab. Drug. Des. Devel. Ther. 2017, 11, 3137–3144. [Google Scholar] [CrossRef] [Green Version]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronica rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef]
- 2020 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf (accessed on 30 June 2022).
- Chan, R.; Kuo, C.R.; Lipworth, B. Disconnect between effects of mepolizumab on severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. Pr. 2020, 8, 1714–1716. [Google Scholar] [CrossRef]
- Detoraki, A.; Tremante, E.; D’Amato, M.; Calabrese, C.; Casella, C.; Maniscalco, M.; Poto, R.; Brancaccio, R.; Boccia, M.; Martino, M.; et al. Mepolizumab improves sino-nasal symptoms and asthma control in severe eosinophilic asthma patients with chronic rhinosinusitis and nasal polyps: A 12-month real-life study. Ther. Adv. Respir. Dis. 2021, 15. [Google Scholar] [CrossRef]
- Harvey, E.S.; Langton, D.; Katelaris, C.; Stevens, S.; Farah, C.S.; Gillman, A.; Harrington, J.; Hew, M.; Kritikos, V.; Radhakrishna, N.; et al. Mepolizumab effectiveness and iden-tification of super-responders in severe asthma. Eur. Respir. J. 2020, 55, 1902420. [Google Scholar] [CrossRef]
- Howarth, P.; Chupp, G.; Nelsen, L.M.; Bradford, E.S.; Bratton, D.J.; Smith, S.G.; Albers, F.C.; Brusselle, G.; Bachert, C. Severe eosinophilic asthma with nasal polyposis: A phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J. Allergy Clin. Immunol. 2020, 145, 1713–1715. [Google Scholar] [CrossRef] [Green Version]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]
- Sposato, B.; Scalese, M.; Camiciottoli, G.; Carpagnano, G.E.; Pelaia, C.; Santus, P.; Maniscalco, M.; Corsico, A.; Grosso, A.; Baglioni, S.; et al. Real-life Mepolizumab effectiveness in severe eosinophilic asthmatics with nasal polyposis. Respir. Med. Res. 2020, 78, 100791. [Google Scholar] [CrossRef]
- Lipworth, B.; Chan, R.; Kuo, C.R. Eosinophil paradox with mepolizumab in chronic rhinosinusitis with nasal polyposis. J. Allergy Clin. Immunol. 2020, 146, 683. [Google Scholar] [CrossRef]
- Bandi, F.; Gallo, S.; Preti, A.; Mozzanica, F.; Visca, D.; Marelli, M.; Maddalone, E.; Gambarini, C.; Vaghi, A.; Spanevello, A.; et al. Effects of biological therapies on chronic rhinosinusitis in severe asthmatic patients. Acta Otorhinolaryngol. Ital. 2020, 40, 435–443. [Google Scholar] [CrossRef]
- 2017 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2019/04/wmsGINA-2017-main-report-final_V2.pdf (accessed on 30 June 2022).
- 2018 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf (accessed on 30 June 2022).
- 2019 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed on 30 June 2022).
- 2021 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 30 June 2022).
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Russo, F.; Mozzanica, F.; Preti, A.; Bandi, F.; Costantino, C.; Gera, R.; Ottaviani, F.; Castelnuovo, P. Prognostic value of the Sinonasal Outcome Test 22 (SNOT-22) in chronic rhinosinusitis. Acta Otorhinolaryngol. Ital. 2020, 40, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Malvezzi, L.; Blasi, F.; Paggiaro, P.; Mantero, M.; Senna, G.; Heffler, E.; Bonavia, M.; Caiaffa, P.; Calabrese, C.; et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: Evidences from the Severe Asthma Network Italy (SANI) registry. Respir. Med. 2020, 166, 105947. [Google Scholar] [CrossRef] [PubMed]
- John Staniorski, C.; Price, C.P.E.; Weibman, A.R.; Welch, K.C.; Conley, D.B.; Shintani-Smith, S.; Stevens, W.W.; Peters, A.T.; Grammer, L.; Lidder, A.K.; et al. Asthma onset pattern and patient outcomes in a chronic rhinosinusitis popu-lation. Int. Forum. Allergy Rhinol. 2018, 8, 495–503. [Google Scholar] [CrossRef]
- Yii, A.C.; Tay, T.; Choo, X.N.; Koh, M.S.; Tee, A.K.; Wang, D. Precision medicine in united airways disease: A “treatable traits” approach. Allergy 2018, 73, 1964–1978. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, J.A.; Virchow, J.C.; Murphy, K.; Maspero, J.F.; Jacobs, J.; Adir, Y.; Humbert, M.; Castro, M.; Marsteller, D.A.; McElhattan, J.; et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid- dependent asthma: Results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2020, 8, 461–474. [Google Scholar]
- Vanderhaegen, T.; Gengler, I.; Dendooven, A.; Chenivesse, C.; Lefèvre, G.; Mortuaire, G. Eosinophils in the Field of Nasal Poly-posis: Towards a Better Understanding of Biologic Therapies. Clin. Rev. Allergy Immunol. 2021, 62, 90–102. [Google Scholar] [CrossRef]
- Schleimer, R.P. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 331–357. [Google Scholar] [CrossRef] [Green Version]
- Takabayashi, T.; Schleimer, R.P. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J. Allergy Clin. Immunol. 2020, 145, 740–750. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int. Forum. Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a humanized anti–IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 2011, 128, 989–995.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, I.; Türk, M.; Nazik Bahçecioğlu, S.; Tutar, N.; Gülmez, I. Efficacy of mepolizumab treatment in oral corticoster-oid-dependent severe eosinophilic asthma patients with chronic rhinosinusitis with nasal polyps: Single center, real life study. Turk. J. Med. Sci. 2020, 50, 433–441. [Google Scholar] [CrossRef] [PubMed]
- da Costa Martins, S.M.; Tinoco, E.; Cabrita, B.; Machado, D.; Franco, I.; Ladeira, I.; Pascoal, I.; Lima, R.; Valente, S. Mepolizumab in the treatment of severe asthma with nasal polyposis: Real-life study. Eur. Respir. J. 2021, 58, PA3727. [Google Scholar] [CrossRef]
- Meier, E.C.; Schmid-Grendelmeier, P.; Steiner, U.C.; Soyka, M.B. Real-Life Experience of Monoclonal Antibody Treatments in Chronic Rhinosinusitis with Nasal Polyposis. Int. Arch. Allergy Immunol. 2021, 182, 736–743. [Google Scholar] [CrossRef]
- Tiotiu, A.; Mendez-Brea, P.; Ioan, I.; Romero-Fernandez, R.; Oster, J.P.; Hoang, T.-C.; Roux, P.; Ochoa-Gutierrez, D.C.; Bonniaud, P.; de Blay, F.; et al. Real-Life Effectiveness of Benralizumab, Mepolizumab and Omalizumab in Severe Allergic Asthma Associated with Nasal Polyps. Clin. Rev. Allergy Immunol. 2022, 1–14. [Google Scholar] [CrossRef]
- Lipworth, B.J.; Chan, R. The Choice of Biologics in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pr. 2021, 9, 4235–4238. [Google Scholar] [CrossRef]
- Lildholdt, T. Surgical versus medical treatment of nasal polyps. Rhinol. Suppl. 1989, 8, 31–33. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Prussin, C.; Panettieri, R.A.; Lee, S.; Ferguson, B.J.; Adappa, N.D.; Lane, A.P.; Ba, M.L.P.; Sullivan, M.; Ba, M.S.; et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope 2018, 129, E61–E66. [Google Scholar] [CrossRef]
- Rudmik, L.; Soler, Z.M.; Mace, J.C.; De Conde, A.S.; Schlosser, R.J.; Smith, T.L. Using preoperative SNOT-22 score to inform patient decision for Endoscopic sinus surgery. Laryngoscope 2014, 125, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
| Variables | N. (%) | |
|---|---|---|
| mean age at baseline (SD) | 52.2 (10.9) | |
| sex M, F n. (%) | 18 (42%), 25 (58%) | |
| smoke habits n. (%) |
smoker—ex smoker nonsmoker |
16 (37%) 27 (63%) |
| respiratory disease onset n. (%) |
concordant early CRS—asthma concordant late CRS—asthma discordant, early CRS—late asthma discordant, late CRS—early asthma |
18 (42%) 18 (42%) 3 (7%) 4 (9%) |
| NSAID sensitivity n. (%) | 15 (35%) | |
| Seasonal and/or perennial inhalant sensitization n. (%) | 24 (56%) | |
| type of ongoing medical therapy |
INCS spray INCS in squeeze bottle continuative OCS intermittent OCS (at least 2 courses/year) antihistamine no therapy |
25 15 7 13 15 3 |
| previous mAb therapy n. (%) | 12 (28%) | |
| nasal surgery n. (%) | 32 (74%) | |
| timing of surgery |
before mAb therapy starting during mAb therapy |
27/32 (84%) 5/32 (16%) |
| type of major surgery |
polypectomy FESS ESS ESS + frontal sinusotomy DRAF type 3 |
4 10 14 4 |
| median number of surgeries n. (IQR) | 1 (3) | |
| mean age at first surgery n. (SD) | 39.9 (12.4) | |
| median baseline IgE kUI/L (IQR) * | 171.5 (329.2) | |
| Study | Type |
N. of Patients |
Weeks of Follow Up |
Analyzed Variables | Statistically Significant Outcomes | Results | Limits |
|---|---|---|---|---|---|---|---|
| Yilmaz et al. 2020 [28] | R | 16 | 24 | OCS, asthma exacerbation, ACT, FEV1, blood eosinophils, NAS |
Asthma exacerbation ACT Blood eosinophils | The number of asthma exacerbations within 24 weeks significantly decreased and a significant increase in ACT scores was observed despite the decrease in daily OCS dosages. There was no significant difference in FEV1. |
Small sample size Short-term study No control groups |
| Chan et al. 2020 [4] | R | 6 | 40 | Lildholt NPS, blood eosinophils, CRS exacerbations | Blood eosinophils | Patients responded favorably to mepolizumab in terms of asthma control, but their CRS disease persisted and, in some cases, continued to worsen |
Absence of PROMS Small sample size No control group |
| Sposato et al. 2020 [9] | R | 69 | 48 (24–53) | Subjective nasal symptoms improvement | - | In severe asthmatic patients, a greater reduction in nasal symptoms was observed in patients with nasal polyps (76%) compared to patients without (45%) |
Absence of rhinologic scoring systems Not all patients were evaluated No control group |
| Bandi et al. 2020 [11] | P | 20 | 52 | SNOT-22, SNOT 1-12, NPS, LKS, CRS clinical control, blood eosinophils |
SNOT-22 SNOT 1-12 NPS CRS clinical control | Improvement in nasal symptoms after 52 weeks of treatment, which was not associated with significant improvement in endoscopic findings |
Small sample size No control groups Lack of respiratory functional data |
| Detoraki et al. 2021 [5] | P | 44 | 52 | SNOT-22, NPS, blood eosinophils |
SNOT-22 Blood eosinophils | Significant reduction in SNOT-22 and a decrease in NPS compared to baseline. Significant decreases in blood eosinophils and mean prednisone intake were also reported |
Small sample size No control groups |
| da Costa Martins et al. 2021 [29] | R | 12 | 52 | OCS, asthma exacerbation, SNOT-22, NCS |
OCS, asthma exacerbation, SNOT-22, NCS | A reduction in asthma exacerbations and systemic corticosteroid therapy was observed. In parallel, there was also a statistically significant improvement in sinonasal symptoms evidenced by a reduction in the average total score on the Sino-Nasal Outcome Test 22 (p = 0.008) and Nasal Congestion scale (p = 0.010). |
Small sample size Short-term study No control groups Endoscopic outcomes not evaluated |
| Meier et al. 2021 [30] | R | 19 | 28 (4–108) | NPS, nasal symptoms | - | Treatments with mepolizumab showed the best success rates compared to other biologics; however, a correlation between biomarkers and treatment success could not be found |
Absence of PROMs Small sample size No control groups Nonhomogeneous follow-up time |
| Tiotiu et al. 2022 [31] | R | 21 | 24 | Nasal symptoms, NPS, CRS exacerbations, CT sinus imaging, blood eosinophils |
Nasal symptoms NPS CRS exacerbations Blood eosinophils | Significant improvement in nasal symptoms (except pruritus) and decrease in endoscopic score, blood eosinophil count, and number of CRS exacerbations |
Absence of PROMs Small sample size Lack of baseline homogeneity No control groups Short-term study |
| Present study | R | 43 | 52 | SNOT-22, SNOT 1-12, SNOT-22 individual symptoms, CRS clinical control, NPS, blood eosinophils |
SNOT-22 SNOT 1-12 SNOT-22 individual symptoms NPS Blood eosinophils | Significant improvement in nasal symptoms and quality of life, significant improvement in endoscopic findings. |
Small sample size No control groups Lack of respiratory functional data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, S.; Castelnuovo, P.; Spirito, L.; Feduzi, M.; Seccia, V.; Visca, D.; Spanevello, A.; Statuti, E.; Latorre, M.; Montuori, C.; et al. Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study. J. Pers. Med. 2022, 12, 1304. https://doi.org/10.3390/jpm12081304
Gallo S, Castelnuovo P, Spirito L, Feduzi M, Seccia V, Visca D, Spanevello A, Statuti E, Latorre M, Montuori C, et al. Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study. Journal of Personalized Medicine. 2022; 12(8):1304. https://doi.org/10.3390/jpm12081304
Chicago/Turabian StyleGallo, Stefania, Paolo Castelnuovo, Luca Spirito, Marta Feduzi, Veronica Seccia, Dina Visca, Antonio Spanevello, Erica Statuti, Manuela Latorre, Claudio Montuori, and et al. 2022. "Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study" Journal of Personalized Medicine 12, no. 8: 1304. https://doi.org/10.3390/jpm12081304
APA StyleGallo, S., Castelnuovo, P., Spirito, L., Feduzi, M., Seccia, V., Visca, D., Spanevello, A., Statuti, E., Latorre, M., Montuori, C., Rizzi, A., Boccabella, C., Bonini, M., & De Corso, E. (2022). Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study. Journal of Personalized Medicine, 12(8), 1304. https://doi.org/10.3390/jpm12081304









