Development and Characterization of an Ex Vivo Testing Platform for Evaluating Automated Central Vascular Access Device Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluated ACVAD Performance Metrics
2.2. Commercially Available Ultrasound Trainers
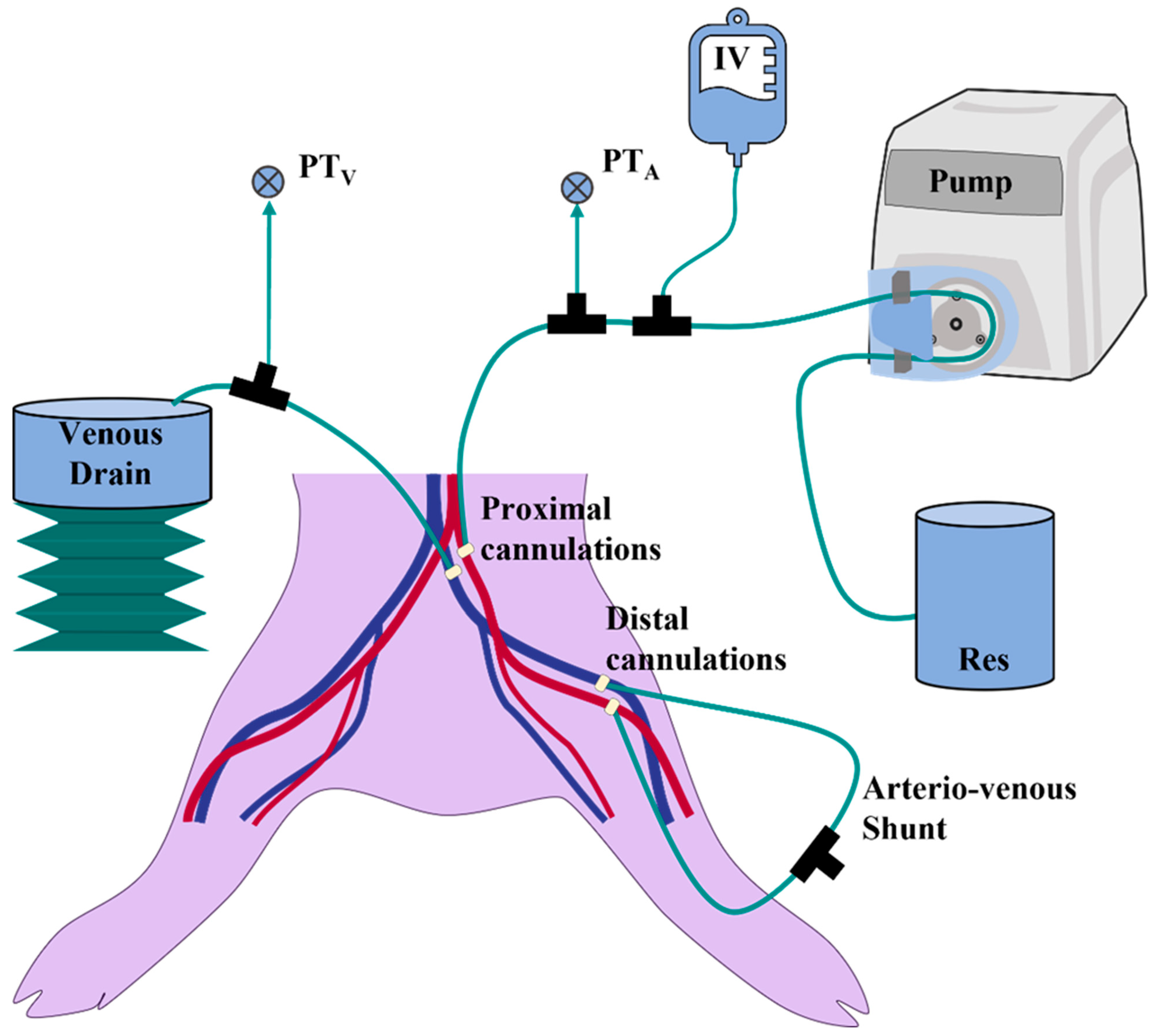
2.3. Lower-Body Ex Vivo Porcine Model
2.4. Ultrasound Imaging and Vascular Access
3. Results
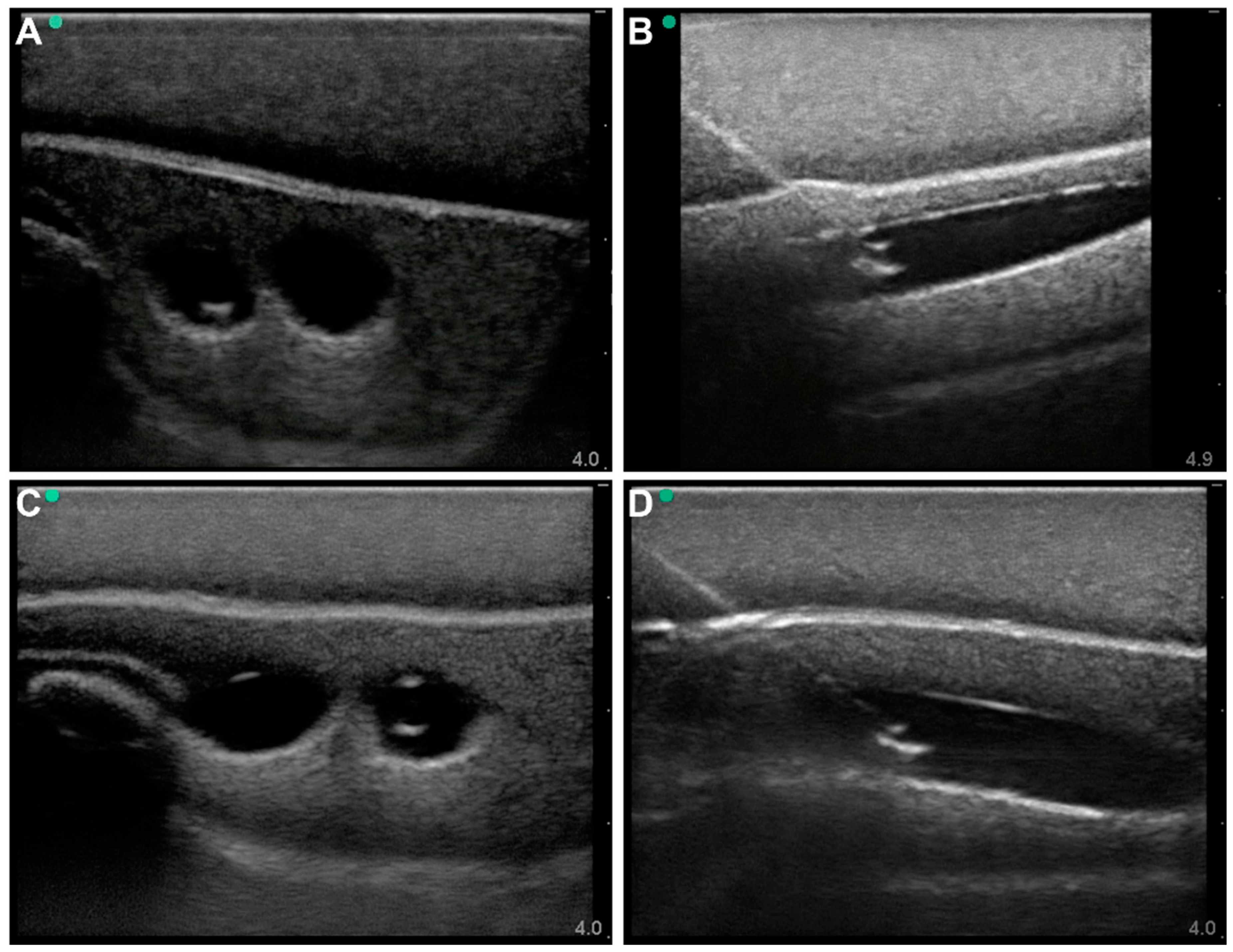
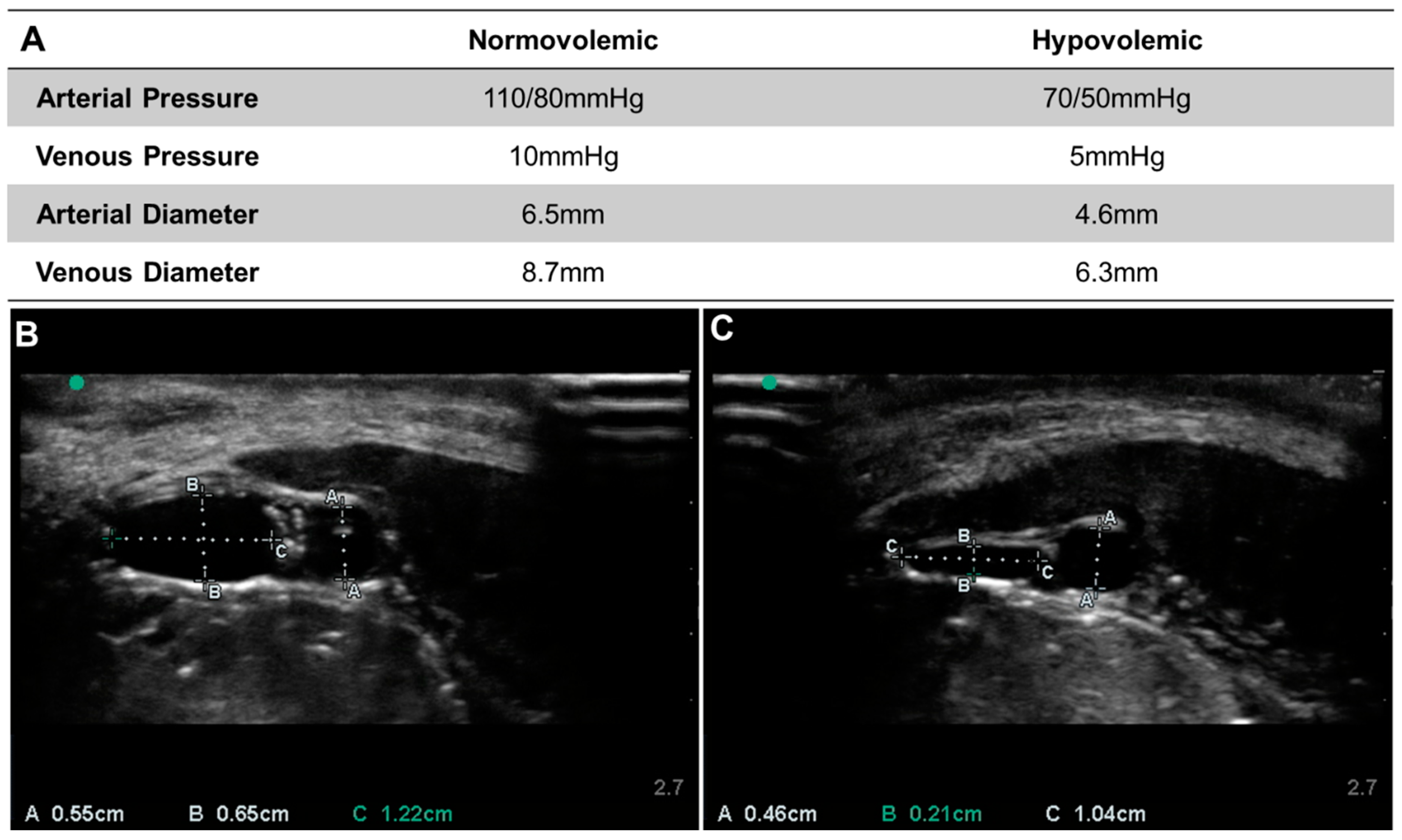
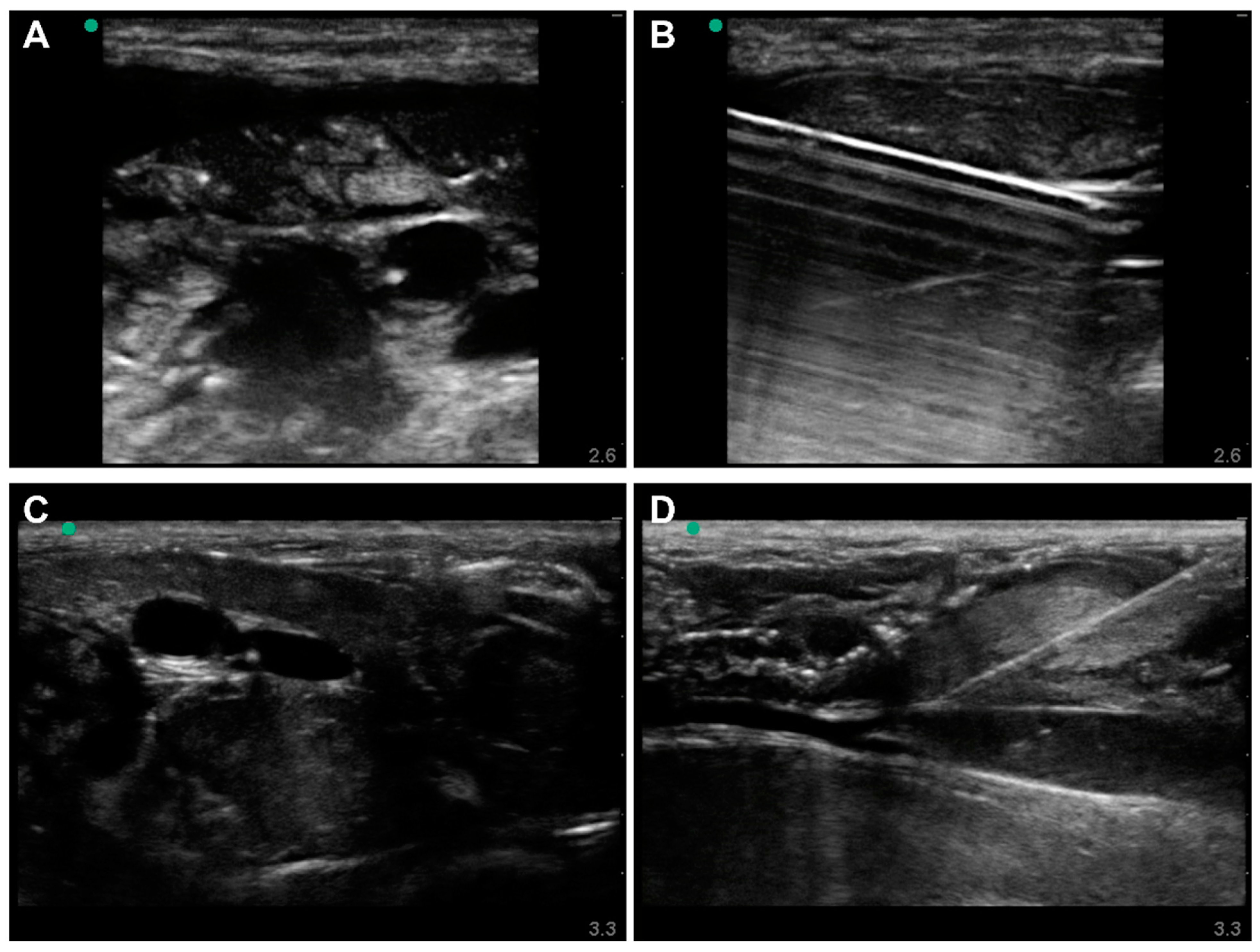
3.1. Commercial Test Results
3.2. Lower-Body Ex Vivo Porcine Model Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
DoD Disclaimer
Conflicts of Interest
References
- Kleber, C.; Giesecke, M.T.; Tsokos, M.; Haas, N.P.; Buschmann, C.T. Trauma-Related Preventable Deaths in Berlin 2010: Need to Change Prehospital Management Strategies and Trauma Management Education. World J. Surg. 2013, 37, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Eastridge, B.J.; Mabry, R.L.; Seguin, P.; Cantrell, J.; Tops, T.; Uribe, P.; Mallett, O.; Zubko, T.; Oetjen-Gerdes, L.; Rasmussen, T.E.; et al. Death on the Battlefield (2001–2011): Implications for the Future of Combat Casualty Care. J. Trauma Acute Care Surg. 2012, 73, S431–S437. [Google Scholar] [CrossRef] [PubMed]
- Cap, A.P.; Spinella, P.C.; Borgman, M.A.; Blackbourne, L.H.; Perkins, J.G. Timing and Location of Blood Product Transfusion and Outcomes in Massively Transfused Combat Casualties. J. Trauma Acute Care Surg. 2012, 73, S89–S94. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.; Gendler, S.; Benov, A.; Shina, A.; Baruch, E.; Twig, G.; Glassberg, E. Intravenous Access in the Prehospital Settings: What Can Be Learned from Point-of-Injury Experience. J. Trauma Acute Care Surg. 2015, 79, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, K.; Saybel, R.; Mathura, P.; Tsang, B.; Fawcett, V.; Widder, S. Ensuring Adequate Vascular Access in Patients with Major Trauma: A Quality Improvement Initiative. BMJ Open Qual. 2018, 7, e000090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, J.; Morrison, J.; Lauer, C.; Grabo, D.; Polk, T.; Blackbourne, L.; Dubose, J.; Rasmussen, T. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Hemorrhagic Shock. Mil. Med. 2018, 183, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.R.; Park, C.Y.; Kim, D.H.; Ma, D.S.; Chang, S.W. A Course on Endovascular Training for Resuscitative Endovascular Balloon Occlusion of the Aorta: A Pilot Study for Residents and Specialists. Ann. Surg. Treat. Res. 2020, 99, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.W.; Mason, P.E.; Batchinsky, A.I. Past and Present Role of Extracorporeal Membrane Oxygenation in Combat Casualty Care: How Far Will We Go? J. Trauma Acute Care Surg. 2018, 84, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Chreiman, K.M.; Dumas, R.P.; Seamon, M.J.; Kim, P.K.; Reilly, P.M.; Kaplan, L.J.; Christie, J.D.; Holena, D.N. The Intraosseous Have It: A Prospective Observational Study of Vascular Access Success Rates in Patients in Extremis Using Video Review. J. Trauma Acute Care Surg. 2018, 84, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Automated, Handheld Device to Rapidly Access Arteries and Veins in Trauma Patients|SBIR.Gov. Available online: https://www.sbir.gov/node/1319149 (accessed on 7 April 2022).
- VuPath: Crystalline Medical. Available online: https://crystallinemed.com/ (accessed on 7 April 2022).
- Brattain, L.J.; Pierce, T.T.; Gjesteby, L.A.; Johnson, M.R.; DeLosa, N.D.; Werblin, J.S.; Gupta, J.F.; Ozturk, A.; Wang, X.; Li, Q.; et al. AI-Enabled, Ultrasound-Guided Handheld Robotic Device for Femoral Vascular Access. Biosensors 2021, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Boice, E.N.; Berard, D.; Gonzalez, J.M.; Hernandez-Torres, S.I.; Knowlton, Z.J.; Avital, G.; Snider, E.J. Development of a Modular Tissue Phantom for Evaluating Vascular Access Devices. Bioengineering 2022, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Trainers, M.S. Gen II Femoral Vascular Access & Regional Anesthesia Ultrasound Training Model—Medical Skills Trainers. Available online: https://medicalskillstrainers.cae.com/gen-ii-femoral-vascular-access-and-regional-anesthesia-ultrasound-training-model/p (accessed on 7 April 2022).
- Regional Anesthesia Femoral Trainer with SmarTissue. Available online: https://simulab.com/products/regional-anesthesia-femoral-trainer-smartissue (accessed on 7 April 2022).
- Kopac, D.S.; Chen, J.; Tang, R.; Sawka, A.; Vaghadia, H. Comparison of a Novel Real-Time SonixGPS Needle-Tracking Ultrasound Technique with Traditional Ultrasound for Vascular Access in a Phantom Gel Model. J. Vasc. Surg. 2013, 58, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janakes, J. The Effects of Real-Time Computerized Needle Tip Location Feedback on State Anxiety and Immediate Performance of Simulated Ultrasound-Guided Regional Anesthesia. Ph.D. Dissertation, University of Nevada, Las Vegas, NV, USA, 2021. [Google Scholar]
- Liu, M.; Salmon, M.; Zaidi, R.; Nagdev, A.; Debebe, F.; Muller, M.F.; Ruhangaza, C.F.; Emiru, H.; Belachew, Y.; Tumebo, A.; et al. Ultrasound-Guided Regional Anesthesia: Feasibility and Effectiveness of Teaching via Telesimulation in Ethiopia. Reg. Anesth. Pain Med. 2021, 46, 722. [Google Scholar] [CrossRef] [PubMed]
- Sander, D.; Schick, V.; Ecker, H.; Lindacher, M.F.; Spelten, O.; Schier, R.; Hinkelbein, J.; Padosch, S. Novel Navigated Ultrasound Compared With Conventional Ultrasound for Vascular Access—A Prospective Study in a Gel Phantom Model. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1261–1265. [Google Scholar] [CrossRef] [PubMed]

| Model Features for Evaluating Vascular Access | Blue Phantom Gen II Femoral Ultrasound Trainer | Simulab Regional Anesthesia Femoral Trainer | Lower-Body Ex Vivo Porcine Model |
|---|---|---|---|
| Can measure needle bore tip relative to all vessel walls | ✓ | ✓ | ✓ |
| Angle of needle to surface can be visualized | ✓ | ✓ | ✓ |
| Needle flashback for successful insertion into vessel | ✓ | ✓ | ✓ |
| Subject vessel depth variability | ✓ | ✓ | |
| Subject distance between vessels variability | ✓ | ✓ | |
| Subject vessel diameter variability | ✓ | ||
| Variety of volume states | ✓ | ||
| Physiological Ultrasound Complexity | ✓ | ||
| Doppler Compliant | ** | ||
| Extent of vessel damage from needle insertion can be measured |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boice, E.N.; Berard, D.; Hernandez Torres, S.I.; Avital, G.; Snider, E.J. Development and Characterization of an Ex Vivo Testing Platform for Evaluating Automated Central Vascular Access Device Performance. J. Pers. Med. 2022, 12, 1287. https://doi.org/10.3390/jpm12081287
Boice EN, Berard D, Hernandez Torres SI, Avital G, Snider EJ. Development and Characterization of an Ex Vivo Testing Platform for Evaluating Automated Central Vascular Access Device Performance. Journal of Personalized Medicine. 2022; 12(8):1287. https://doi.org/10.3390/jpm12081287
Chicago/Turabian StyleBoice, Emily N., David Berard, Sofia I. Hernandez Torres, Guy Avital, and Eric J. Snider. 2022. "Development and Characterization of an Ex Vivo Testing Platform for Evaluating Automated Central Vascular Access Device Performance" Journal of Personalized Medicine 12, no. 8: 1287. https://doi.org/10.3390/jpm12081287
APA StyleBoice, E. N., Berard, D., Hernandez Torres, S. I., Avital, G., & Snider, E. J. (2022). Development and Characterization of an Ex Vivo Testing Platform for Evaluating Automated Central Vascular Access Device Performance. Journal of Personalized Medicine, 12(8), 1287. https://doi.org/10.3390/jpm12081287






