Alteration of Skin Sympathetic Nerve Activity after Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Catheter Ablation
2.2.1. Electrophysiological Study and Mapping Strategy
2.2.2. RFA
2.2.3. Cryoablation (CBA)
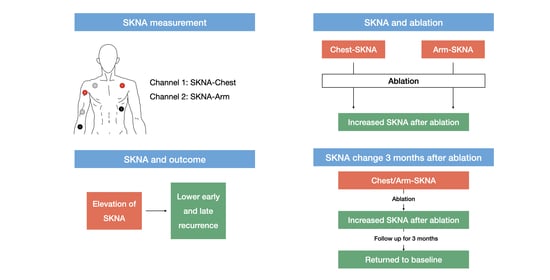
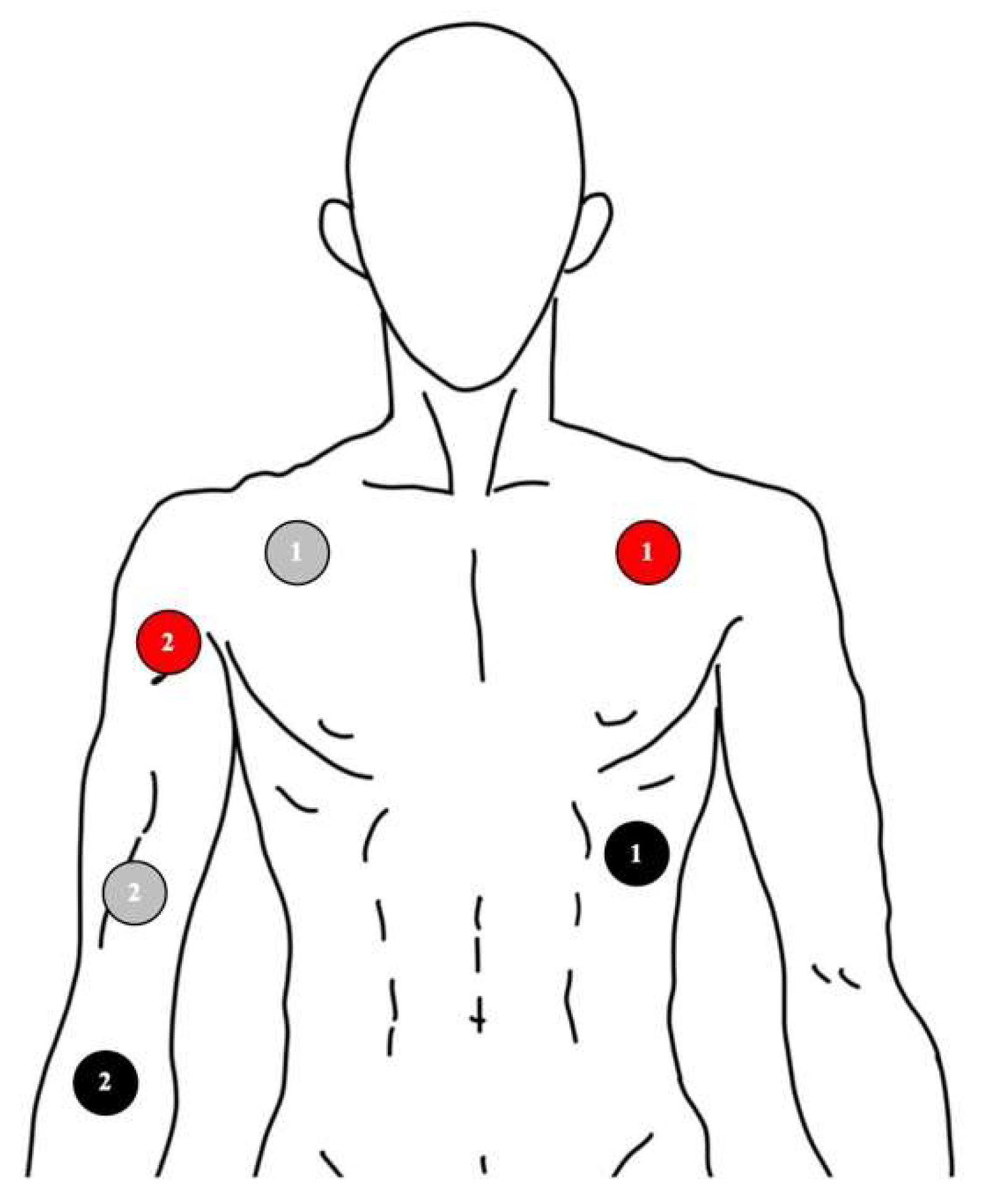
2.3. Signal Recording of Skin Nerve Activity
2.4. Follow-Up
2.5. Statistics
3. Results
3.1. Baseline Characteristics and Ablation Results
3.2. SKNA Results
3.2.1. SKNA One Day before Ablation Procedure
3.2.2. SKNA One Day after the Ablation
All Patients
Patients with/without Early Recurrence
Patients with/without Late Recurrence
3.2.3. SKNA 3 Months after Ablation
4. Discussion
4.1. Main Findings
4.2. Neuromodulation Effect of PVI
4.3. Neuromodulation Effect and Ablation Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.S.; Tai, C.T.; Hsieh, M.H.; Tsai, C.F.; Lin, Y.K.; Tsao, H.M.; Huang, J.L.; Yu, W.C.; Yang, S.P.; Ding, Y.A.; et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 107, 3176–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucker, W.J.; Singh, J.P.; Parks, K.; Armoundas, A.A. Device-Based Approaches to Modulate the Autonomic Nervous System and Cardiac Electrophysiology. Arrhythm. Electrophysiol. Rev. 2014, 3, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.K.; Shen, M.J.; Han, S.; Kim, D.; Hwang, S.; Sayfo, S.; Piccirillo, G.; Frick, K.; Fishbein, M.C.; Hwang, C.; et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 2010, 121, 2615–2623. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ. Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 2005, 209, 425–438. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Y.; Doytchinova, A.; Kamp, N.J.; Tsai, W.C.; Yuan, Y.; Adams, D.; Wagner, D.; Shen, C.; Chen, L.S.; et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm 2015, 12, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Doytchinova, A.; Hassel, J.L.; Yuan, Y.; Lin, H.; Yin, D.; Adams, D.; Straka, S.; Wright, K.; Smith, K.; Wagner, D.; et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 2017, 14, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Everett, T.H.; Doytchinova, A.; Cha, Y.M.; Chen, P.S. Recording sympathetic nerve activity from the skin. Trends Cardiovasc. Med. 2017, 27, 463–472. [Google Scholar] [CrossRef]
- Uradu, A.; Wan, J.; Doytchinova, A.; Wright, K.C.; Lin, A.Y.T.; Chen, L.S.; Shen, C.; Lin, S.F.; Everett, T.H.; Chen, P.S. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 2017, 14, 964–971. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.J.; Tai, C.T.; Chang, S.L.; Lo, L.W.; Tuan, T.C.; Wongcharoen, W.; Udyavar, A.R.; Hu, Y.F.; Chang, C.J.; Tsai, W.C.; et al. Efficacy of additional ablation of complex fractionated atrial electrograms for catheter ablation of nonparoxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 607–615. [Google Scholar] [CrossRef]
- Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chao, T.F.; Chung, F.P.; Liao, J.N.; Chiou, C.W.; Tsao, H.M.; Chen, S.A. Predictors and Characteristics of Multiple (More Than 2) Catheter Ablation Procedures for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2015, 26, 1048–1056. [Google Scholar] [CrossRef]
- Chen, S.A.; Hsieh, M.H.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; Ding, Y.A.; Chang, M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef] [Green Version]
- Higa, S.; Tai, C.T.; Chen, S.A. Catheter ablation of atrial fibrillation originating from extrapulmonary vein areas: Taipei approach. Heart Rhythm 2006, 3, 1386–1390. [Google Scholar] [CrossRef]
- Tsai, C.F.; Tai, C.T.; Hsieh, M.H.; Lin, W.S.; Yu, W.C.; Ueng, K.C.; Ding, Y.A.; Chang, M.S.; Chen, S.A. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: Electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000, 102, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.H.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Hung, Y.; Chung, F.P.; Liao, J.N.; Tuan, T.C.; Chao, T.F.; et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm 2019, 16, 1327–1333. [Google Scholar] [CrossRef]
- Chang, T.Y.; Lo, L.W.; Te, A.L.D.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chung, F.P.; Chao, T.F.; Liao, J.N.; Tuan, T.C.; et al. The importance of extrapulmonary vein triggers and atypical atrial flutter in atrial fibrillation recurrence after cryoablation: Insights from repeat ablation procedures. J. Cardiovasc. Electrophysiol. 2019, 30, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Pappone, C.; Santinelli, V.; Manguso, F.; Vicedomini, G.; Gugliotta, F.; Augello, G.; Mazzone, P.; Tortoriello, V.; Landoni, G.; Zangrillo, A.; et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004, 109, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Seaborn, G.E.J.; Todd, K.; Michael, K.A.; Baranchuk, A.; Abdollah, H.; Simpson, C.S.; Akl, S.G.; Redfearn, D.P. Heart rate variability and procedural outcome in catheter ablation for atrial fibrillation. Ann. Noninvasive Electrocardiol. 2014, 19, 23–33. [Google Scholar] [CrossRef]
- Cui, J.; Gonzalez, M.D.; Blaha, C.; Hill, A.; Sinoway, L.I. Sympathetic responses induced by radiofrequency catheter ablation of atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H476–H484. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Chen, J.; Wang, L.; Li, Y.; Deng, N.; Tang, Q.; Wu, L.; Zhou, B.; Li, W. Study of the Distribution of Epicardial Vagal Ganglion and the Relationship Between Delayed Enhancement Magnetic Resonance Imaging and Radiofrequency Ablation in Patients with Atrial Fibrillation. World Neurosurg. 2020, 138, 732–739. [Google Scholar] [CrossRef]
- Furlan, R.; Jacob, G.; Snell, M.; Robertson, D.; Porta, A.; Harris, P.; Mosqueda-Garcia, R. Chronic orthostatic intolerance: A disorder with discordant cardiac and vascular sympathetic control. Circulation 1998, 98, 2154–2159. [Google Scholar] [CrossRef] [Green Version]
- Pearson, M.J.; Smart, N.A. Exercise therapy and autonomic function in heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 91–108. [Google Scholar] [CrossRef]
- Tan, A.Y.; Chen, P.-S.; Chen, L.S.; Fishbein, M.C. Autonomic nerves in pulmonary veins. Heart Rhythm 2007, 4 (Suppl. 3), S57–S60. [Google Scholar] [CrossRef] [Green Version]
- Pokushalov, E.; Romanov, A.; Artyomenko, S.; Turov, A.; Shirokova, N.; Katritsis, D.G. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2010, 33, 1231–1238. [Google Scholar] [CrossRef]
- Scherschel, K.; Hedenus, K.; Jungen, C.; Lemoine, M.D.; Rübsamen, N.; Veldkamp, M.W.; Klatt, N.; Lindner, D.; Westermann, D.; Casini, S.; et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci. Transl. Med. 2019, 11, eaav7770. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhang, Y.; Xie, X.; Wang, W.; Li, Z.; Gao, M.; Wang, Z.; Hou, Y. Long-Term Effects of Ganglionated Plexi Ablation on Electrophysiological Characteristics and Neuron Remodeling in Target Atrial Tissues in a Canine Model. Circ. Arrhythm. Electrophysiol. 2015, 8, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.; van Delft, R.; Notenboom, R.G.E.; Wouters, P.F.; DeRuiter, M.C.; Plevier, J.W.M.; Jongbloed, M.R.M. Human adult cardiac autonomic innervation: Controversies in anatomical knowledge and relevance for cardiac neuromodulation. Auton. Neurosci. 2020, 227, 102674. [Google Scholar] [CrossRef] [PubMed]

| Results | |
|---|---|
| Male, n (%) | 34 (91.9%) |
| Age (years old), mean ± SD | 58.9 ± 9.0 |
| LVEF (%), mean ± SD | 57.8 ± 6.1 |
| LAD (mm), mean ± SD | 39.4 ± 4.8 |
| RA enlargement, n (%) | 5 (14.3%) |
| Hypertension, n (%) | 15 (40.5%) |
| Diabetes mellitus, n (%) | 3 (8.1%) |
| CAD, n (%) | 5 (13.5%) |
| HFrEF, n (%) | 3 (8.1%) |
| Ablation Type | |
| RFA, n (%) | 23 (62.2%) |
| CBA, n (%) | 14 (37.8%) |
| Follow up time (days), mean ± SD | 973.0 ± 229.7 |
| Circumferential PVI, n (%) | 28 (75.7%) |
| Segmental PVI, n (%) | 9 (24.3%) |
| RSPV, n (%) | 37 (100%) |
| RIPV, n (%) | 35 (94.6%) |
| LSPV, n (%) | 37 (100%) |
| LIPV, n (%) | 35 (94.6%) |
| Complete isolation of PV, n (%) | 37 (100%) |
| Additional ablation, n (%) | 5 (13.5%) |
| Early recurrence, n (%) | 4 (10.8%) |
| Late recurrence, n (%) | 7 (18.9%) |
| Before | After | p-Value | Percentage of Change | |
|---|---|---|---|---|
| Median SKNA-Arm (Q1 and Q3) (µV) | ||||
| All | 517.1 (396.0; 728.0) | 1226.2 (555.2; 2281.0) | <0.001 | 179.8% (125.0%, 376.0%) |
| With early recurrence | 682.6 (470.4; 923.9) | 892.7 (539.0; 1936.3) | 0.465 | 136.5% (91.1%;318.5%) |
| Without early recurrence | 481.5 (394.7; 708.1) | 1338.9 (555.2; 2281.0) | <0.001 | 204.2% (125.0%; 376.0%) |
| With late recurrence | 517.1 (396.8; 705.3) | 531.0 (462.4; 1510.7) | 0.063 | 147.0% (92.2%; 346.8%) |
| Without late recurrence | 503.3 (395.0; 777.1) | 1438.0 (671.4; 2480.3) | <0.001 | 187.5% (125.3%; 446.1%) |
| Mean SKNA-Chest (Q1; Q3) (µV) | ||||
| All | 538.2 (432.9; 663.9) | 640.0 (474.2; 925.6) | 0.004 | 108.3% (95.6%; 167.9%) |
| With early recurrence | 474.3 (408.1; 569.7) | 497.4 (469.7; 697.7) | 0.465 | 118.1% (97.3%; 145.9%) |
| Without early recurrence | 544.1 (437.7; 707.0) | 658.9 (474.2; 970.6) | 0.006 | 108.1% (95.6%; 167.9%) |
| With late recurrence | 625.9 (544.1; 819.2) | 596.4 (509.4; 1040.9) | 0.499 | 116.1% (77.6%; 124.7%) |
| Without late recurrence | 520.6 (420.5; 643.6) | 649.5 (448.8; 905.8) | 0.003 | 108.2% (98.4%; 172.5%) |
| Median SKNA-Arm (Q1; Q3) (µV) | p-Value * | Median SKNA-Chest (Q1; Q3) (µV) | p-Value * | |
|---|---|---|---|---|
| Before ablation | 446.7 (396.9; 680.3) | 535.1 (420.7; 663.9) | ||
| 1 Day after ablation | 1660.4 (978.9; 2439.7) | 0.006 | 688.2 (443.7; 854.2) | 0.034 |
| 3 Months after ablation | 546.4 (428.4; 1109.6) | 0.308 | 483.7 (306.0; 613.7) | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, W.-T.; Lo, L.-W.; Lin, Y.-J.; Chang, S.-L.; Hu, Y.-F.; Chung, F.-P.; Liao, J.-N.; Tuan, T.-C.; Chao, T.-F.; Lin, C.-Y.; et al. Alteration of Skin Sympathetic Nerve Activity after Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation. J. Pers. Med. 2022, 12, 1286. https://doi.org/10.3390/jpm12081286
Sung W-T, Lo L-W, Lin Y-J, Chang S-L, Hu Y-F, Chung F-P, Liao J-N, Tuan T-C, Chao T-F, Lin C-Y, et al. Alteration of Skin Sympathetic Nerve Activity after Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine. 2022; 12(8):1286. https://doi.org/10.3390/jpm12081286
Chicago/Turabian StyleSung, Wei-Ting, Li-Wei Lo, Yenn-Jiang Lin, Shih-Lin Chang, Yu-Feng Hu, Fa-Po Chung, Jo-Nan Liao, Ta-Chuan Tuan, Tze-Fan Chao, Chin-Yu Lin, and et al. 2022. "Alteration of Skin Sympathetic Nerve Activity after Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation" Journal of Personalized Medicine 12, no. 8: 1286. https://doi.org/10.3390/jpm12081286
APA StyleSung, W.-T., Lo, L.-W., Lin, Y.-J., Chang, S.-L., Hu, Y.-F., Chung, F.-P., Liao, J.-N., Tuan, T.-C., Chao, T.-F., Lin, C.-Y., Chang, T.-Y., Kuo, L., Liu, C.-M., Liu, S.-H., Cheng, W.-H., Ton, A. K.-N., Hsu, C.-Y., Chhay, C., Elimam, A. M., ... Chen, S.-A. (2022). Alteration of Skin Sympathetic Nerve Activity after Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine, 12(8), 1286. https://doi.org/10.3390/jpm12081286