Use of Sonophoresis with Corticosteroids in Carpal Tunnel Syndrome: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Elegibility Criteria
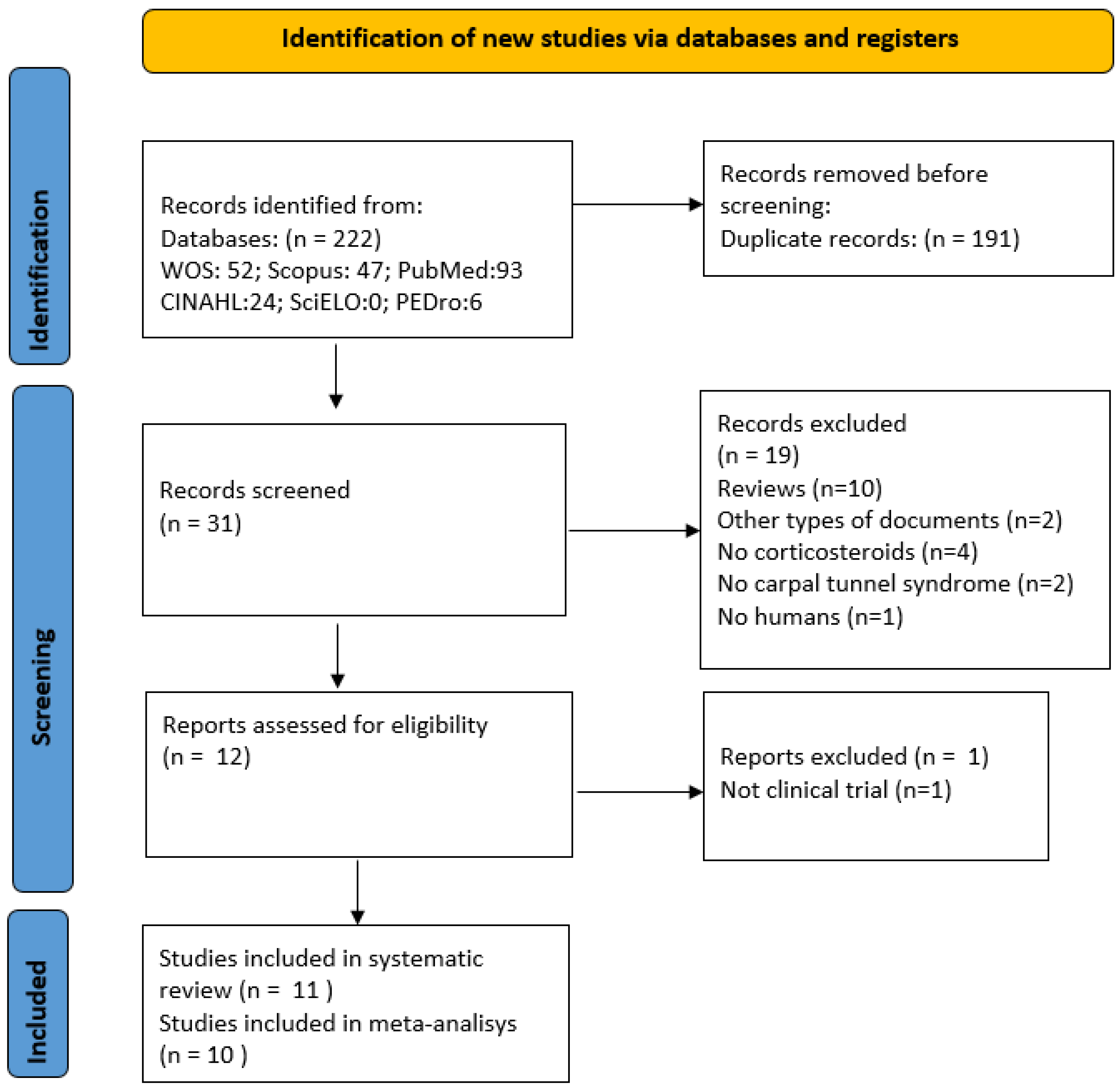
2.4. Studies Selection and Data Abstraction
2.5. Assessment of the Methodological Quality of the Studies
2.6. Statistical Analysis
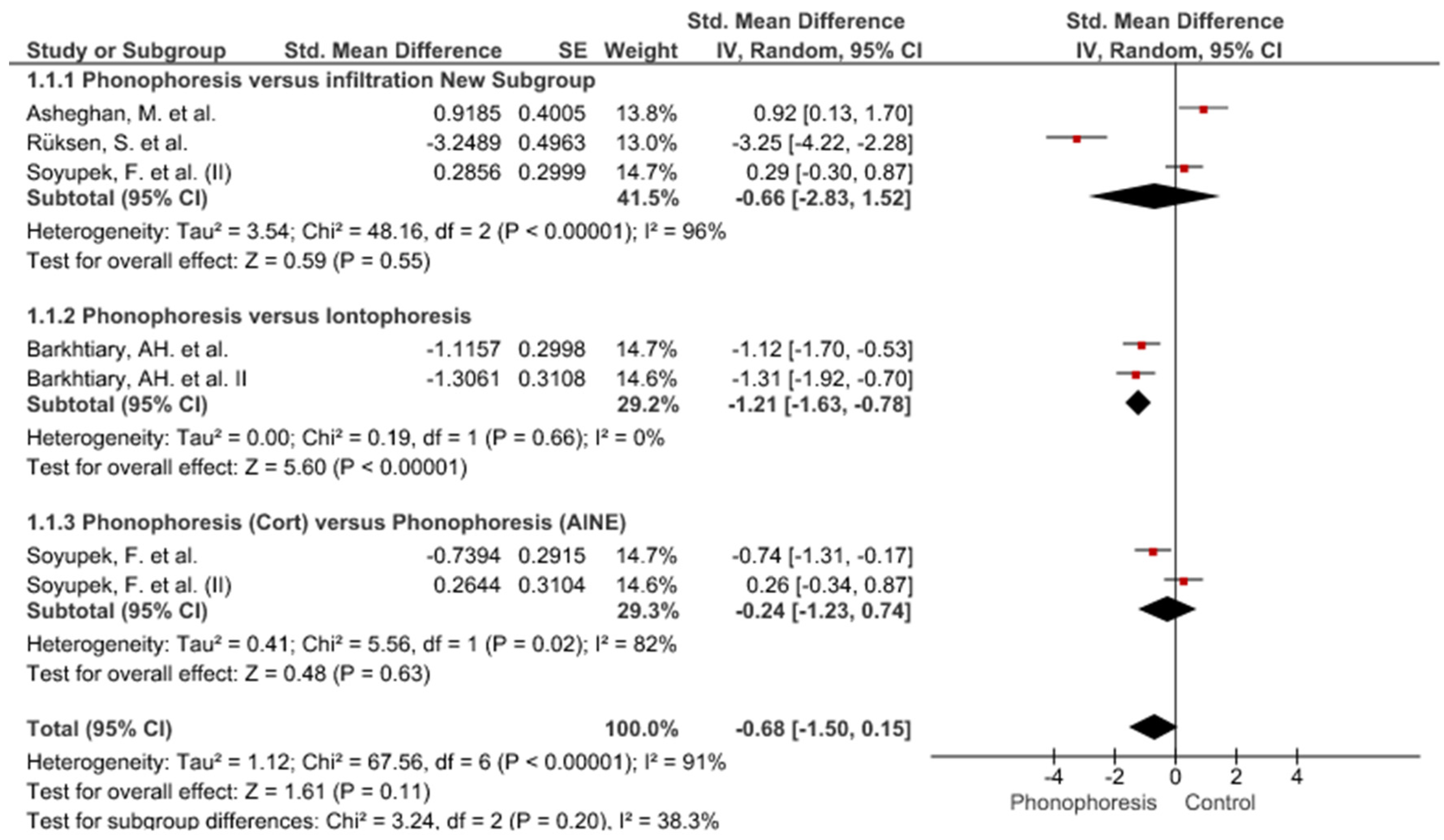
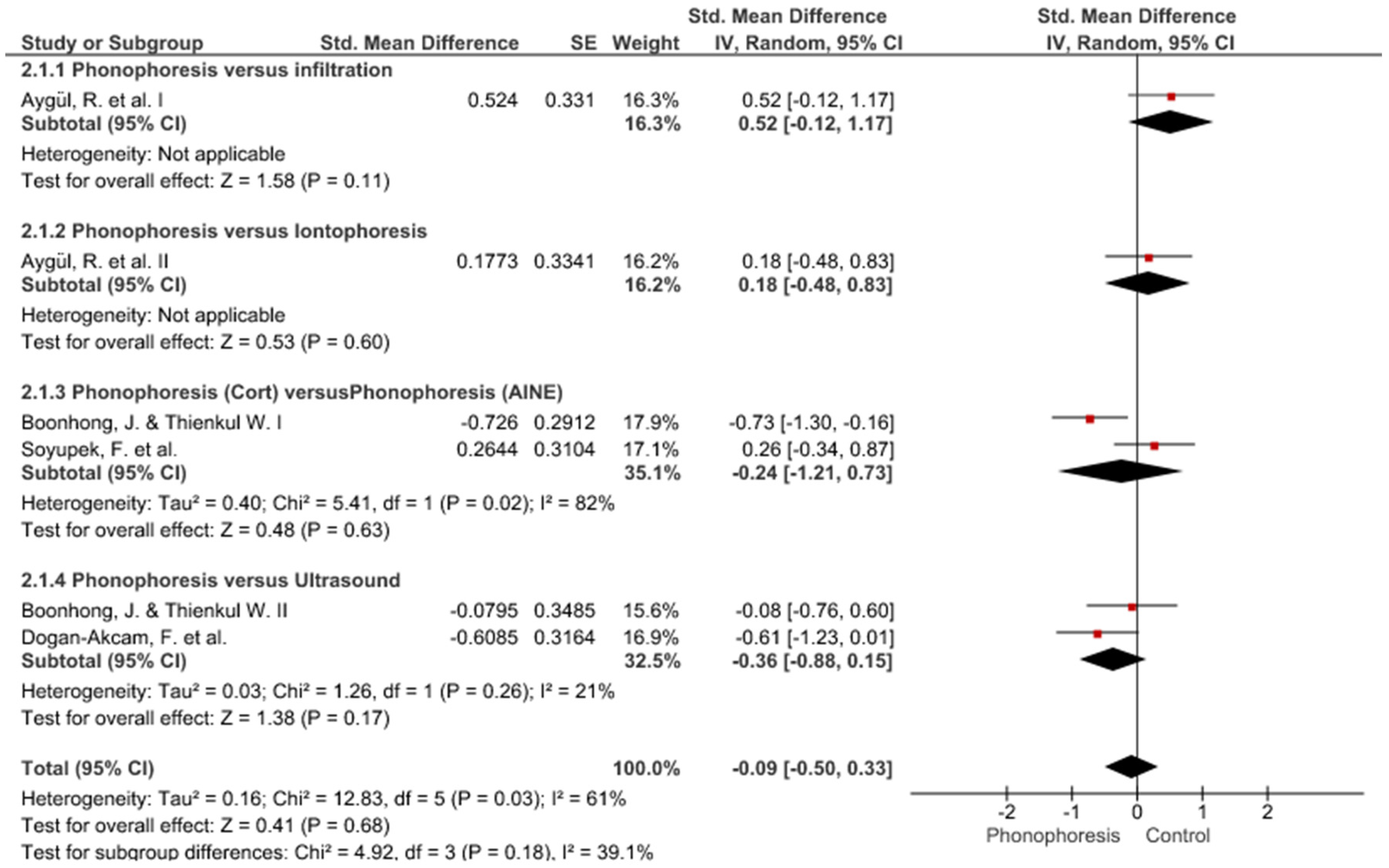
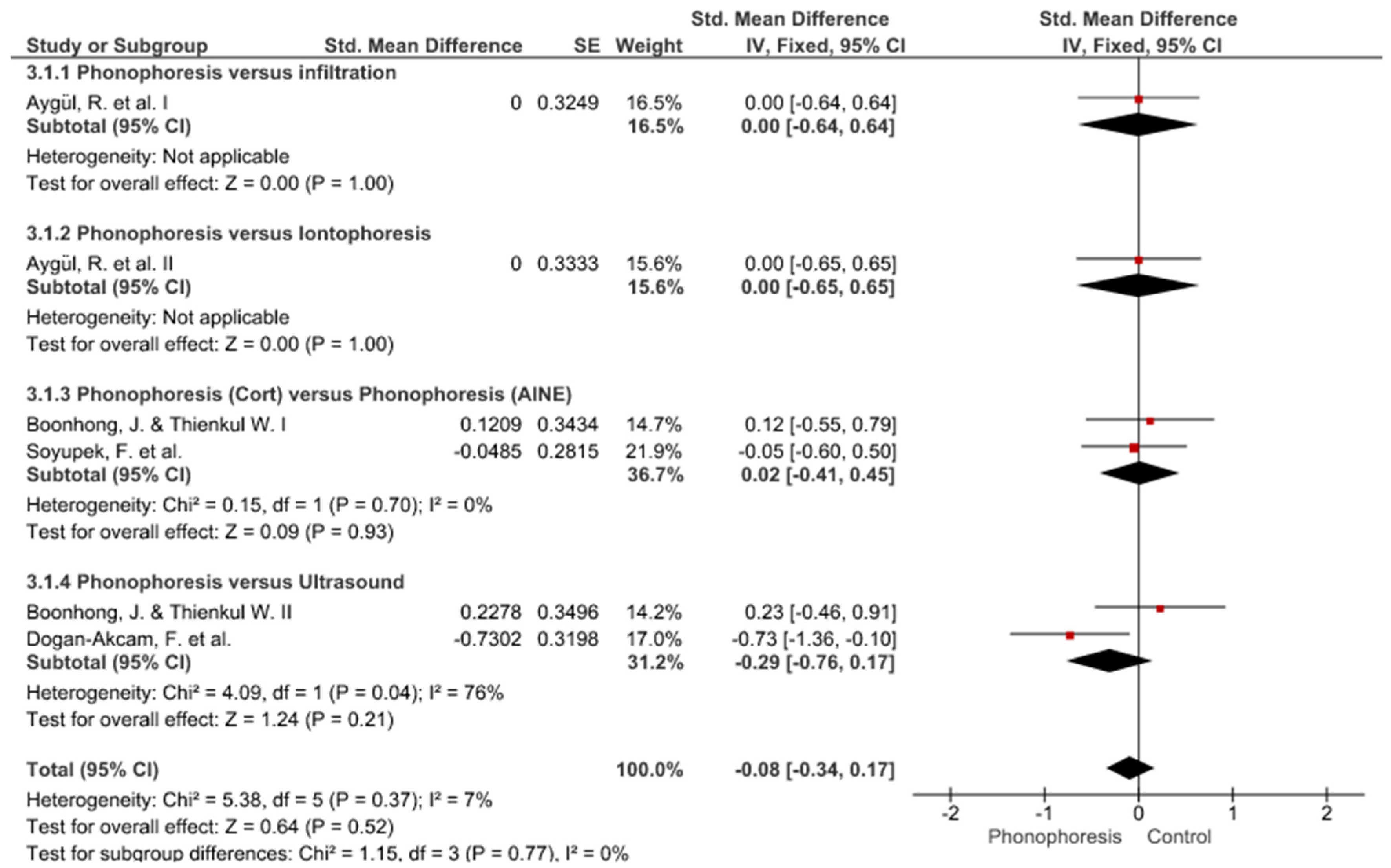
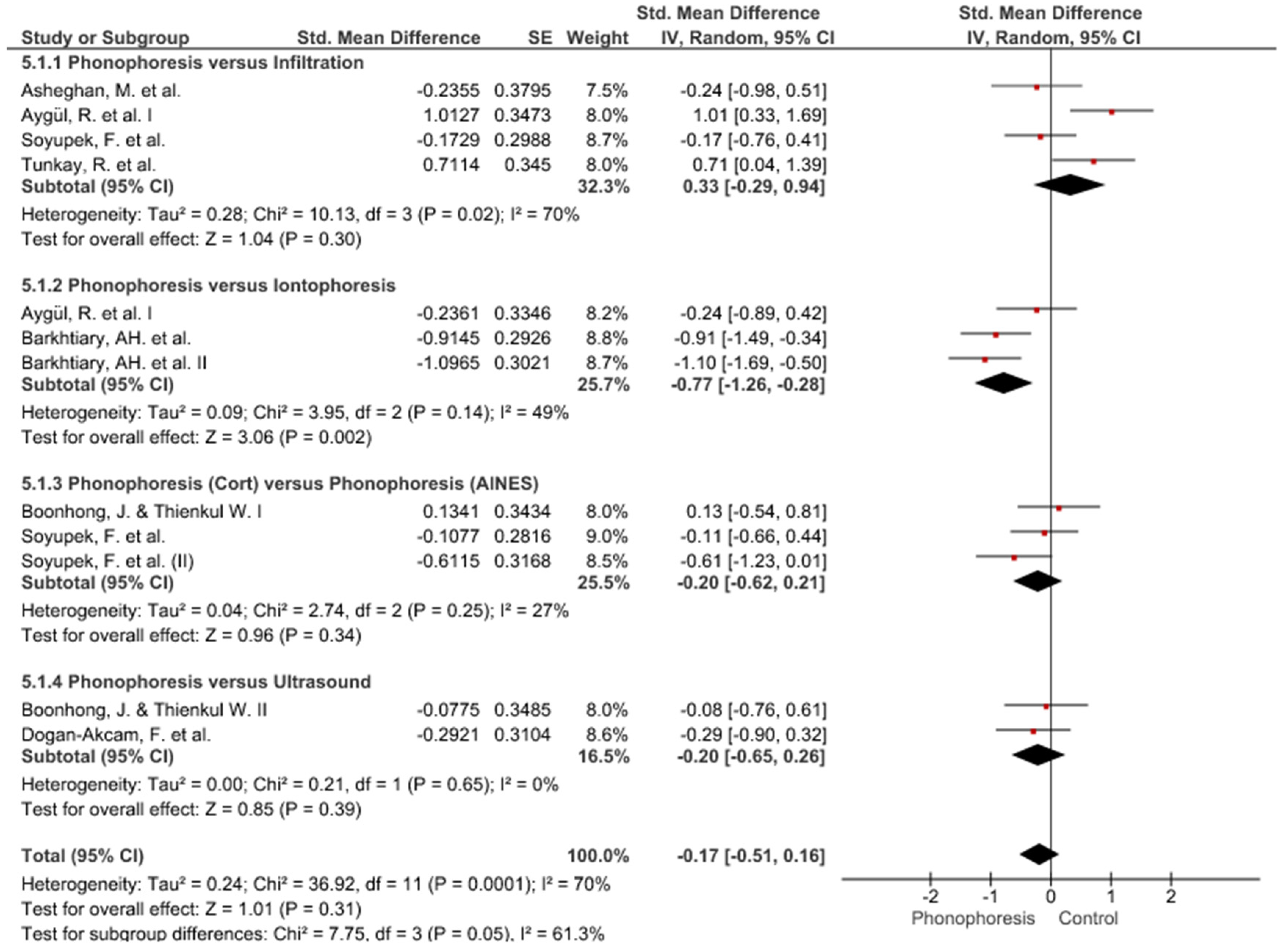
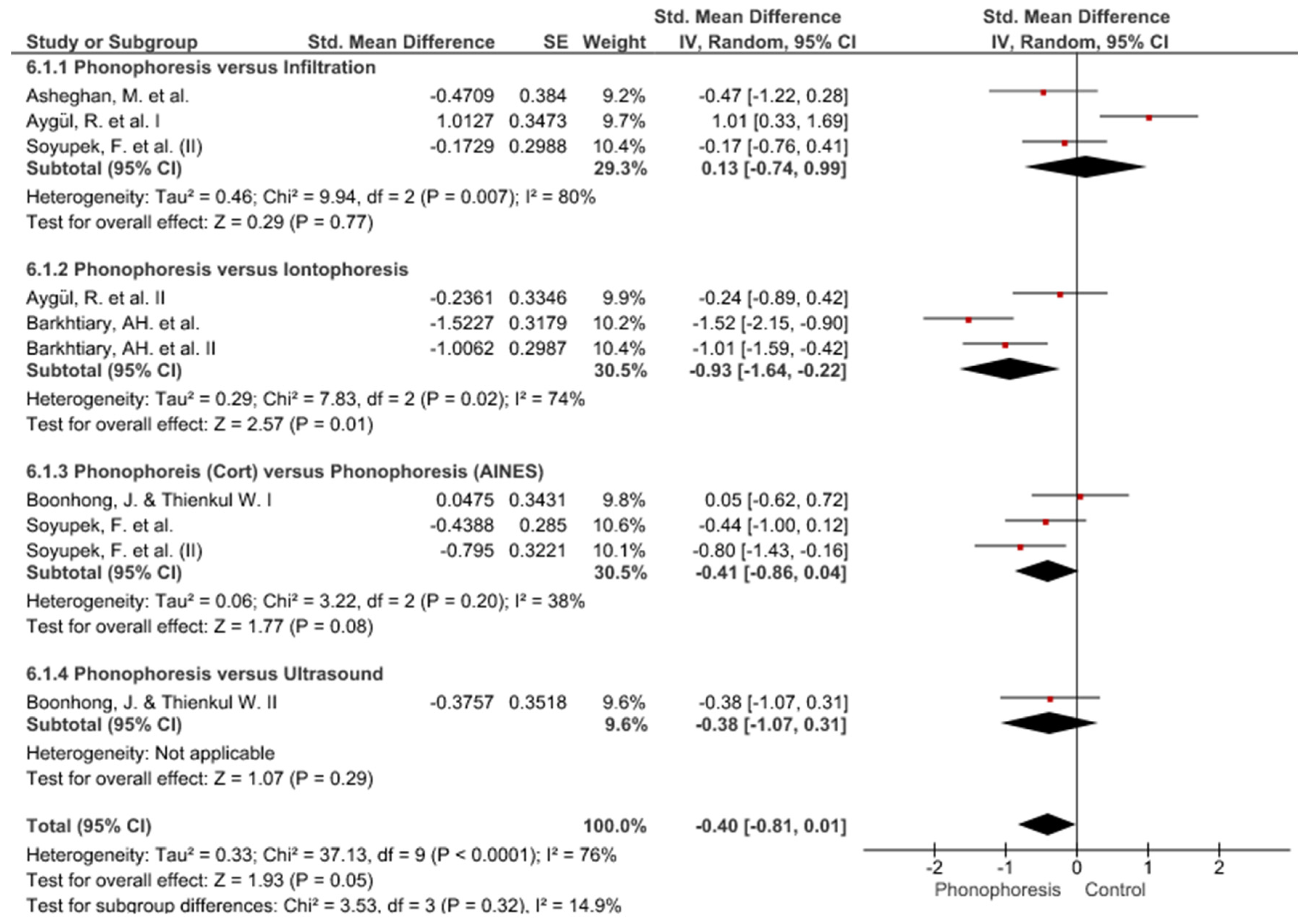
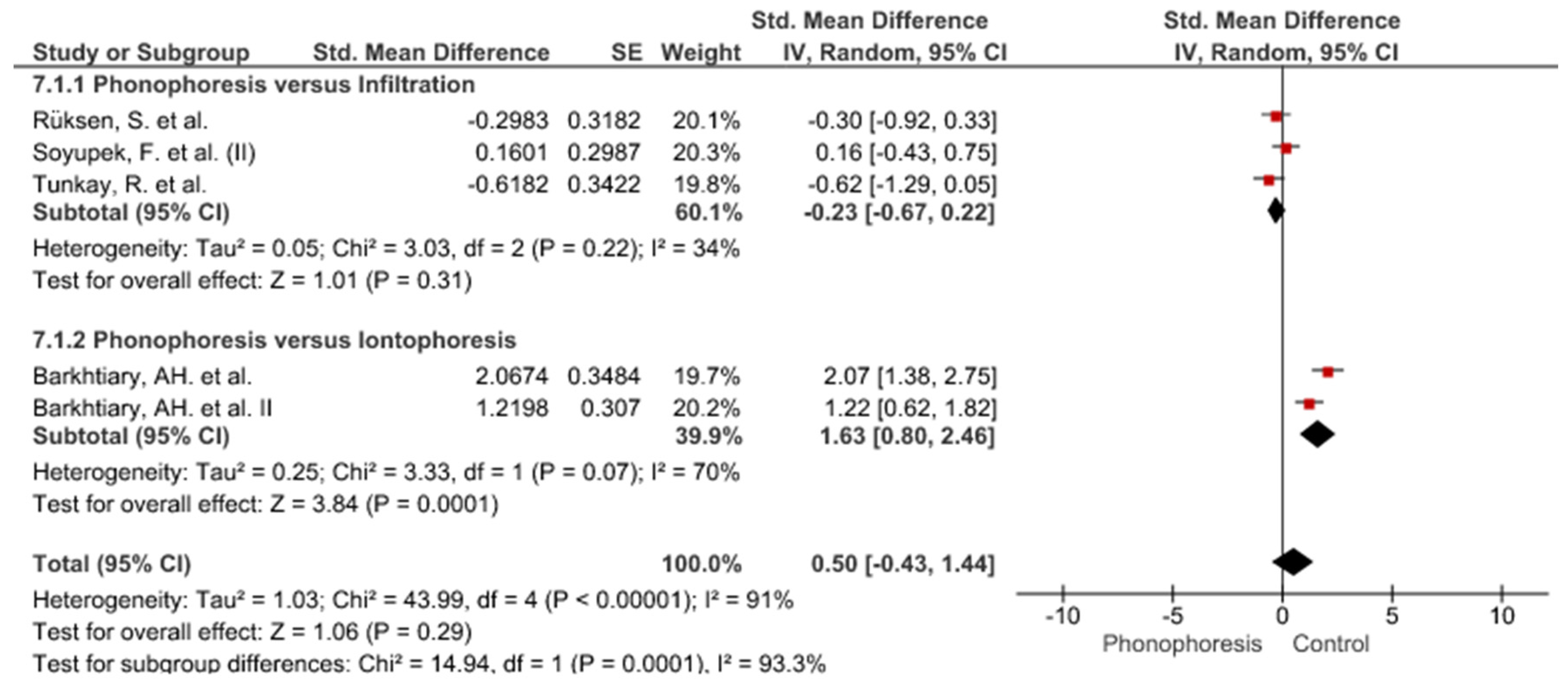
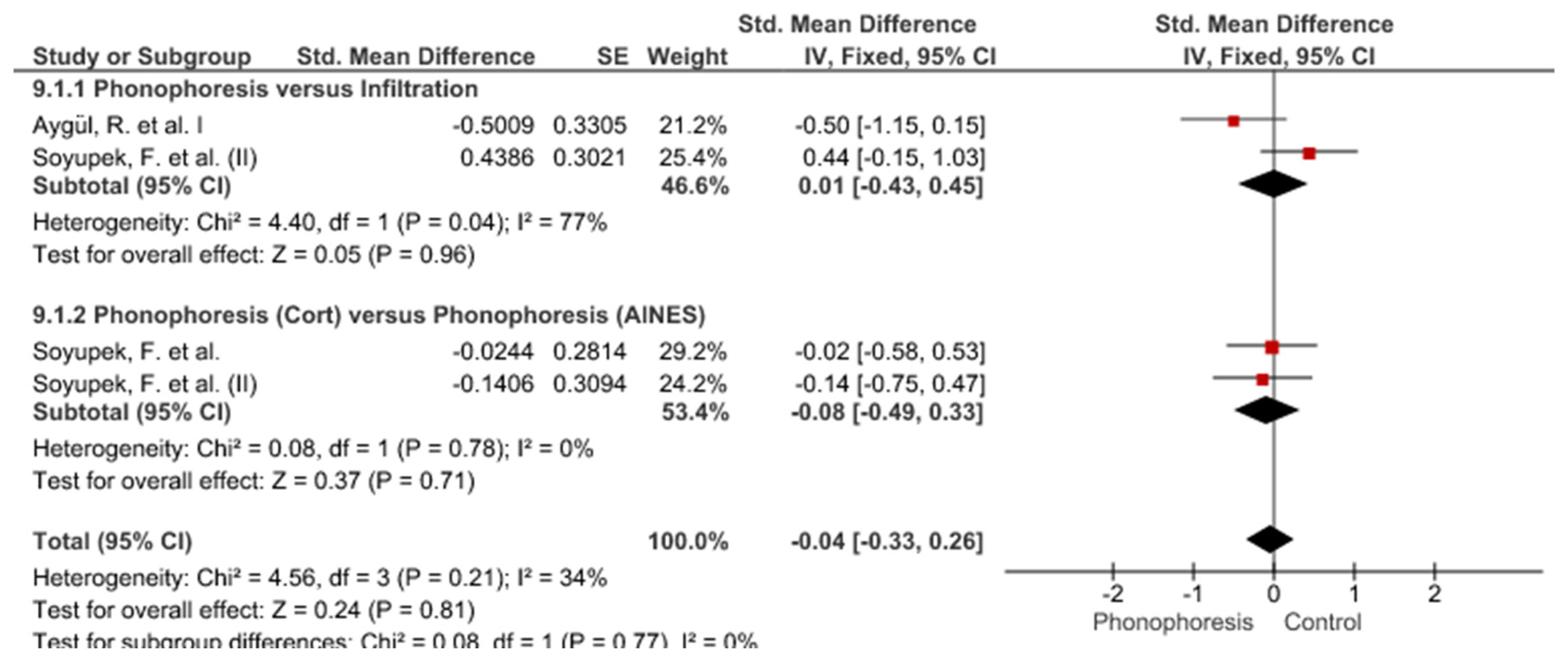
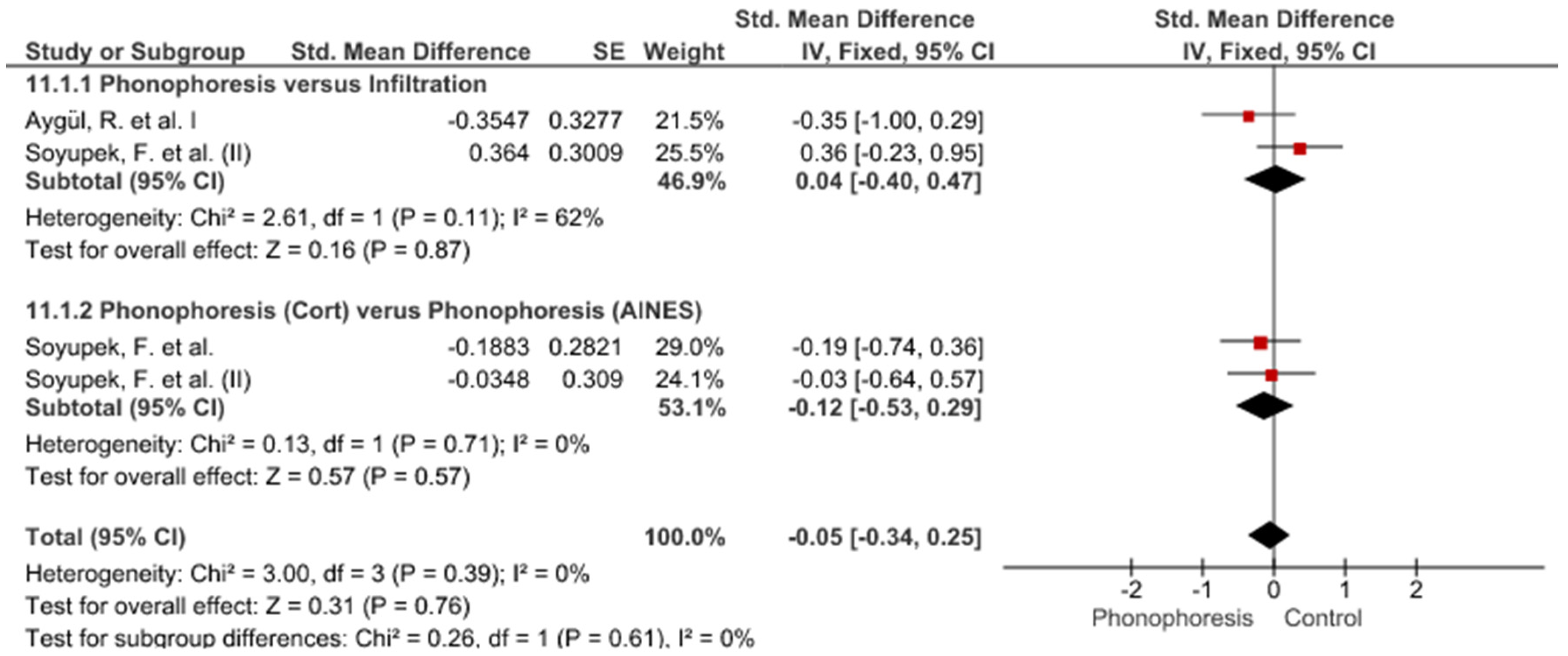
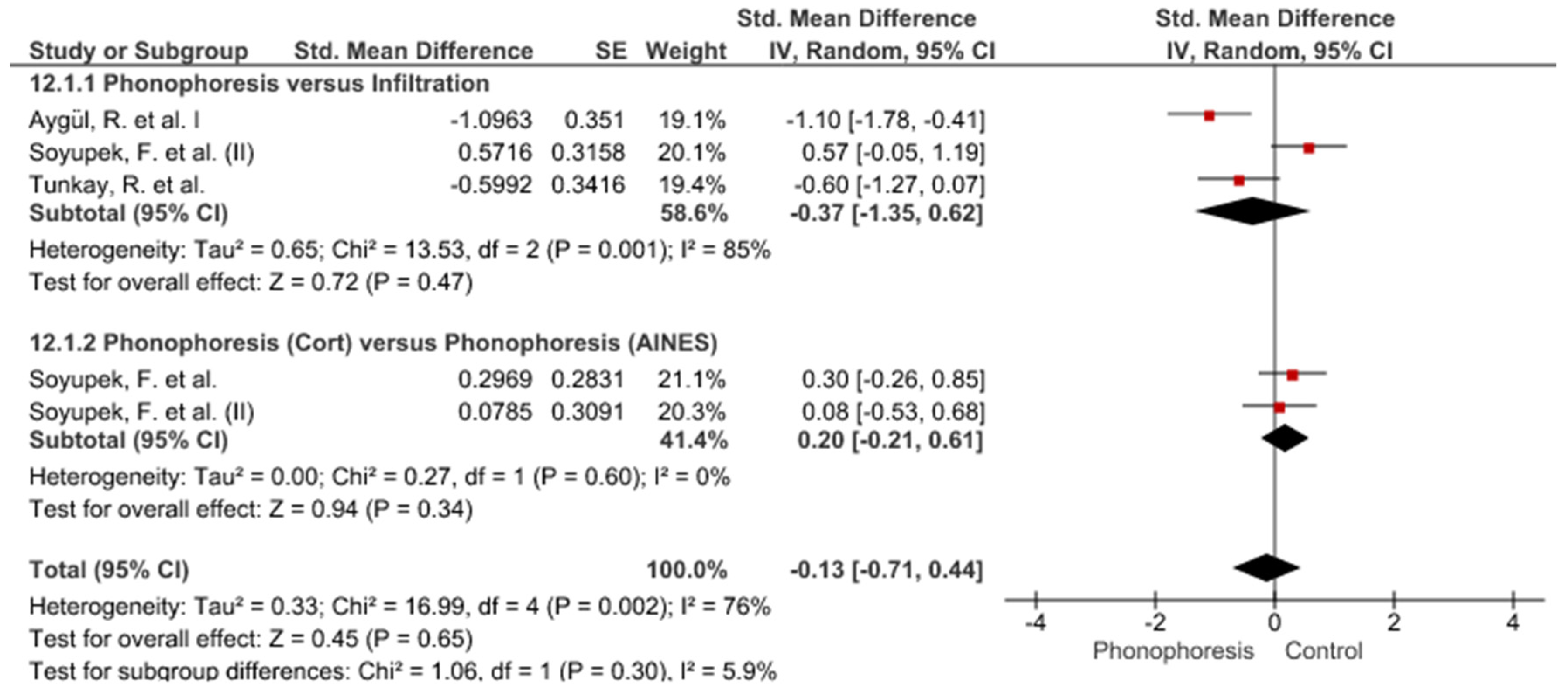
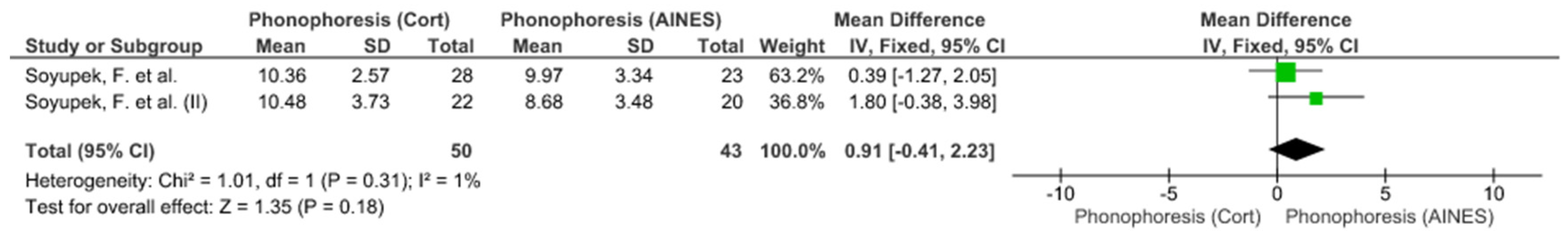
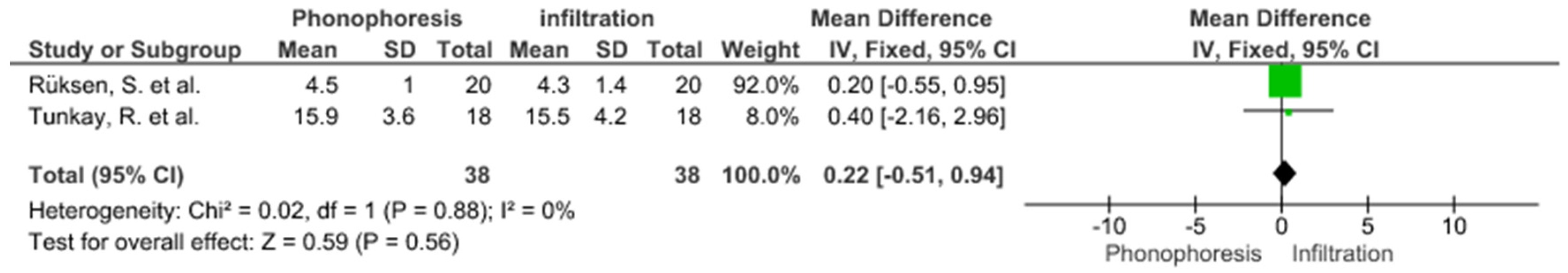
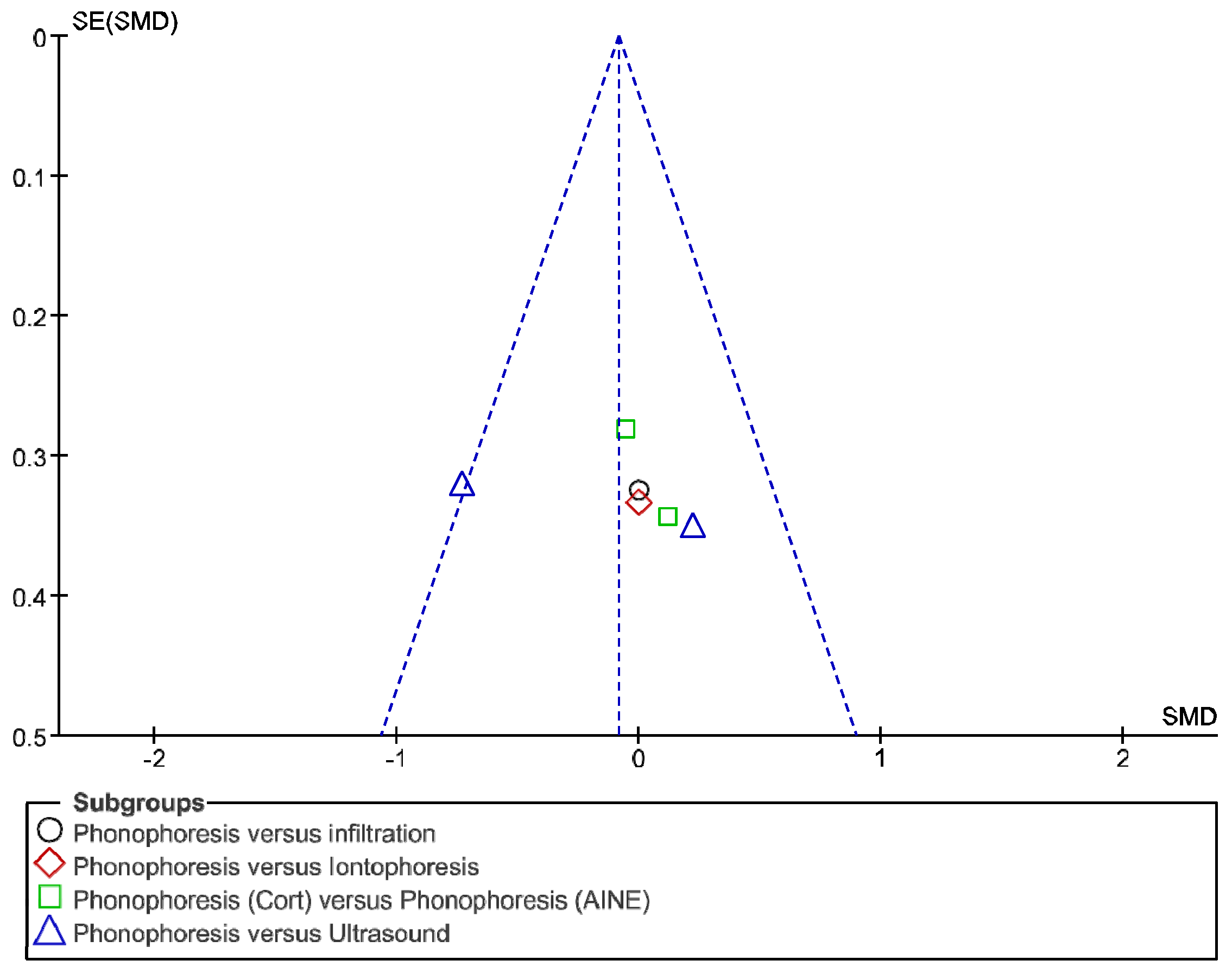
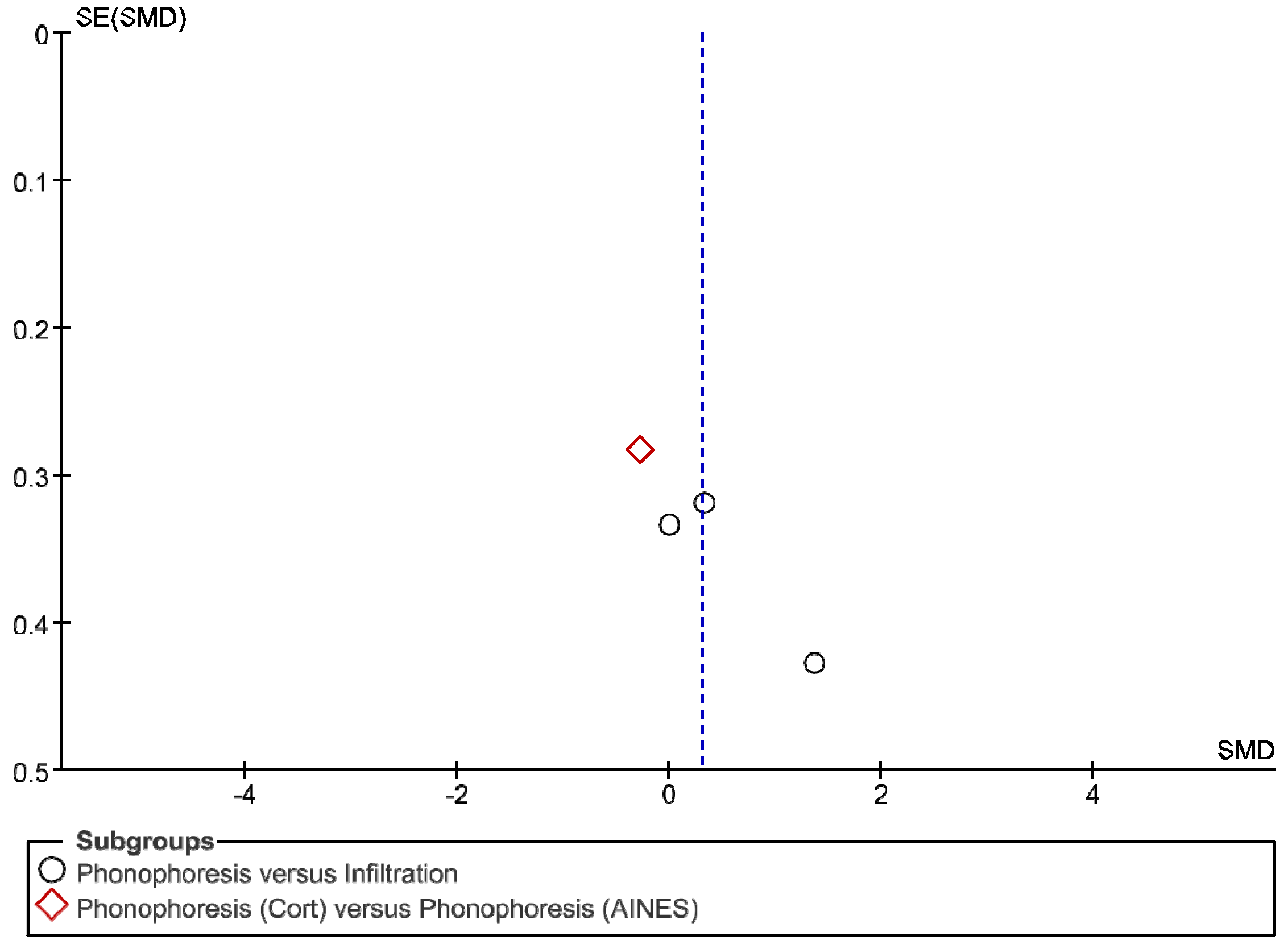
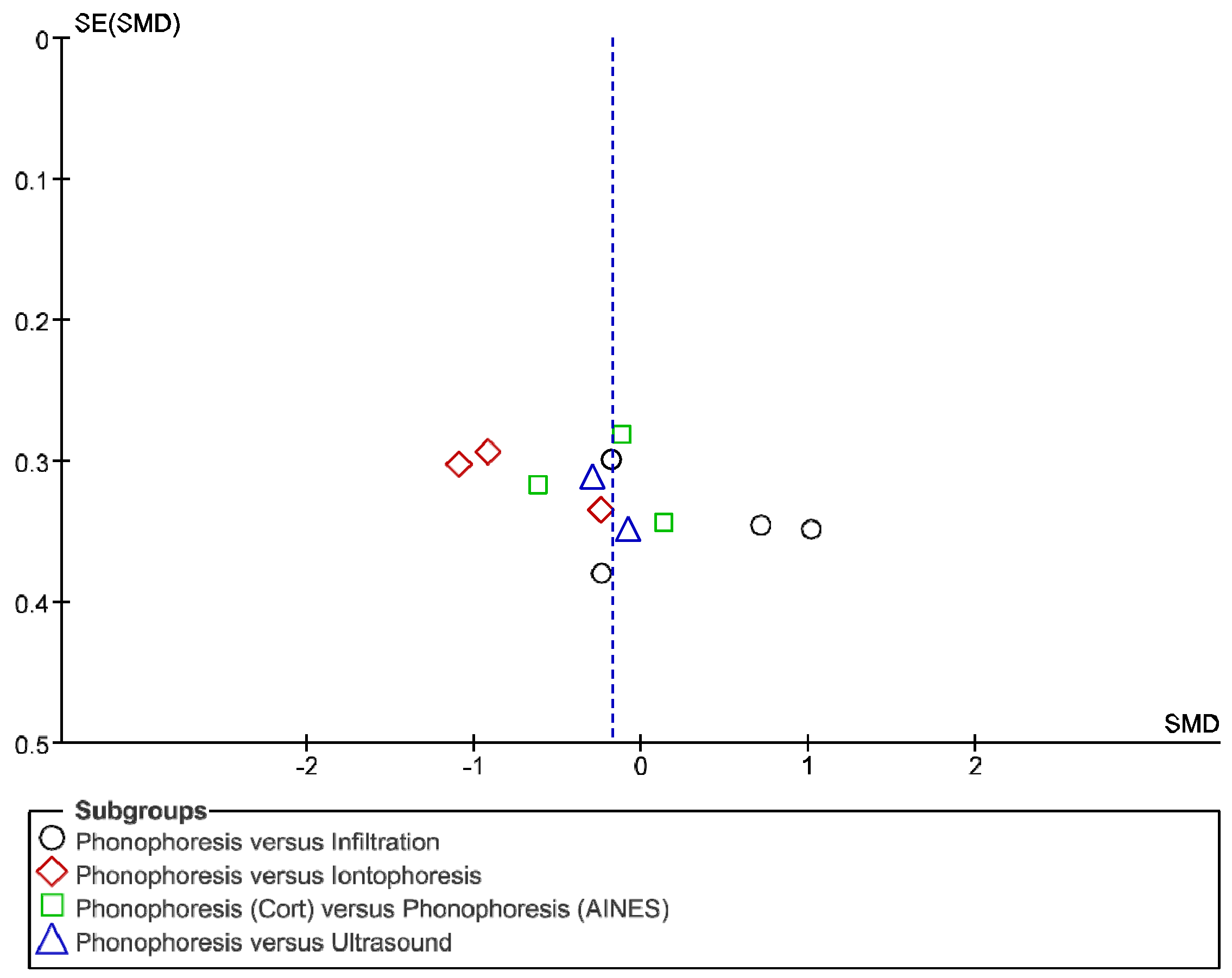
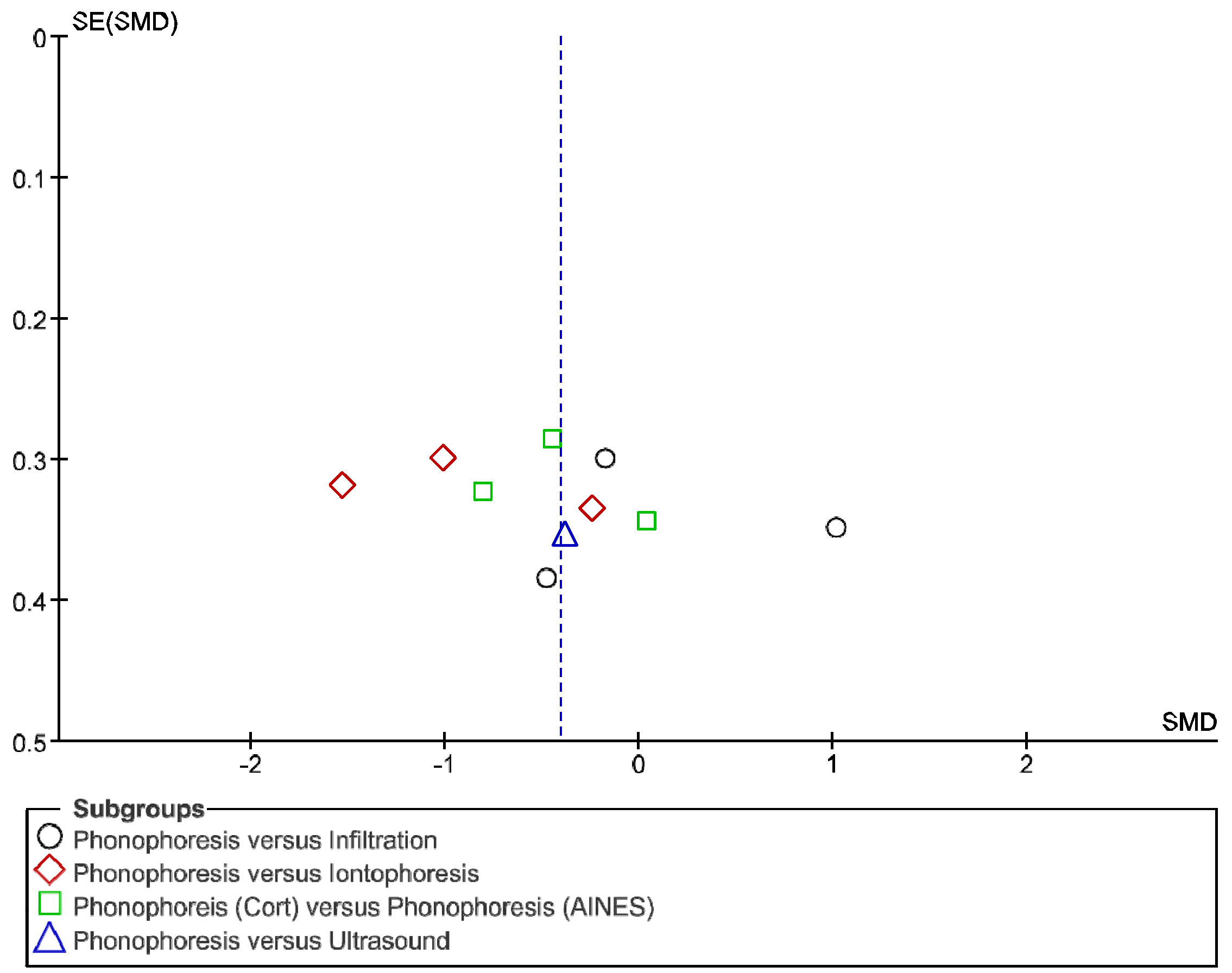
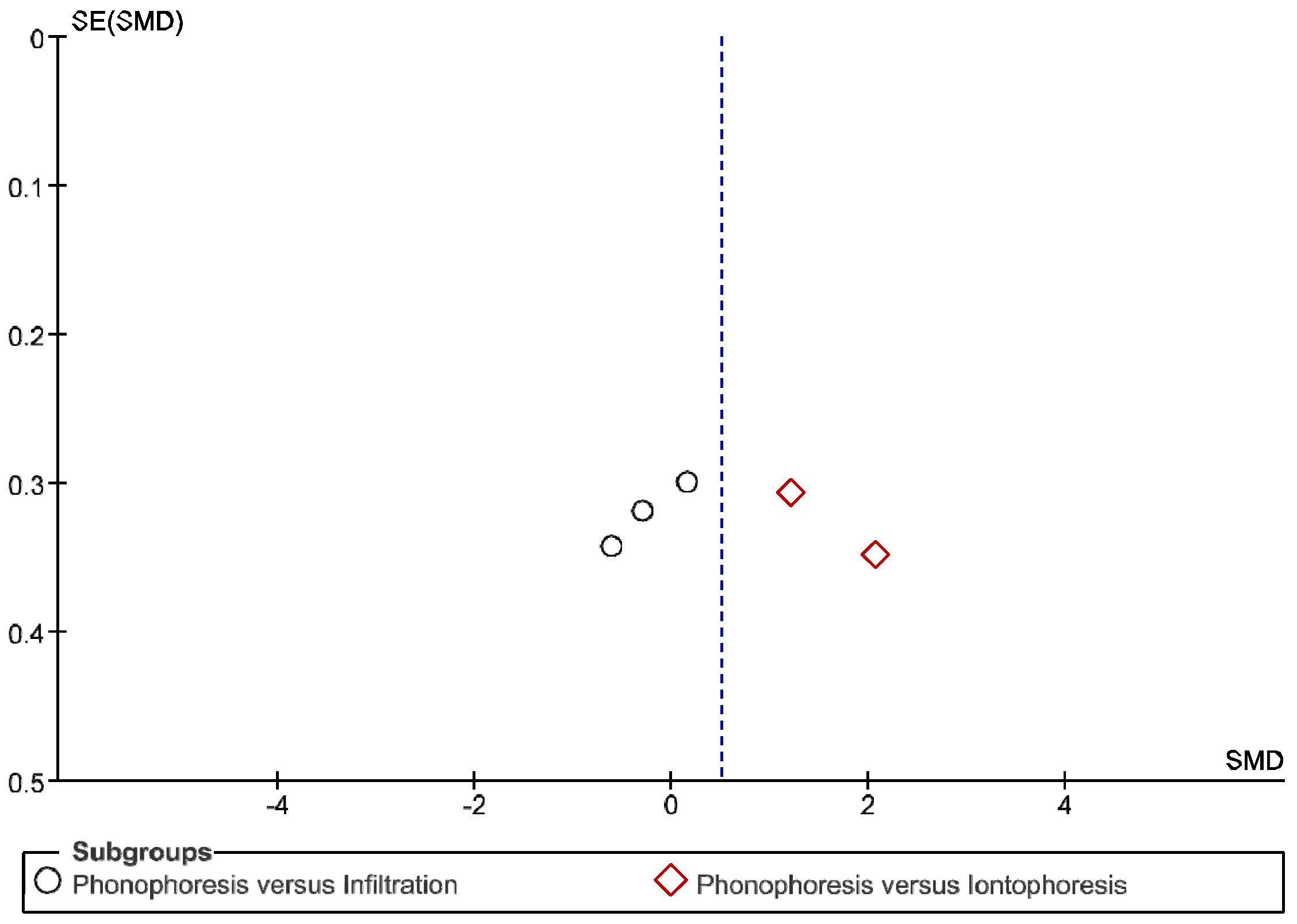
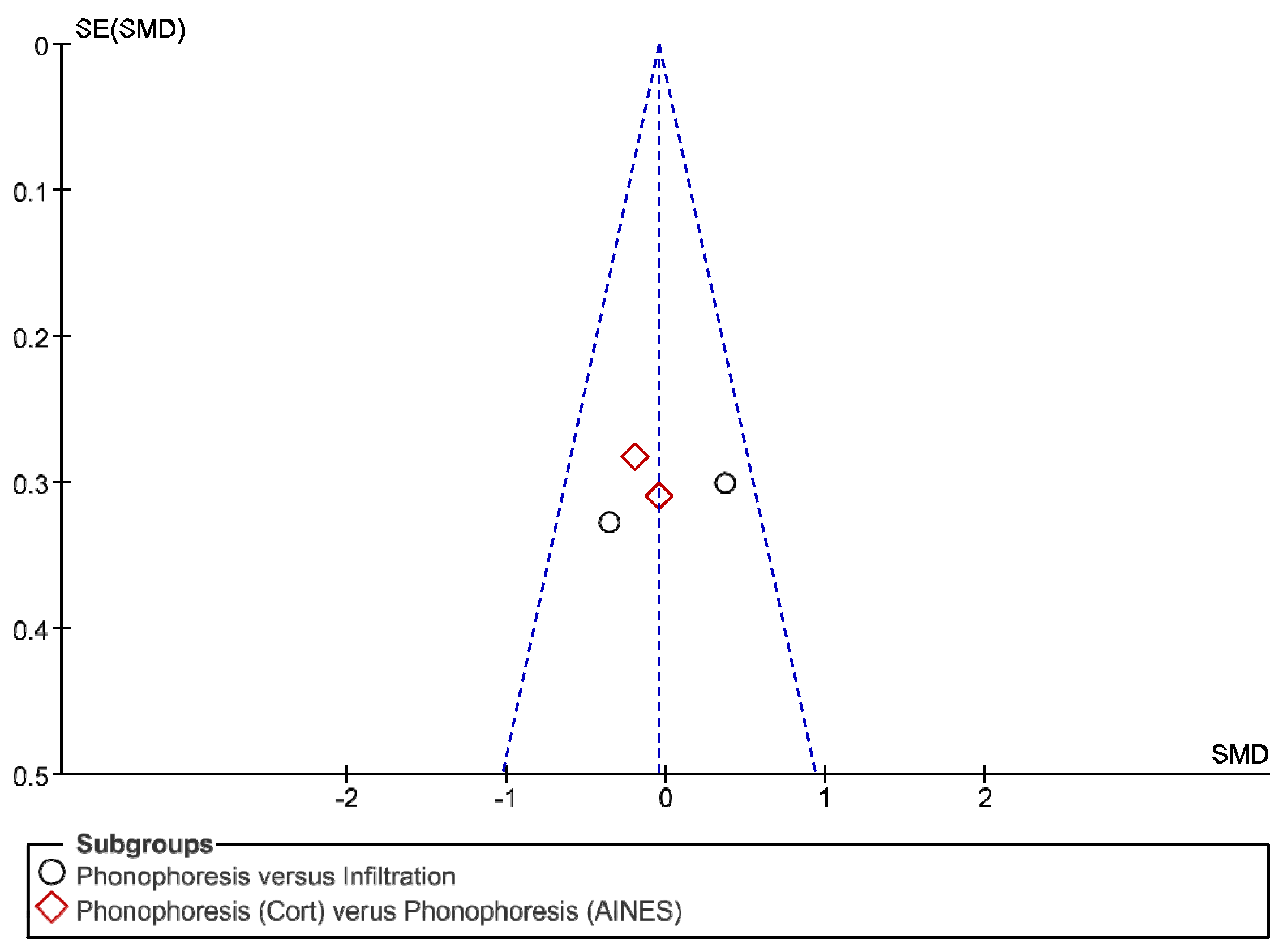
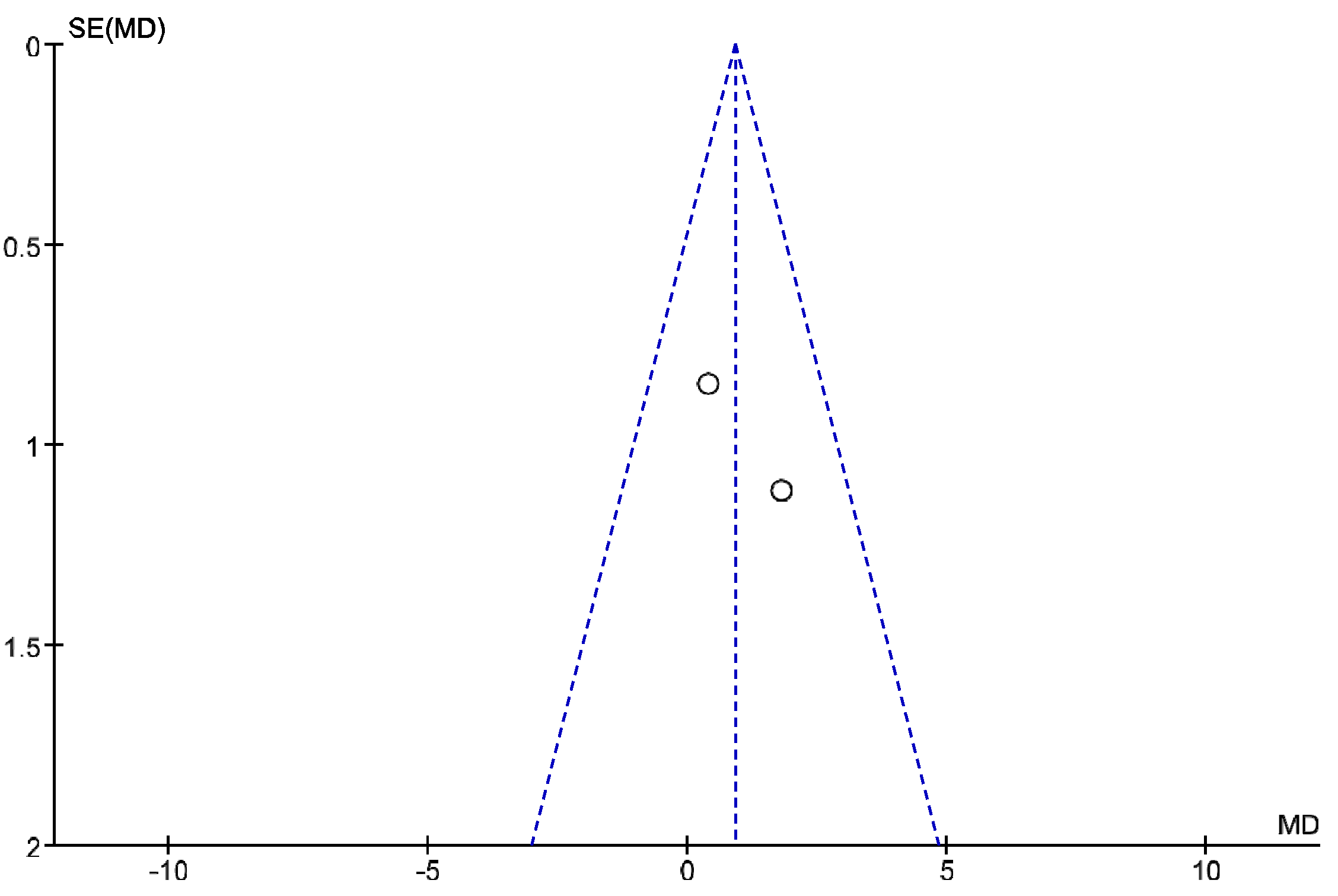
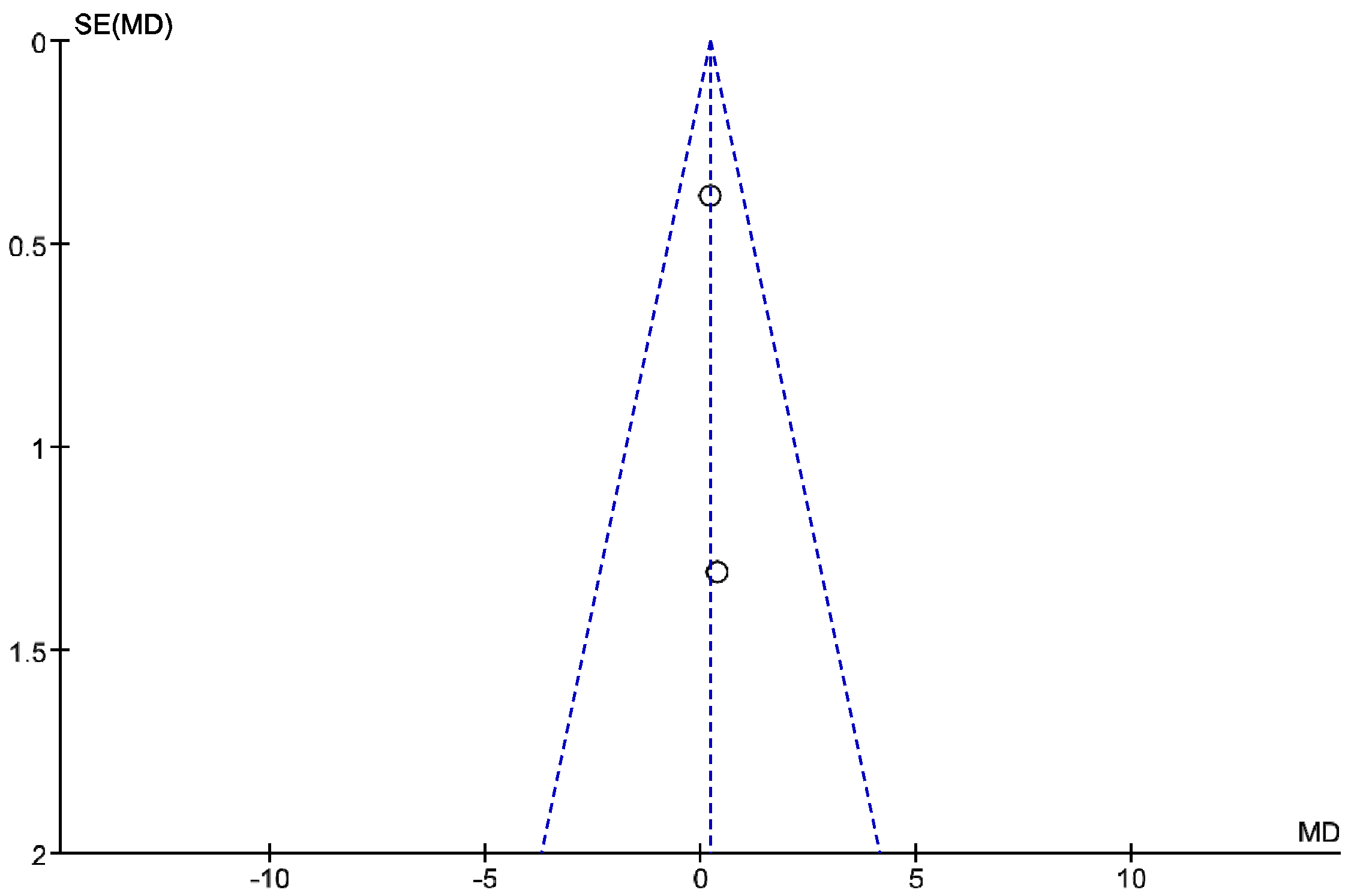
3. Results
Study Groups Included in the Meta-Analysis
4. Discussion
Review Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristiani-Winer, M.; Allende-Nores, C.; Aguirre, G.L.; Robles, C.O.; Ré, R. Sensitivity and specificity of three complementary methods in the diagnosis of carpal tunnel syndrome. Acta Ortop. Mex. 2020, 34, 31–37. [Google Scholar] [PubMed]
- Vicuña, P.; Idiáquez, J.F.; Jara, P.; Pino, F.; Cárcamo, M.; Cavada, G.; Verdugo, R. Electrophysiological severity of carpal tunnel syndrome according to age in adult patients. Rev. Med. Chile 2017, 145, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.A.; Shih, Y.C.; Hong, J.P.; Wu, C.W.; Liao, C.D.; Chen, H. Ultrasound-guided corticosteroid injection for patients with carpal tunnel syndrome: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 10417. [Google Scholar] [CrossRef] [PubMed]
- del Barrio, S.J.; Gracia, E.B.; García, C.H.; de Miguel, E.E.; Tricás Moreno, J.M.; Rodríguez Marco, S.; Ceballos Laita, L. Tratamiento conservador en pacientes con síndrome del túnel carpiano con intensidad leve o moderada. Revisión sistemática. Neurología 2018, 33, 590–601. [Google Scholar] [CrossRef]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Graham, B.; Peljovich, A.E.; Afra, R.; Cho, M.S.; Gray, R.; Stephenson, J.; Gurman, A.; MacDermid, J.; Mlady, G.; Patel, A.T.; et al. The American Academy of Orthopaedic Surgeons Evidence-Based Clinical Practice Guideline on: Management of Carpal Tunnel Syndrome. J. Bone Jt. Surg. Am. 2016, 98, 1750–1754. [Google Scholar] [CrossRef]
- Taheri, P.; Naderi, M.; Khosravi, S. Extracorporeal Shock Wave Therapy Versus Phonophoresis Therapy for Neck Myofascial Pain Syndrome: A Randomized Clinical Trial. Anesthesiol. Pain Med. 2021, 11, e112592. [Google Scholar] [CrossRef]
- Mitragotri, S.; Edwards, D.A.; Blankschtein, D.; Langer, R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J. Pharm. Sci. 1995, 84, 697–706. [Google Scholar] [CrossRef]
- Rigby, J.H.; Hagan, A.M.; Kelcher, A.R.; Ji, C. Dexamethasone Sodium Phosphate Penetration during Phonophoresis at 2 Ultrasound Frequencies. J. Athl. Train. 2020, 55, 628–635. [Google Scholar] [CrossRef]
- Knight, K.; Knight, K.L.; Draper, D.O. Therapeutic Modalities: The Art and Science; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Hu, L.; Batheja, P.; Meidan, V.; Michniak-Kohn, B.B. Iontophoretic transdermal drug delivery. In Handbook of NonInvasive Drug Delivery Systems; Vitthal, S.K., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 95–118. ISBN 9780815520252. [Google Scholar]
- Huisstede, B.M.; Hoogvliet, P.; Franke, T.P.; Randsdorp, M.S.; Koes, B.W. Carpal Tunnel Syndrome: Effectiveness of Physical Therapy and Electrophysical Modalities. An Updated Systematic Review of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2018, 99, 1623–1634. [Google Scholar] [CrossRef]
- Hartzell, T.L.; Rubinstein, R.; Herman, M. Therapeutic modalities—An updated review for the hand surgeon. J. Hand Surg. Am. 2012, 37, 597–621. [Google Scholar] [CrossRef]
- Marshall, S.; Tardif, G.; Ashworth, N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2007, 18, CD001554. [Google Scholar] [CrossRef]
- Osterman, A.L.; Whitman, M.; Della Porta, L. Nonoperative carpal tunnel syndrome treatment. Hand Clin. 2002, 18, 279–289. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Cumpston, M.S.; McKenzie, J.E.; Thomas, J.; Brennan, S.E. Current practice in systematic reviews including the “PICO for each synthesis” and methods other than meta-analysis: Protocol for a cross-sectional study. F1000Research 2020, 9, 678. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review: Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef]
- Cochrane Cochrane Training. 20 June. Available online: https://training.cochrane.org/online-learning/core/software/revman (accessed on 20 June 2022).
- Epidat: Programa Para Análisis Epidemiológico de Datos; Versión 4.2, Julio 2016; Consellería de Sanidade; Xunta de Galicia, España; Organización Panamericana de la salud (OPS-OMS); Universidad CES: Medellín, Colombia, 2016.
- Asheghan, M.; Aghda, A.K.; Sobhani, V.; Hashemi, S.E.; Hollisaz, M.T. A randomized comparative trial of corticosteroid phonophoresis, local corticosteroid injection, and low-level laser in the treatment of carpal tunnel syndrome. Laser Ther. 2020, 29, 11–17. [Google Scholar] [CrossRef]
- Ruksen, S.; Oz, B.; Olmez, N.; Memis, A. Comparison of Clinical Effectiveness of Corticosteroid Phonophoresis and Local Steroid Injection Treatment in Carpal Tunnel Syndrome. Turk. Fiz. Tip Rehabil. Derg.-Turk. J. Phys. Med. Rehabil. 2011, 57, 119–123. [Google Scholar] [CrossRef]
- Aygül, R.; Ulvi, H.; Karatay, S.; Deniz, O.; Varoglu, A.O. Determination of sensitive electrophysiologic parameters at follow-up of different steroid treatments of carpal tunnel syndrome. J. Clin. Neurophysiol. 2005, 22, 222–230. [Google Scholar]
- Tunkay, R.; Unlu, E.; Gurkay, E. The effects of Phonophoresis and Local Corticosteroid Injection to Boston Symptom Severity Scale, Grip Strength, Pinch Strength and Electrophysiological Findings in Patients with Carpal Tunnel Syndrome. Nobel Med. 2005, 1, 11–14. [Google Scholar]
- Bakhtiary, A.H.; Fatemi, E.; Emami, M.; Malek, M. Comparing the effects of iontophoresis and phonophoresis of dexamethasone on the treatment of carpal tunnel syndrome. KOOMESH-J. Semnan Univ. Med. Sci. 2011, 13, 83–92. [Google Scholar]
- Gurcay, E.; Unlu, E.; Gurcay, A.G.; Tuncay, R.; Cakci, A. Assessment of phonophoresis and iontophoresis in the treatment of carpal tunnel syndrome: A randomized controlled trial. Rheumatol. Int. 2012, 32, 717–722. [Google Scholar] [CrossRef]
- Soyupek, F.; Yesildag, A.; Kutluhan, S.; Askin, A.; Ozden, A.; Uslusoy, G.A.; Demirci, S. Determining the effectiveness of various treatment modalities in carpal tunnel syndrome by ultrasonography and comparing ultrasonographic findings with other outcomes. Rheumatol. Int. 2012, 32, 3229–3234. [Google Scholar] [CrossRef]
- Soyupek, F.; Kutluhan, S.; Uslusoy, G.; Ilgun, E.; Eris, S.; Aşkın, A. The efficacy of phonophoresis on electrophysiological studies of the patients with carpal tunnel syndrome. Rheumatol. Int. 2012, 32, 3235–3242. [Google Scholar] [CrossRef]
- Dogan-Akcam, F.; Basaran, S.; Guzel, R.; Uysal, F.G. The Effects of Steroid Phonophoresis on Clinical Parameters and Nerve Conduction Velocities in Carpal Tunnel Syndrome. Cukurova Med. J. 2012, 37, 17–26. [Google Scholar]
- Bakhtiary, A.H.; Fatemi, E.; Emami, M.; Malek, M. Phonophoresis of dexamethasone sodium phosphate may manage pain and symptoms of patients with carpal tunnel syndrome. Clin. J. Pain 2013, 29, 348–353. [Google Scholar] [CrossRef]
- Boonhong, J.; Thienkul, W. Effectiveness of Phonophoresis Treatment in Carpal Tunnel Syndrome: A Randomized Double-blind, Controlled Trial. PM R 2020, 12, 8–15. [Google Scholar] [CrossRef]
- Levine, D.W.; Simmons, B.P.; Koris, M.J.; Daltroy, L.H.; Hohl, G.G.; Fossel, A.H.; Katz, J.N. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J. Bone Jt. Surg. Am. 1993, 75, 1585–1592. [Google Scholar] [CrossRef]
- American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve 1992, 15, 229–253. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale. Arthritis Care Res. 2011, 63 (Suppl. S1), S240–S252. [Google Scholar] [CrossRef]
- Multanen, J.; Uimonen, M.M.; Repo, J.P.; Häkkinen, A.; Ylinen, J. Use of conservative therapy before and after surgery for carpal tunnel syndrome. BMC Musculoskelet. Disord. 2021, 22, 484. [Google Scholar] [CrossRef]
- Paiva, H.R.; Paiva, V.D.G.N.; Oliveira, E.F.; Rocha, M.A. Profile of Patients with Carpal Tunnel Syndrome Treated at a Referral Service. Acta Ortop. Bras. 2020, 28, 117–120. [Google Scholar] [CrossRef]
- Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Sweeney, M.H.; Sestito, J.P.; Calvert, G.M. Prevalence and work-relatedness of carpal tunnel syndrome in the working population, United States, 2010 National Health Interview Survey. Am. J. Ind. Med. 2010, 56, 615–624. [Google Scholar] [CrossRef]
- Roel-Valdés, J.; Arizo-Luque, V.; Ronda-Pérez, E. Epidemiology of occupationally-caused carpal tunnel syndrome in the province of Alicante, Spain 1996–2004. Rev. Esp. Salud Publica 2006, 80, 395–409. [Google Scholar] [CrossRef]
- Pires, P.R.; Andrade, R.P.; Pardini, A. Síndromes compressivas no membro superior. In Cirurgia da mão: Lesões não Traumáticas; Medbook: Río de Janeiro, Brazil, 2008; pp. 163–169. ISBN 8599977261. [Google Scholar]
- Stone, S.; Malanga, G.A.; Capella, T. Corticosteroids: Review of the History, the Effectiveness, and Adverse Effects in the Treatment of Joint Pain. Pain Physician 2021, 24, S233–S246. [Google Scholar]
- Hamzeh, H.H.; Alworikat, N.A. Cross cultural adaptation, reliability and construct validity of the Boston Carpal Tunnel Questionnaire in standard Arabic language. Disabil. Rehabil. 2021, 43, 430–435. [Google Scholar] [CrossRef]
- Ebenbichler, G.R.R.K.; Nicolakis, P.; Wiesinger, G.F.; Uhl, F.; Ghanem, A.H.; Fialka, V. Ultrasound treatment for treating the carpal tunnel syndrome: Randomised “sham” controlled trial. Br. Med. J. 1998, 7, 731–735. [Google Scholar] [CrossRef]
- Multanen, J.; Ylinen, J.; Karjalainen, T.; Kautiainen, H.; Repo, J.P.; Häkkinen, A. Reliability and Validity of The Finnish Version of the Boston Carpal Tunnel Questionnaire among Surgically Treated Carpal Tunnel Syndrome Patients. Scand. J. Surg. 2020, 109, 343–350. [Google Scholar] [CrossRef]
- Multanen, J.; Ylinen, J.; Karjalainen, T.; Ikonen, J.; Häkkinen, A.; Repo, J.P. Structural validity of the Boston Carpal Tunnel Questionnaire and its short version, the 6-Item CTS symptoms scale: A Rasch analysis one year after surgery. BMC Musculoskelet. Disord. 2020, 21, 609. [Google Scholar] [CrossRef]
- Oteo-Álvaro, Á.; Marín, M.T.; Matas, J.A.; Vaquero, J. Spanish validation of the Boston Carpal Tunnel Questionnaire. Med. Clin. 2016, 146, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Valles-Figueroa, J.F.; Obil-Chavarría, C.A.; Suárez-Ahedo, C.E.; Rodríguez-Reséndiz, F. Surgical treatment of carpal tunnel syndrome and its correlation with preoperative nerve conduction tests. Acta Ortop. Mex. 2012, 26, 303–306. [Google Scholar] [PubMed]
- Madrazo, J.; Marín, I.; Bringas, A.; Fernández, A. The efficacy of neurophysiological examination in the diagnosis of carpal tunnel syndrome. Rev. Neurol. 2000, 30, 1005–1008. [Google Scholar]
- Kahl, C.; Cleland, J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005, 10, 123–128. [Google Scholar] [CrossRef]
- Li Pi Shan, R.; Nicolle, M.; Chan, M.; Ashworth, N.; White, C.; Winston, P.; Dukelow, S. Electrodiagnostic Testing and Treatment for Carpal Tunnel Syndrome in Canada. Can. J. Neurol. Sci. 2016, 43, 178–182. [Google Scholar] [CrossRef]
- Suda, M.; Kawakami, M.; Okuyama, K.; Ishii, R.; Oshima, O.; Hijikata, N.; Nakamura, T.; Oka, A.; Kondo, K.; Liu, M. Validity and Reliability of the Semmes-Weinstein Monofilament Test and the Thumb Localizing Test in Patients with Stroke. Front. Neurol. 2021, 11, 625917. [Google Scholar] [CrossRef] [PubMed]
- Hersh, B.; D’Auria, J.; Scott, M.; Fowler, J.R. A Comparison of Ultrasound and MRI Measurements of the Cross-Sectional Area of the Median Nerve at the Wrist. Hand 2019, 14, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Küçük, E.B.; Taşkıran, Ö. Evaluation of Duruöz Hand Index in diagnosis and staging of Carpal tunnel syndrome. J. Clin. Neurosci. 2020, 82, 111–114. [Google Scholar] [CrossRef]
- Cunha, T.A.L.; Oliveira, O.M.; Ribeiro, M.B. Phalen Test Positivation Time and Its Correlation with Electroneuromyography. Acta Ortop. Bras. 2020, 28, 114–116. [Google Scholar] [CrossRef]
- Nakashian, M.N.; Ireland, D.; Kane, P.M. Cubital Tunnel Syndrome: Current Concepts. Curr. Rev. Musculoskelet. Med. 2020, 13, 520–524. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker for Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Kuan, T.S.; Yang, H.C.; Tsai, C.L.; Yeh, C.H.; Lin, C.C.; Kuo, L.-C. Effects of the Surface Texture and Weight of a Pinch Apparatus on the Reliability and Validity of a Hand Sensorimotor Control Assessment. Arch. Phys. Med. Rehabil. 2019, 100, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Mose, K.F.; Andersen, F.; Røpke, M.A.; Skov, L.; Friedmann, P.S.; Andersen, K.E. Anti-inflammatory potency testing of topical corticosteroids and calcineurin inhibitors in human volunteers sensitized to diphenylcyclopropenone. Br. J. Clin. Pharmacol. 2018, 84, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; McDermott, M.F.; Laurino, C.; Malagoli, A.; Palmieri, B. Corticosteroid transdermal delivery significantly improves arthritis pain and functional disability. Drug Deliv. Transl. Res. 2017, 7, 156–161. [Google Scholar] [CrossRef][Green Version]
- Akinbo, S.R.; Aiyejusunle, C.B.; Akinyemi, O.A.; Adesegun, S.A.; Danesi, M.A. Comparison of the therapeutic efficacy of phonophoresis and iontophoresis using dexamethasone sodium phosphate in the management of patients with knee osteoarthritis. Niger. Postgr. Med. J. 2007, 14, 190–194. [Google Scholar]
- Boucaud, A.; Garrigue, M.A.; Machet, L.; Vaillant, L.; Patat, F. Effect of sonication parameters on transdermal delivery of insulin to hairless rats. J. Control Release 2002, 81, 113–119. [Google Scholar] [CrossRef]
- Tezel, A.; Sens, A.; Tuchscherer, J.; Mitragotri, S. Frequency dependence of sonophoresis. Pharm. Res. 2001, 18, 1694–1700. [Google Scholar] [CrossRef]





| Search Formulae | Databases |
|---|---|
| Phonophoresis AND carpal tunnel syndrome Phonophoresis AND carpal tunnel syndrome AND treatment(s) Phonophoresis AND median neuropathy Phonophoresis AND median neuropathy AND treatment(s) Sonophoresis AND carpal tunnel syndrome Sonophoresis AND carpal tunnel syndrome AND treatment(s) Sonophoresis AND median neuropathy Sonophoresis AND median neuropathy AND treatment(s) | Web of Science |
| SCOPUS | |
| PUBMED | |
| CINAHL Complete | |
| SciELO | |
| “Phonophoresis” “carpal tunnel syndrome” “Phonophoresis” “carpal tunnel syndrome” “treatment(s)” “Phonophoresis” “median neuropathy” “Phonophoresis” “median neuropathy” “treatment(s)” “Sonophoresis” “carpal tunnel syndrome” “Sonophoresis” “carpal tunnel syndrome” “treatment(s)” “Sonophoresis” “median neuropathy” “Sonophoresis” “median neuropathy” “treatment(s)” | PEDro |
| TOTAL |
| Authors (Year)/Design | Study Groups | Measuring and Evaluation Tools | Intervention | Parameters | Results |
|---|---|---|---|---|---|
| Asheghan M. et al., 2020 [22] RCT | No. = 42 (31 women. 36 right-handed). Average age: LCI: 48.6 (±11.6); LLLT: 49.4 (±5.2); PCS: 52.4 (±3.8) LCI: no = 14 (11 women) LLLT: no = 14 (9 women) PCS: no = 14 (11 women) CTS: Mild to moderate | Pain (VAS) BCTQ NCS Evaluation: Before- 4th week | LCI: methylprednisolone with lidocaine LLLT PCS: hydrocortisone acetate (10%) | LLLT: 10 sessions. (10 s/session) PCS: 10 sessions (3 times/week), Frequency: 1 MHz. Intensity: 1 W/cm2. ERA: 5 cm2. 5 min/session | All the three methods were effective. Statistically significant differences in terms of pain for LCI (p = 0.003) and for sensory latency (p = 0.001) |
| Boonhong J & Thienkul W. 2020 [32] RCT | No. = 33 (50 hands. 17 bilateral) Average age: 51.5 (±10.5) US: no = 16 hands PNSAI: no = 17 hands PCS: no = 17 hands CTS: Mild to moderate | BCTQ NCS Evaluation: Before 4th week | US: Contact gel PNSAI: Piroxicam (0.5%) PCS: Dexamethasone sodium phosphate (0.4%) | For all the treatments: 10 sessions (2–3 times/week) for four weeks. Continuous mode. Frequency: 1 MHz. Intensity: 1 W/cm2. 10 min/session | All the three methods improve clinical symptoms and functionality, but not the electrophysiologic parameters. Statistically significant differences are not observed among methods (p < 0.05) |
| Soyupek F. et al., 2012 [28] RCT | No. = 47 (74 hands. 28 bilateral. 14 right-handed. 4 left-handed) Average age: Splint: 47.9 (±6.9); PNSAI: 53.7 (±10.4); PCS: 50.5 (±8.7) Splint: no = 23 hands PNSAI: no = 23 hands PCS: no = 28 hands | Pain (VAS) Ecography (cross-sectional area of median nerve) Phalen and Tinel tests BCTQ NCS Evaluation: Before- 3 months | Splint: Neutral position PNSAI: diclofenac diethylammonium PCS: betamethasone valerate (0.1%) | PNSAI/PCS: 5 sessions/week for 3 weeks. Frequency: 3 MHZ. Intensity: 1.5 W/cm2. ERA: 5 cm2. 10 min/session | PCS proved more efficient, although no correlation was established between symptoms severity, functionality and ecographic and electrophysiologic findings. (p < 0.05) |
| Aygül R et al., 2005 [24] RCT | No. = 31 (56 hands. 31 women. 27 bilateral) Average age: LCI: 46 (±13.5); Ionto: 46.1 (±13.5); PCS: 44.1 (±5.7) LCI: no = 12 Ionto: no = 9 PCS: no = 10 CTS: Mild to moderate | BCTQ NCS Evaluation: Before 2 months & 3 months | LCI: dexamethasone sodium phosphate Ionto: dexamethasone sodium phosphate (0.1%) PCS: dexamethasone sodium phosphate (0.1%) for 3 weeks | Ionto: 3 weeks (5 days/week). Galvanic current from 1 to 4 mA. 10 min/session PCS: 3 weeks (5 days/week). Frequency: 3 MHz. Intensity: 1 W/cm2. ERA: 5 cm2. 10 min/session | LCI is more effective compared to electrophysiologic parameters. Statistically significant differences are not observed between PCS and Ionto. (p < 0.05) |
| Bakhtiary AH et al., 2013 [31] RCT | No. = 34 (52 hands. 18 bilateral. 16 right-handed). Average age: Ionto: 48.2(±14.5); PCS: 44.6 (±12.8) Ionto: no = 26 hands PCS: no = 26 hands CTS: Mild to moderate | Pain (VAS) Pinch and grip strength (Dynamometer) NCS Evaluation: Before-after and in week 4th | Ionto & PCS: dexamethasone sodium phosphate (0.4%) | Ionto: 2 mA/minute galvanic current. Dosage: 40 mA. 20 min PCS: 10 sessions (5 sessions/week). Pulsed emission (25%) Frequency: 1 MHz. Intensity: 1 W/cm2. 5 min/session | PCS is more efficient than Ionto (p < 0.05) |
| Soyupek F et al., 2012 [29] (II)EC | No. = 51 (84 hands. 33 bilateral) Average age: LCI: 51.34 (±10.18); PNSAI: 48.3 (±8.66); PCS: 49.24 (±12.27); Splint:47.52 (±8.36) Splint: 19 hands LCI: no = 23 hands PNSAI: no = 20 hands PCS: no = 22 hands | Pain (VAS) Grip strength (Dynamometer) Manual dexterity (The grooved pegboard) Semmes-Weinstein test Duruoz Hand Index Phalen and Tinel tests NCS Evaluation: Before- 3 months | Splint: Neutral position LCI: betamethasone dipropionate (0.5 mg) PNSAI: diclofenac diethylammonium (0.1%) PCS: betamethasone valerate (0.1%) | PNSAI/PCS: 3 weeks (5 sessions/week). Frequency: 3 MHz. Intensity: 1.5 W/cm2. ERA: 5 cm2. 10 min/session | For PCS improved NCS parameters are recorded, but not for pain and other subjective parameters (p < 0.05) |
| Gurkay E et al., 2012 [27] RCT | No. = 54 (45 right-handed. 7 left-handed) Average age: Splint: 43 (±6.9); Ionto: 44.1 (±9.5); PCS: 44 (±8.7) Splint: no = 18 hands Ionto: no = 16 hands PCS: no = 18 hands | BCTQ Grip strength (Dynamometer) Manual dexterity and function (Nine-holepeg test) Evaluation: Before- 3 months | Splint (all the groups): Neutral position Ionto: Betamethasone (0.1%) PCS: Betamethasone (0.1%) | Ionto: 3 weeks. (3 sessions/week). 4 mA galvanic current. 10 min/session PCS: 3 weeks. (3 sessions/week). Frequency: Continuous mode. 1 MHz. Intensity: 1 W/cm2. 10 min/session | All three methods were effective. Statistically significant difference in PCS BCTQ compared to splint. Variations are not observed concerning grip strength, manual dexterity, and function (p > 0.05) |
| Rüksen S et al., 2011 [23] RCT | No. = 32 (40 hands. 29 women. 9 bilateral) Average age: LCI: 41.3 (±11.2); PCS: 45.7 (±10.3) LCI: no = 20 hands (19 women) PCS: no = 20 hands (18 women) CTS: Mild to moderate | Pain (VAS) BCTQ Pinch and grip strength (Dynamometer) Paresthesia (Likert Scale) Manual dexterity (Test Grooved Pegboard) Evaluation: Before-after and in 3 months | LCI: (6.43 mg of betamethasone dipropionate) + splint + exercises PCS: (2.63 mg of betamethasone valerate) + splint + exercises | PCS: 2 weeks (5 sessions/week). Intensity: 1 W/cm2. 10 min/session | After treatment completion both methods recorded a statistically significant improvement. No statistically significant differences were observed in relation to the degree of efficacy of both treatments. (p < 0.05) |
| Tuncay R et al., 2005 [25] RCT | No. = 36 women Average age: LCI: 39.16 (±13.03) PCS: 44.05 (±8.73) LCI: no = 18 PCS: no = 18 | BCTQ Pinch and grip strength (Dynamometer) Evaluation: Before- 3 months | LCI: (Betamethasone 1 mg) + splint in a neutral position at night PCS: (Betamethasone) + splint at night in a neutral position | PCS: 3 weeks. (3 sessions/week). Continuous mode. Frequency: 1 MHz. Intensity: 1 W/cm2. 10 min/session | Both methods were effective (p < 0.001). LCI improves nerve conduction velocity (p < 0.05) |
| Bakhtiary AH et al., 2014 [26] RCT | No. = 35 (51 hands) Ionto: no = 19 (25 hands) PCS: no = 16 (26 hands) CTS: Mild to moderate | Pain (VAS) Pinch and grip strength (Dynamometer) Paresthesia NCS Evaluation: Before-after and in week 4th | Ionto: dexamethasone sodium phosphate (0.4%) PCS: dexamethasone (0.4%) | Ionto: 2 weeks (1 session/week). 0.4 mA/cm2 continuous current. 10 min/session PCS: 2 weeks (1 session/week). Pulsed mode. Frequency: 1 MHz. Intensity: 1 W/cm2. 5 min/session | More efficacy of PCS. Improved grip strength (p = 0.006), reduced pain (p = 0.001) and improved NCS parameters (sensory: p = 0.001, motor: p = 0.008). |
| Dogan-Akcam F et al., 2012 [30] RCT | No. = 39 (69 hands. 30 bilateral) Average age: US simulation: 49.8 (±5.3); US: 46.2 (±12.1); PCS: 46.1 (±7.7) US simulation: no = 13 (24 hands) US: no = 13 (21 hands) PCS: no = 13 (21 hands) | Pain (VAS) BCTQ NCS Evaluation: Before- 2 weeks and 12 weeks | US: simulation (harmless contact gel) + exercises US: (harmless contact gel) + exercises PCS: (dexamethasone 0.1%) + exercises | For all the groups: 2 weeks (5 sessions/week). Intensity: 0.1 W/cm2 (except for US simulation: 0.0 W/cm2). 5 min/session | All the methods are effective in relation to clinical parameters and evaluations. PCS is more efficient and long-lasting compared to NCS parameters (p < 0.05) |
| Author (Year) | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asheghan M. et al., 2020 [22] | - | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9/10 |
| Boonhong J & Thienkul W., 2020 [32] | - | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9/10 |
| Soyupek F. et al., 2012 [28] | - | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7/10 |
| Aygül R et al., 2005 [24] | - | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7/10 |
| Bakhtiary AH et al., 2013 [31] | - | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9/10 |
| Soyupek F et al., 2012 (II) [29] | - | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6/10 |
| Gurkay E et al., 2012 [27] | - | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6/10 |
| Rüksen S et al., 2011 [23] | - | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7/10 |
| Tuncay R et al., 2005 [25] | - | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6/10 |
| Bakhtiary AH et al., 2014 [26] | - | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 6/10 |
| Dogan-Akcam F et al., 2012 [30] | - | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Vega, F.J.; Vinolo-Gil, M.J.; Perez-Cabezas, V.; Rodríguez-Huguet, M.; Garcia-Munoz, C.; Gonzalez Medina, G. Use of Sonophoresis with Corticosteroids in Carpal Tunnel Syndrome: Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1160. https://doi.org/10.3390/jpm12071160
Martin-Vega FJ, Vinolo-Gil MJ, Perez-Cabezas V, Rodríguez-Huguet M, Garcia-Munoz C, Gonzalez Medina G. Use of Sonophoresis with Corticosteroids in Carpal Tunnel Syndrome: Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(7):1160. https://doi.org/10.3390/jpm12071160
Chicago/Turabian StyleMartin-Vega, Francisco Javier, Maria Jesus Vinolo-Gil, Veronica Perez-Cabezas, Manuel Rodríguez-Huguet, Cristina Garcia-Munoz, and Gloria Gonzalez Medina. 2022. "Use of Sonophoresis with Corticosteroids in Carpal Tunnel Syndrome: Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 7: 1160. https://doi.org/10.3390/jpm12071160
APA StyleMartin-Vega, F. J., Vinolo-Gil, M. J., Perez-Cabezas, V., Rodríguez-Huguet, M., Garcia-Munoz, C., & Gonzalez Medina, G. (2022). Use of Sonophoresis with Corticosteroids in Carpal Tunnel Syndrome: Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 12(7), 1160. https://doi.org/10.3390/jpm12071160








