Fasting Proinsulin Independently Predicts Incident Type 2 Diabetes in the General Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Data Collection
2.3. Laboratory Measurements
2.4. Outcome Ascertainment
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Cross-Sectional Associations
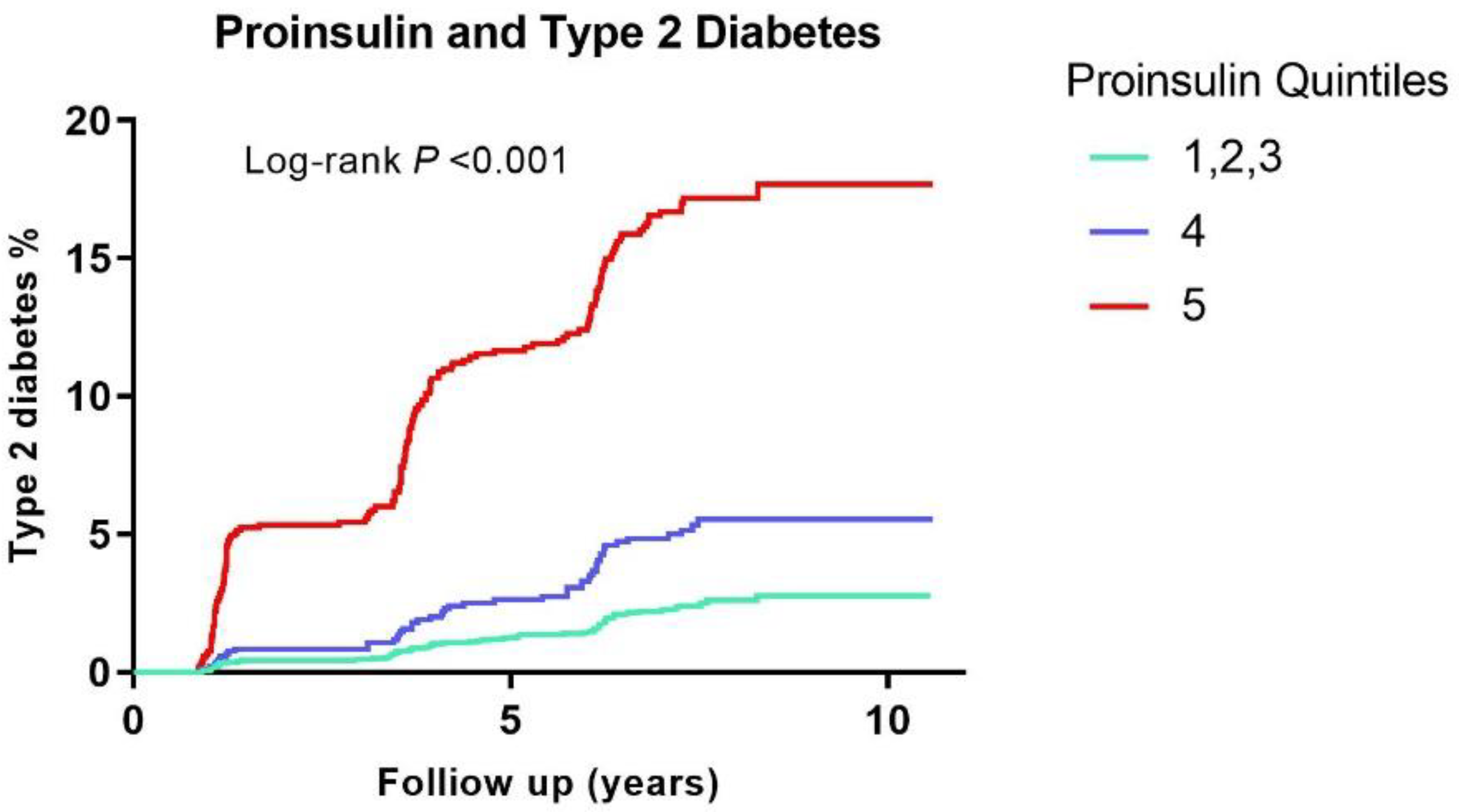
3.3. Association of Proinsulin with Risk of Developing T2D
3.4. Model Performance Compared with the FOS Risk Score
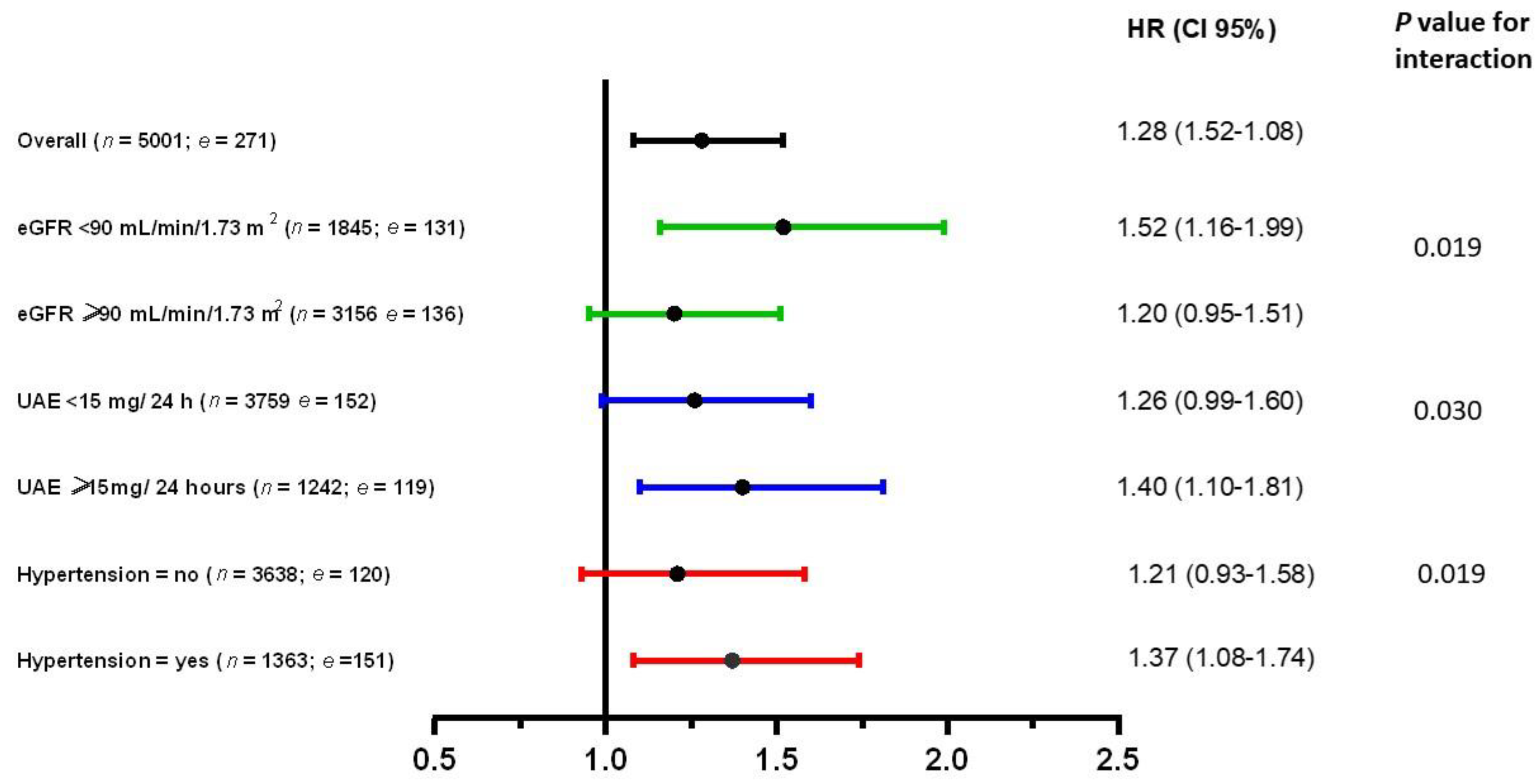
3.5. Secondary Analyses of Proinsulin with Incident T2D in Various Groups
3.6. Sensitivity Analyses on Proinsulin and T2D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and Treatment of Diabetes. Nutr. Diabetes 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The Natural History of Insulin Secretory Dysfunction and Insulin Resistance in the Pathogenesis of Type 2 Diabetes Mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Tomono, S.; Kato, N.; Utsugi, T.; Ohno, T.; Shimizu, M.; Fukuda, M.; Itoh, Y.; Ishii, C.; Kawazu, S. The Role of Insulin in Coronary Atherosclerosis. Diabetes Res. Clin. Pract. 1994, 22, 117–122. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, R.E.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2012, 9, 25–53. [Google Scholar] [CrossRef]
- Steiner, D.F.; Park, S.Y.; Støy, J.; Philipson, L.H.; Bell, G.I. A Brief Perspective on Insulin Production. Diabetes Obes. Metab. 2009, 11, 189–196. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, D.L.; Choi, K.M.; Baik, S.H.; Choi, D.S. Serum Insulin, Proinsulin and Proinsulin/Insulin Ratio in Type 2 Diabetic Patients: As an Index of Beta-Cell Function or Insulin Resistance. Korean J. Intern. Med. 2000, 15, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, K.I.; Torjesen, P.A.; Eriksson, J.; Vaaler, S.; Groop, L. Hyperproinsulinemia of Type II Diabetes Is Not Present Before the Development of Hyperglycemia. Diabetes Care 1994, 17, 1307–1310. [Google Scholar] [CrossRef]
- Pfützner, A.; Kann, P.H.; Pfützner, A.H.; Kunt, T.; Larbig, M.; Weber, M.M.; Forst, T. Intact and Total Proinsulin: New Aspects for Diagnosis and Treatment of Type 2 Diabetes Mellitus and Insulin Resistance. Clin. Lab. 2004, 50, 567–573. [Google Scholar] [PubMed]
- Patel, N.; Taveira, T.H.; Choudhary, G.; Whitlatch, H.; Wu, W.C. Fasting Serum C-Peptide Levels Predict Cardiovascular and Overall Death in Nondiabetic Adults. J. Am. Heart Assoc. 2012, 1, e003152. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Tronier, B.; Bülow, J.B. Kinetics of Circulating Endogenous Insulin, C-Peptide, and Proinsulin in Fasting Nondiabetic Man. Metabolism 1987, 36, 463–468. [Google Scholar] [CrossRef]
- Katz, A.I.; Rubenstein, A.H. Metabolism of Proinsulin, Insulin, and C-Peptide in the Rat. J. Clin. Investig. 1973, 52, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Sokooti, S.; Kieneker, L.M.; de Borst, M.H.; Kobold, A.M.; Kootstra-Ros, J.E.; Gloerich, J.; van Gool, A.J.; Heerspink, H.J.L.; Gansevoort, R.T.; Dullaart, R.P.F.; et al. Plasma C-Peptide and Risk of Developing Type 2 Diabetes in the General Population. J. Clin. Med. 2020, 9, 3001. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; Brantsma, A.H.; de Zeeuw, D.; Bakker, S.J.L.; de Jong, P.E.; Gansevoort, R.T. Albuminuria Assessed from First-Morning-Void Urine Samples versus 24-Hour Urine Collections as a Predictor of Cardiovascular Morbidity and Mortality. Am. J. Epidemiol. 2008, 168, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.B.; Struck, J.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Plasma Procalcitonin and Risk of Type 2 Diabetes in the General Population. Diabetologia 2011, 54, 2463–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruppen, E.G.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum Paraoxonase-1 Activity Is Associated with Light to Moderate Alcohol Consumption: The PREVEND Cohort Study. Am. J. Clin. Nutr. 2018, 108, 1283–1290. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; Kootstra-Ros, J.E.; Blokzijl, H.; Gansevoort, R.T.; Dullaart, R.P.F. Inverse Linear Associations between Liver Aminotransferases and Incident Cardiovascular Disease Risk: The PREVEND Study. Atherosclerosis 2015, 243, 138–147. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W.F.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B. Prediction of Incident Diabetes Mellitus in Middle-Aged Adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- Pencina, M.J.; Steyerberg, E.W.; D’Agostino, R.B., Sr. Extensions of Net Reclassification Improvement Calculations to Measure Usefulness of New Biomarker. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef]
- Kahn, S.E.; Leonetti, D.L.; Prigeon, R.L.; Boyko, E.J.; Bergstrom, R.W.; Fujimoto, W.Y. Relationship of Proinsulin and Insulin with Noninsulin-Dependent Diabetes Mellitus and Coronary Heart Disease in Japanese-American Men: Impact of Obesity—Clinical Research Center Study. J. Clin. Endocrinol. Metab. 1995, 80, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Wareham, N.J.; Byrne, C.D.; Williams, R.; Day, N.E.; Hales, C.N. Fasting Proinsulin Concentrations Predict the Development of Type 2 Diabetes. Diabetes Care 1999, 22, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.J.G.; D’Agostino, R.; Wagenknecht, L.E.; Saad, M.F.; Savage, P.J.; Bergman, R.; Haffner, S.M. Increased Proinsulin Levels and Decreased Acute Insulin Response Independently Predict the Incidence of Type 2 Diabetes in the Insulin Resistance Atherosclerosis Study. Diabetes 2002, 51, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Buring, J.E.; Liu, S.; Ridker, P.M. Insulin, Proinsulin, Proinsulin: Insulin Ratio, and the Risk of Developing Type 2 Diabetes Mellitus in Women. Am. J. Med. 2003, 114, 438–444. [Google Scholar] [CrossRef]
- Haffner, S.M.; Mykkänen, L.; Valdez, R.A.; Stern, M.P.; Holloway, D.L.; Monterrosa, A.; Bowsher, R.R. Disproportionately Increased Proinsulin Levels Are Associated with the Insulin Resistance Syndrome. J. Clin. Endocrinol Metab. 1994, 79, 1806–1810. [Google Scholar]
- Vangipurapu, J.; Áková, A.S.; Kuulasmaa, T.; Kuusisto, J.; Laakso, M. Both Fasting and Glucose-Stimulated Proinsulin Levels Predict Hyperglycemia and Incident Type 2 Diabetes: A Population-Based Study of 9396 Finnish Men. PLoS ONE 2015, 10, e0124028. [Google Scholar] [CrossRef]
- Martin, B.C.; Warram, J.H.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R.; Martin, B.C.; Bergman, R.N. Role of Glucose and Insulin Resistance in Development of Type 2 Diabetes Mellitus: Results of a 25-Year Follow-up Study. Lancet 1992, 340, 925–929. [Google Scholar] [CrossRef]
- Pfützner, A.; Kunt, T.; Hohberg, C.; Mondok, A.; Pahler, S.; Konrad, T.; Lübben, G.; Forst, T. Fasting Intact Proinsulin Is a Highly Specific Predictor of Insulin Resistance in Type 2 Diabetes. Diabetes Care 2004, 27, 682–687. [Google Scholar] [CrossRef] [Green Version]
- Pfützner, A.; Hermanns, I.; Ramljak, S.; Demircik, F.; Pfützner, A.H.; Kann, P.H.; Weber, M.M. Elevated Intact Proinsulin Levels during an Oral Glucose Challenge Indicate Progressive SS-Cell Dysfunction and May Be Predictive for Development of Type 2 Diabetes. J. Diabetes Sci. Technol. 2015, 9, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Pfützner, A.; Pfützner, A.H.; Larbig, M.; Forst, T. Role of Intact Proinsulin in Diagnosis and Treatment of Type 2 Diabetes Mellitus. Diabetes Technol. Ther. 2004, 6, 405–411. [Google Scholar] [CrossRef]
- Martínez, M.S.; Manzano, A.; Olivar, L.C.; Nava, M.; Salazar, J.; D’Marco, L.; Ortiz, R.; Chacín, M.; Guerrero-Wyss, M.; Cabrera de Bravo, M.; et al. The Role of the α Cell in the Pathogenesis of Diabetes: A World beyond the Mirror. Int. J. Mol. Sci. 2021, 22, 9504. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, A.; Pfützner, A.H.; Kann, P.H.; Stute, R.; Löbig, M.; Yang, J.W.; Mistry, J.; Forst, T. Clinical and Laboratory Evaluation of a New Specific ELISA for Intact Proinsulin. Clin. Lab. 2005, 51, 243–249. [Google Scholar] [PubMed]
- Pfützner, A.; Forst, T. Elevated Intact Proinsulin Levels Are Indicative of Beta-Cell Dysfunction, Insulin Resistance, and Cardiovascular Risk: Impact of the Antidiabetic Agent Pioglitazone. J. Diabetes Sci. Technol. 2011, 5, 784–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-D.; Kang, S.J.; Lee, M.K.; Park, S.E.; Rhee, E.J.; Park, C.-Y.; Oh, K.-W.; Park, S.-W.; Lee, W.-Y. C-Peptide-Based Index Is More Related to Incident Type 2 Diabetes in Non-Diabetic Subjects than Insulin-Based Index. Endocrinol. Metab. 2016, 31, 320. [Google Scholar] [CrossRef] [Green Version]
- Haffner, S.M.; Goldberg, R.; Ratner, R.; Lachin, J.; Temprosa, M.; Orchard, T.; Molitch, M.; Saad, M. Hypertension, Insulin, and Proinsulin in Participants with Impaired Glucose Tolerance. Hypertension 2002, 40, 679–686. [Google Scholar]
- DeFronzo, R.A.; Goldberg, M.; Agus, Z.S. The Effects of Glucose and Insulin on Renal Electrolyte Transport. J. Clin. Investig. 1976, 58, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Rubenstein, A.H.; Pottenger, L.A.; Mako, M.; Getz, G.S.; Steiner, D.F. The Metabolism of Proinsulin and Insulin by the Liver. J. Clin. Investig. 1972, 51, 912–921. [Google Scholar] [CrossRef]
- Zelle, D.M.; Corpeleijn, E.; Deinum, J.; Stolk, R.P.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Pancreatic β-Cell Dysfunction and Risk of New-Onset Diabetes after Kidney Transplantation. Diabetes Care 2013, 36, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Chisalita, S.I.; Dahlström, U.; Arnqvist, H.J.; Alehagen, U. Proinsulin and IGFBP-1 Predicts Mortality in an Elderly Population. Int. J. Cardiol. 2014, 174, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Alssema, M.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J. Proinsulin Concentration Is an Independent Predictor of All-Cause and Cardiovascular Mortality: An 11-Year Follow-up of the Hoorn Study. Diabetes Care 2005, 28, 860–865. [Google Scholar] [CrossRef] [Green Version]
| Quintiles | 1,2,3 | 4 | 5 | p-Value for Trend * | |
|---|---|---|---|---|---|
| Proinsulin (pmol/L) | <7.78 | 7.78–10.37 | >10.37 | ||
| Participants, N | 3001 | 1000 | 1000 | ||
| Female (%) | 58.0 | 43.6 | 35.5 | <0.001 | |
| Age | 49.9 ± 10.8 | 54.4 ± 11.5 | 57.6 ± 11.3 | <0.001 | |
| Race, white (%) | 99.2 | 99.0 | 99.6 | 0.329 | |
| Family history of diabetes (%) | 15.5 | 18.3 | 22.1 | <0.001 | |
| Smoking status, | 0.004 | ||||
| Never (%) | 31.0 | 29.5 | 26.2 | ||
| Current (%) | 29.4 | 25.0 | 23.6 | ||
| Former (%) | 39.0 | 45.5 | 50.0 | ||
| Alcohol consumption, | <0.001 | ||||
| None (%) | 21.7 | 23.8 | 28.4 | ||
| 1–4 units per month (%) | 16.5 | 17.9 | 17.5 | ||
| 2–7 units per week (%) | 34.6 | 30.9 | 28.9 | ||
| 1–3 units per day (%) | 23.3 | 22.7 | 21.2 | ||
| >3 units per day (%) | 4.0 | 4.6 | 3.9 | ||
| Gestational diabetes (%) | 1.0 | 2.3 | 2.8 | 0.002 | |
| Length (cm) | 172 ± 8 | 173 ± 9 | 173 ± 8 | 0.002 | |
| Weight (kg) | 75.4 ± 12.4 | 81.4 ± 13.0 | 88.9 ± 15.3 | <0.001 | |
| BMI (kg/m2) | 25.2 ± 3.6 | 27.1 ± 3.8 | 29.4 ± 4.5 | <0.001 | |
| Systolic blood pressure (mmHg) | 121.0 ± 16.5 | 128.1 ± 17.7 | 133.5 ± 19.0 | <0.001 | |
| Diastolic blood pressure (mmHg) | 71.4 ± 8.7 | 74.7 ± 8.8 | 76.5 ± 8.5 | <0.001 | |
| Use of antihypertension medication (%) | 9.6 | 18.3 | 28.2 | <0.001 | |
| Hypertension (%) | 18.9 | 33.1 | 46.4 | <0.001 | |
| Cholesterol (mmol/L) | 5.3 ± 1.0 | 5.6 ± 0.9 | 5.5 ± 1 | <0.001 | |
| HDL (mmol/L) | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 10.3 | <0.001 | |
| Triglycerides (mmol/L) | 0.9 (0.7–1.3) | 1.2 (0.8–1.7) | 1.5 (1.1–2.1) | <0.001 | |
| Use of lipid-lowering medication (%) | 4.9 | 9.2 | 15.3 | <0.001 | |
| Glucose (mmol/L) | 4.7 ± 0.5 | 4.9 ± 0.6 | 5.2 ± 0.7 | <0.001 | |
| Insulin (mU/L) | 6.7 (5.0–9.1) | 9.1 (6.7–12.2) | 13.2 (9.2–19.3) | <0.001 | |
| C-peptide (pmol/L) | 624 (514–771) | 789 (654–984) | 1078 (861–1353) | <0.001 | |
| HOMA-IR ((mU mmol/L)/22.5) | 1.4 (1.0–1.9) | 1.9 (1.4–2.7) | 3.0 (2.0–4.5) | <0.001 | |
| Serum creatinine (μmol/L) | 70.2 ± 12.8 | 73.6 ± 14.0 | 78.5 ± 30.7 | <0.001 | |
| Plasma albumin (g/L) | 43.9 ± 5.5 | 43.8 ± 2.6 | 43.9 ± 2.6 | 0.22 | |
| eGFR (mL/min/1.73 m2) | 97.5 (86.9–106.9) | 94.1 (81.6–104.4) | 87.7 (75.6–98.9) | <0.001 | |
| UAE (mg/24 h) | 7.7 (5.8–11.8) | 9.4 (6.3–17.3) | 11.7 (7.1–25.4) | <0.001 |
| Quintiles of Plasma Proinsulin, pmol/L | Proinsulin Per 1 SD Increase | |||||
|---|---|---|---|---|---|---|
| p-Value | ||||||
| 1,2,3 | 4 | 5 | ||||
| Cases | 66 | 49 | 156 | 271 | ||
| Crude analysis | 1.00 (ref) | 2.24 (1.55–3.25) | 8.10 (6.07–10.81) | 2.66 (2.41–2.93) | <0.001 | |
| Model 1 | 1.00 (ref) | 1.99 (1.37–2.90) | 6.69 (4.94–9.06) | 2.55 (2.29–2.84) | <0.001 | |
| Model 2 | 1.00 (ref) | 1.99 (1.37–2.90) | 6.62 (4.89–8.97) | 2.53 (2.27–2.81) | <0.001 | |
| Model 3 | 1.00 (ref) | 1.56 (1.07–2.27) | 3.84 (2.79–5.29) | 2.05 (1.81–2.32) | <0.001 | |
| Model 4 | 1.00 (ref) | 1.44 (0.98–2.09) | 3.14 (2.27–4.35) | 1.88 (1.65–2.15) | <0.001 | |
| Model 5 | 1.00 (ref) | 1.41 (0.96–2.08) | 3.18 (2.28–4.44) | 1.90 (1.66–2.18) | <0.001 | |
| Model 6 | 1.00 (ref) | 1.08 (0.73–1.60) | 1.90 (1.34–2.68) | 1.37 (1.18–1.59) | <0.001 | |
| Model 7 | 1.00 (ref) | 1.06 (0.71–1.57) | 1.84 (1.27–2.65) | 1.37 (1.16–1.61) | <0.001 | |
| Model 8 | 1.00 (ref) | 1.05 (0.70–1.56) | 1.51 (1.02–2.23) | 1.28 (1.08–1.52) | 0.005 | |
| C-Statistics | p-Value for Change in C-Statistics | IDI | p-Value | |
|---|---|---|---|---|
| FOS risk model | 0.886 (0.867–0.906) | - | - | - |
| + Proinsulin | 0.888 (0.869–0.907) | 0.019 | 0.0069 | 0.004 |
| + C-peptide | 0.888 (0.870–0.908) | 0.018 | 0.0056 | 0.02 |
| + Insulin | 0.887 (0.867–0.906) | 0.241 | 0.0020 | 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokooti, S.; Dam, W.A.; Szili-Torok, T.; Gloerich, J.; van Gool, A.J.; Post, A.; de Borst, M.H.; Gansevoort, R.T.; Heerspink, H.J.L.; Dullaart, R.P.F.; et al. Fasting Proinsulin Independently Predicts Incident Type 2 Diabetes in the General Population. J. Pers. Med. 2022, 12, 1131. https://doi.org/10.3390/jpm12071131
Sokooti S, Dam WA, Szili-Torok T, Gloerich J, van Gool AJ, Post A, de Borst MH, Gansevoort RT, Heerspink HJL, Dullaart RPF, et al. Fasting Proinsulin Independently Predicts Incident Type 2 Diabetes in the General Population. Journal of Personalized Medicine. 2022; 12(7):1131. https://doi.org/10.3390/jpm12071131
Chicago/Turabian StyleSokooti, Sara, Wendy A. Dam, Tamas Szili-Torok, Jolein Gloerich, Alain J. van Gool, Adrian Post, Martin H. de Borst, Ron T. Gansevoort, Hiddo J. L. Heerspink, Robin P. F. Dullaart, and et al. 2022. "Fasting Proinsulin Independently Predicts Incident Type 2 Diabetes in the General Population" Journal of Personalized Medicine 12, no. 7: 1131. https://doi.org/10.3390/jpm12071131
APA StyleSokooti, S., Dam, W. A., Szili-Torok, T., Gloerich, J., van Gool, A. J., Post, A., de Borst, M. H., Gansevoort, R. T., Heerspink, H. J. L., Dullaart, R. P. F., & Bakker, S. J. L. (2022). Fasting Proinsulin Independently Predicts Incident Type 2 Diabetes in the General Population. Journal of Personalized Medicine, 12(7), 1131. https://doi.org/10.3390/jpm12071131






