Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model
Abstract
:1. Introduction
2. Operant Conditioning
3. Motivational Factors and Setting Events
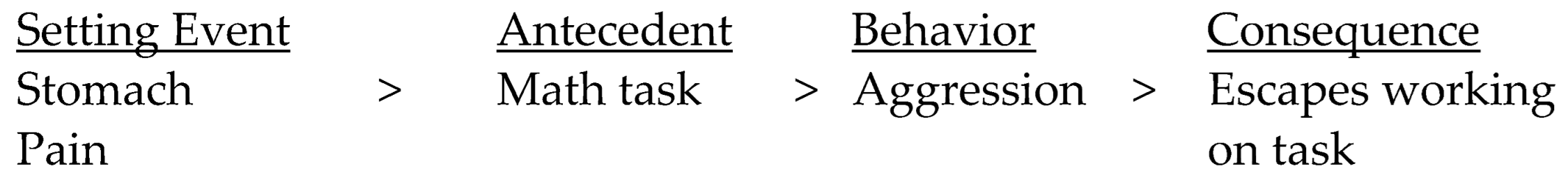
4. Interoception
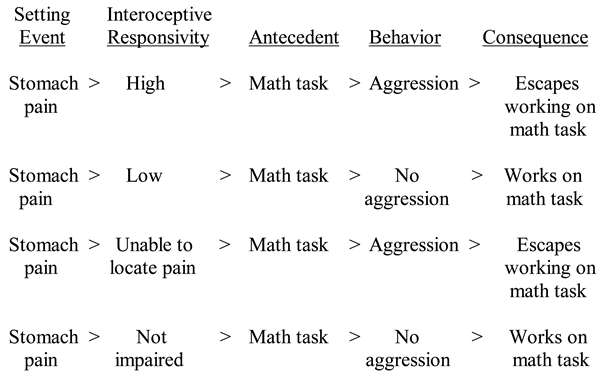
5. Interoception and Anxiety
6. Assessing Individuals with Challenging Behaviors
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampton, L.H.; Roberts, M.Y.; Anderson, E.; Hobson, A.N.; Kaat, A.J.; Bishop, S.L.; Krogh-Jespersen, S.; Wakschlag, L.S.; Bevans, K.B. Brief report: What diagnostic observation can teach us about disruptive behavior in young children with autism. J. Dev. Behav. Pediatr. 2021, 42, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mathesis, M.; Estabillo, J.A.; Matson, J.L. Managing challenging behavior in adolescents with autism spectrum disorder. In Adolescents with Autism Spectrum Disorder: A Clinical Handbook; Gelbar, N.W., Ed.; Oxford University Press: Oxford, UK, 2017; pp. 242–274. [Google Scholar]
- Edelson, S.M. Comparison of autistic individuals who engage in self-injurious behavior, aggression, and both behaviors. Pediatr. Rep. 2021, 13, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Durand, V.M. Severe Behavior Problems: A Functional Communication Training Approach; The Guilford Press: New York, NY, USA, 1990. [Google Scholar]
- Fitzpatrick, S.E.; Srivorakiat, L.; Wink, L.K.; Pedapati, E.V.; Erickson, C.A. Aggression in autism spectrum disorder: Presentation and treatment options. Neuropsychiatr. Dis. Treat. 2016, 12, 1525–1538. [Google Scholar] [PubMed] [Green Version]
- Ward-Hawkes, J.; Rodi, M. Managing Meltdowns and Tantrums on the Autism Spectrum: A Parent and Caregiver’s Guide; Jessica Kingsley Publishers: London, UK, 2019. [Google Scholar]
- Barrett, R.P. Atypical behavior: Self-injury and pica. In Developmental-Behavioral Pediatrics: Evidence and Practice; Wolraich, M.L., Drotar, D.D., Dworkin, P.H., Perrin, E.C., Eds.; C.V. Mosby Co.: St. Louis, MO, USA, 2008; pp. 871–885. [Google Scholar]
- Laverty, C.; Oliver, C.; Moss, J.; Nelson, L.; Richards, C. Persistence and predictors of self-injurious behavior in autism: A ten-year prospective cohort study. Mol. Autism 2020, 11, 8. [Google Scholar]
- Edelson, S.M. Overview of various treatment approaches and their impact on several difficult-to-treat conditions. Autism Dev. Disord. 2020, 18, 38–45. [Google Scholar] [CrossRef]
- Edelson, S.M.; Johnson, J.B. (Eds.) Understanding and Treating Self-Injurious Behavior in Autism; Jessica Kingsley Publishers: London, UK, 2016. [Google Scholar]
- Moskowitz, L.J.; Walsh, C.E.; Durand, V.M. Assessment and intervention for self-injurious behavior using positive behavior support. In Understanding and Treating Self-Injurious Behavior in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2016; pp. 151–185. [Google Scholar]
- Carr, E.G. The motivation of self-injurious behavior: A review of some hypotheses. Psychol. Bull. 1977, 84, 800–816. [Google Scholar] [CrossRef]
- Lovaas, O.I.; Freitag, G.; Gold, V.; Kassorla, I. Experimental studies in childhood schizophrenia: Analysis of self-destructive behavior. J. Exp. Child Psychol. 1965, 2, 67–84. [Google Scholar] [CrossRef]
- Moskowitz, L.J.; Edelson, S.M. Introduction. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 12–20. [Google Scholar]
- Carr, E.G.; Newsom, C.D.; Binkoff, J.A. Stimulus control of self-destructive behavior in a psychotic child. J. Abnorm. Child Psychol. 1976, 4, 139–153. [Google Scholar] [CrossRef]
- Edelson, S.M.; Taubman, M.T.; Lovaas, O.I. Some social contexts to self-destructive behavior. J. Abnorm. Child Psychol. 1983, 11, 299–311. [Google Scholar] [CrossRef]
- Carr, E.G.; McDowell, J.J. Social control of self-injurious behavior of organic etiology. Behav. Ther. 1980, 11, 402–409. [Google Scholar] [CrossRef]
- Lovaas, O.I.; Simmons, J.Q. Manipulation of self-destruction in three retarded children. J. Appl. Behav. Anal. 1969, 2, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winton, A.S.W.; Singh, N.N.; Dawson, M.J. Effects of facial screening and blindfold on self-injurious behavior. Appl. Res. Ment. Retard. 1984, 5, 29–42. [Google Scholar] [PubMed]
- Linscheid, T.R.; Iwata, B.A.; Ricketts, R.W.; Williams, D.E.; Griffin, J.C. Clinical evaluation of the self-injurious behavior inhibiting system (SIBIS). J. Appl. Behav. Anal. 1990, 23, 53–78. [Google Scholar] [PubMed] [Green Version]
- Baumeister, A.A.; Baumeister, A.A. Suppression of repetitive self-injurious behavior by contingent inhalation of aromatic ammonia. J. Autism Child. Schizophr. 1978, 8, 71–77. [Google Scholar] [CrossRef] [PubMed]
- De Lissovoy, V. Head banging in early childhood. Child Dev. 1962, 33, 43–56. [Google Scholar] [CrossRef]
- Baumeister, A.A.; Forehand, R. Stereotyped acts. In International Review of Research in Mental Retardation; Ellis, N.R., Ed.; Academic Press: New York, NY, USA, 1973; Volume 6, pp. 55–96. [Google Scholar]
- Edelson, S.M. Implications of sensory stimulation in self-destructive behavior. Am. J. Ment. Defic. 1984, 89, 140–145. [Google Scholar]
- Sandman, C.A.; Touchette, P. Opioids and the maintenance of self-injurious behavior. In Self-Injurious Behavior: Gene-Brain-Behavior Relationships; Schroeder, S.R., Oster-Granite, M.L., Thompson, T., Eds.; American Psychological Association: Washington, DC, USA, 2002; pp. 191–204. [Google Scholar]
- Bijou, S.W.; Baer, D.M. Child Development I: Systematic and Empirical Theory; Prentice-Hall: Englewood Cliffs, NJ, USA, 1961. [Google Scholar]
- Carr, E.G.; Dunlap, G.; Horner, R.H.; Koegel, R.L.; Turnbull, A.P.; Sailor, W.; Anderson, J.L.; Albin, R.W.; Koegel, L.K.; Fox, L. Positive behavior support: Evolution of an applied science. J. Posit. Behav. Interv. 2002, 4, 4–16. [Google Scholar]
- Lekkas, C.N.; Lentino, W. Symptom-producing interposition of the colon: Clinical syndrome in mentally deficient adults. JAMA 1978, 240, 747–750. [Google Scholar] [CrossRef]
- Smith, C.E.; Carr, E.G.; Moskowitz, L.J. Fatigue as a biological setting event for severe problem behavior in autism spectrum disorder. Res. Autism Spectr. Disord. 2016, 23, 131–144. [Google Scholar] [CrossRef]
- Carr, E.G.; Smith, C.E.; Giacin, T.A.; Whelan, B.M.; Pancari, J. Menstrual discomfort as a biological setting event for severe problem behavior: Assessment and intervention. Am. J. Ment. Retard. 2003, 108, 117–133. [Google Scholar]
- O’Reilly, M.F. Functional analysis of episodic self-injury correlated with recurrent otitis media. J. Appl. Behav. Anal. 1997, 30, 165–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelson, S.M.; Johnson, J.B. (Eds.) Understanding and Treating Anxiety in Autism; Jessica Kingsley Publishers: London, UK, 2021. [Google Scholar]
- Edelson, S.M.; Johnson, J.B. (Eds.) Understanding and Treating Sleep Disturbances in Autism; Jessica Kingsley Publishers: London, UK, 2022. [Google Scholar]
- Neumeyer, A.M.; Anixt, J.; Chan, J.; Perrin, J.M.; Murray, D.; Coury, D.L.; Bennett, A.; Farmer, J.; Parker, R.A. Identifying associations among co-occurring medical conditions in children with autism spectrum disorders. Acad. Pediatr. 2019, 19, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Soke, G.N.; Maenner, M.J.; Christensen, D.; Kurzius-Spencer, M.; Schieve, L.A. Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J. Autism Dev. Disord. 2018, 48, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Carpenter, K.L.; Major, S.; Deaver, M.; Vermeer, S.; Herold, B.; Franz, L.; Howard, J.; Dawson, G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 2020, 25, 405–415. [Google Scholar] [CrossRef]
- Restrepo, B.; Angkustsiri, K.; Taylor, S.L.; Rogers, S.J.; Cabral, J.; Heath, B.; Hechtman, A.; Solomon, M.; Ashwood, P.; Amaral, D.G.; et al. Developmental-behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Res. 2020, 13, 1778–1789. [Google Scholar] [CrossRef]
- Schwichtenberg, A.J.; Young, G.S.; Hutman, T.; Iosif, A.M.; Sigman, M.; Rogers, S.J.; Ozonoff, S. Behavior and sleep problems in children with a family history of autism. Autism Res. 2013, 6, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Mazureka, M.O.; Kannea, S.M.; Wodkab, E.L. Physical aggression in children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2013, 7, 455–465. [Google Scholar] [CrossRef]
- Sherrington, C.S. The Integrative Action of the Nervous System; Yale University Press: New Haven, CT, USA, 1906. [Google Scholar]
- DuBois, D.; Ameis, S.H.; Lai, M.C.; Casanova, M.F.; Desarkar, P. Interoception in autism spectrum disorder: A review. Int. J. Neurosci. 2016, 52, 104–111. [Google Scholar] [CrossRef]
- Murakami, Y.; Sakai, S.; Takeda, K.; Sawamura, D.; Yoshida, K.; Hirose, T.; Ikeda, C.; Mani, H.; Yamamoto, T.; Ito, A. Autistic traits modulate the activity of the ventromedial prefrontal cortex in response to female faces. Neurosci. Res. 2018, 133, 28–37. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Tiley, C.; O’Keeffe, S.; Harrison, N.A.; Seth, A.K.; Critchley, H.D. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol. Psychol. 2016, 114, 117–126. [Google Scholar] [CrossRef]
- Yang, H.X.; Zhou, H.Y.; Li, Y.; Cui, Y.H.; Xiang, Y.; Yuan, R.M.; Lui, S.S.; Chan, R.C. Decreased interoceptive accuracy in children with autism spectrum disorder with co-morbid attention-deficit/hyperactivity disorder. Autism Res. 2022, 15, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Elwin, M.; Ek, L.; Schroder, A.; Kjellin, L. Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Arch. Psychiatr. Nurs. 2012, 26, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Fiene, L.; Brownlow, C. Investigating interoception and body awareness in adults with and without autism spectrum disorders. Autism Res. 2015, 8, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Schauder, K.B.; Mash, L.E.; Bryant, L.K.; Cascio, C.J. Interoceptive ability and body awareness in autism spectrum disorder. J. Exp. Child Psychol. 2014, 131, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahler, K. Interoception: The Eighth Sensory System; AAPC Publishing: Lenexa, KS, USA, 2017. [Google Scholar]
- Kolevzon, A.; Lim, T.; Schmeidler, J.; Martello, T.; Cook, E.H.; Silverman, J.M. Self-injury in autism spectrum disorder: An effect of serotonin transporter gene promoter variants. Psychiatry Res. 2014, 220, 987–990. [Google Scholar] [CrossRef]
- Casanova, M.F.; Casanova, E.L. Self-injurious behavior, aggression, and epilepsy in autism spectrum disorder. In Understanding and Treating Self-Injurious Behavior in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2016; pp. 38–54. [Google Scholar]
- Coleman, M. Clinical presentation of patients with autism and hypocalcinuria. Dev. Brain Dysfunct. 1994, 7, 63–70. [Google Scholar]
- van Steensel, F.J.; Bogel, S.M.; Perrin, S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clin. Child Fam. Psychol. Rev. 2011, 14, 302. [Google Scholar] [CrossRef] [Green Version]
- White, S.W.; Oswald, D.; Ollendick, T.; Scahill, L. Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Rev. 2009, 29, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Paulus, M.P.; Stein, M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Casanova, E.L.; Casanova, M.F.; Sokhadze, E.M.; Lamina, E. Crosstalk between the immune and autonomic nervous systems and their relationship to anxiety in autism. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 44–56. [Google Scholar]
- Barnhill, K.M. Dietary and nutrition intervention to address issues of anxiety. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 78–90. [Google Scholar]
- Hirtoa, T.; Brooks, J.; Hendren, R.L. Pharmacotherapy for anxiety in individuals with autism spectrum disorder. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 133–146. [Google Scholar]
- Edelson, S.M.; Van de Water, J.; Edelson, M.S.G. The immune system and anxiety: A case for toxic exposure. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 57–66. [Google Scholar]
- Cipani, E. Functional Behavioral Assessment, Diagnosis, and Treatment: A Complete System for Education and Mental Health Settings, 3rd ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Bauman, M.L. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Margolis, K.G.; Buie, T.M.; Turner, J.B.; Silberman, A.E.; Feldman, J.F.; Murray, K.F.; McSwiggan-Hardin, M.; Levy, J.; Bauman, M.L.; Veenstra-VanderWeele, J.; et al. Development of a brief parent-report screen for common gastrointestinal disorders in autism spectrum disorder. J. Autism Dev. Disord. 2019, 49, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Zeevenhooven, J.; Koppen, I.J.N.; Benninga, M.A. The new Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr. Gastroenterol. Hepatol. Nutr. 2017, 20, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., III; Furuta, G.T.; Levy, J.; VandeWater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A Consensus Report. Pediatrics 2010, 125 (Suppl. S1), S1–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buie, T.; Fuchs, G.J., III; Furuta, G.T.; Kooros, K.; Levy, J.; Lewis, J.D.; Wershil, B.K.; Winter, H. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics 2010, 125 (Suppl. S1), S19–S29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyonouchi, H. Self-injurious behaviors in children with autism spectrum disorder: Impact of allergic diseases. In Understanding and Treating Self-Injurious Behavior in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2016; pp. 91–108. [Google Scholar]
- Malow, B.A.; Crowe, C.; Henderson, L.; McGrew, S.G.; Wang, L.; Song, Y.; Stone, W.L. A sleep habits questionnaire for children with autism spectrum disorders. J. Child Neurol. 2009, 24, 19–24. [Google Scholar] [CrossRef]
- Sassower, K.C.; Bauman, M.B. The role of clinical polysomnography in the evaluation of sleep difficulties in patients on the autism spectrum. In Understanding and Treating Sleep Disturbances in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2022; pp. 37–50. [Google Scholar]
- Moskowitz, L.J.; Rosen, T.; Lerner, M.D.; Levine, K. Assessment of anxiety in youth with autism spectrum disorder. In Evidence Based Assessment and Treatment of Anxiety in Children and Adolescents with Autism Spectrum Disorder; Kerns, C., Storch, E., Kendall, P., Wood, J.J., Renno, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–104. [Google Scholar]
- Rosen, T.E.; Connell, J.E.; Kerns, C.M. A review of behavioral interventions for anxiety-related behaviors in lower-functioning individuals with autism. Behav. Interv. 2016, 31, 120–143. [Google Scholar] [CrossRef]
- Scahill, L.; Lecavalier, L.; Schultz, R.T.; Evans, A.N.; Maddox, B.; Pritchett, J.; Herrington, J.; Gillespie, S.; Miller, J.; Amoss, R.T.; et al. Development of the parent-rated anxiety scale for youth with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 887–896. [Google Scholar] [CrossRef]
- Sokhadze, E.M.; Casanova, E.L.; Lamina, E.V.; Kelly, D.; Casanova, M.F. Psychophysiological markers of arousal and anxiety in children with autism spectrum disorder. In Understanding and Treating Anxiety in Autism; Edelson, S.M., Johnson, J.B., Eds.; Jessica Kingsley Publishers: London, UK, 2021; pp. 21–43. [Google Scholar]
- Ferentzi, E.; Olaru, G.; Geiger, M.; Vig, L.; Köteles, F.; Wilhelm, O. Examining the factor structure and validity of the Multidimensional Assessment of Interoceptive Awareness. J. Personal. Assess. 2021, 103, 675–684. [Google Scholar] [CrossRef]
- Grabauskaitė, A.; Baranauskas, M.; Griškova-Bulanova, I. Interoception and gender: What aspects should we pay attention to? Conscious. Cogn. 2017, 48, 129–137. [Google Scholar] [CrossRef]
- Purcell, J.R.; Chen, J.; Moussa-Tooks, A.B.; Hetrick, W.P. Psychometric evaluation of the Pinocchio Illusion Questionnaire. Atten. Percept. Psychophys. 2020, 82, 2728–2737. [Google Scholar] [CrossRef]
- Goodall, E.; Brownlow, C. Interoception and Regulation; Jessica Kingsley Publishers: London, UK, 2022. [Google Scholar]
- Mahler, K.; Hample, K.; Jones, C.; Sensenig, J.; Thomasco, P.; Hilton, C. Impact of an interoception-based program on emotion regulation in autistic children. Occup. Ther. Int. 2022, 2022, 9328967. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Egloff, B.; Gerlach, A.L.; Witthoft, M. Improving heartbeat perception in patients with medically unexplained symptoms reduces symptom distress. Biol. Psychol. 2014, 101, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Edelson, S.M.; Natowicz, M.R. Challenging behaviors in adults with autism. Autism Res. Rev. Int. 2021, 35. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelson, S.M. Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model. J. Pers. Med. 2022, 12, 1127. https://doi.org/10.3390/jpm12071127
Edelson SM. Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model. Journal of Personalized Medicine. 2022; 12(7):1127. https://doi.org/10.3390/jpm12071127
Chicago/Turabian StyleEdelson, Stephen M. 2022. "Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model" Journal of Personalized Medicine 12, no. 7: 1127. https://doi.org/10.3390/jpm12071127
APA StyleEdelson, S. M. (2022). Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model. Journal of Personalized Medicine, 12(7), 1127. https://doi.org/10.3390/jpm12071127




