Silver Nanoparticles Selectively Treat Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors in a Neurofibromin-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Silver Nanoparticles
2.3. MTT Assays for Cytotoxicity and Viability
2.4. Lentivirus Production
2.5. Neurofibromin Knockdown
2.6. Restoration of Functional Neurofibromin Expression
2.7. Realtime Quantitative PCR
2.8. Fluorescently Labeled Cells
2.9. Other Reagents
2.10. Imaging Studies
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Silver Nanoparticles Are Selectively Cytotoxic to NF1-Associated MPNSTs
3.2. Intact Silver Nanoparticles Are Required for NF1-Associated MPNST Selectivity
3.3. Increased Oxidative Stress Sensitizes Normal Schwann and Sporadic MPNST Cells to Silver Nanoparticles
3.4. Restoration of Functional Neurofibromin Expression in NF1-Associated MPNST Increases Tolerance to Silver Nanoparticles
3.5. Knockdown of Neurofibromin Sensitizes Normal Schwann Cells, but Not Sporadic MPNSTs, to Silver Nanoparticles
3.6. Silver Nanoparticles Selectively Remove NF1-Associated MPNST Cells in Co-Culture with Normal Schwann Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, M.; Cooper, D.N. Neurofibromatosis Type 1: Molecular and Cellular Biology; Spring: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Widemann, B.C. Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr. Oncol. Rep. 2009, 11, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, M.; Holand, M.; Agesen, T.H.; Brekke, H.R.; Liestol, K.; Hall, K.S.; Mertens, F.; Picci, P.; Smeland, S.; Lothe, R.A. Survival meta-analyses for >1800 msalignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013, 15, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Le, L.Q.; Parada, L.F. Tumor microenvironment and neurofibromatosis type I: Connecting the GAPs. Oncogene 2007, 26, 4609–4616. [Google Scholar] [CrossRef] [Green Version]
- Scheffzek, K.; Welti, S. Neurofibromin: Protein Domains and Functional Characteristics. In Neurofibromatosis Type 1: Molecular and Cellular Biology; Upadhyaya, M., Cooper, D.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 305–326. [Google Scholar]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Goodsell, D.S. The molecular perspective: The ras oncogene. Oncologist 1999, 4, 263–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baines, A.T.; Xu, D.; Der, C.J. Inhibition of Ras for cancer treatment: The search continues. Future Med. Chem. 2011, 3, 1787–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brohl, A.S.; Kahen, E.; Yoder, S.J.; Teer, J.K.; Reed, D.R. The genomic landscape of malignant peripheral nerve sheath tumors: Diverse drivers of Ras pathway activation. Sci. Rep. 2017, 7, 14992. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.J.; Belzberg, A.J.; de Blank, P.; De Raedt, T.; Elefteriou, F.; Ferner, R.E.; Giovannini, M.; Harris, G.J.; Kalamarides, M.; Karajannis, M.A.; et al. 2016 Children’s Tumor Foundation conference on neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Am. J. Med. Genet. A 2018, 176, 1258–1269. [Google Scholar] [CrossRef]
- Ogrunc, M.; Di Micco, R.; Liontos, M.; Bombardelli, L.; Mione, M.; Fumagalli, M.; Gorgoulis, V.G.; d’Adda di Fagagna, F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014, 21, 998–1012. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K.; et al. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B Biointerfaces 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zhao, Y.X.; Guo, Q.Q.; Wang, Z.; Wang, H.Y.; Yang, Y.X.; Huang, Y.Z. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Naddaka, M.; Uboldi, C.; Loudos, G.; Fragogeorgi, E.; Molinari, V.; Pucci, A.; Tsotakos, T.; Psimadas, D.; Ponti, J.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Chockalingam, S.; Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Silver nanoparticles impregnated alginate-chitosan-blended nanocarrier induces apoptosis in human glioblastoma cells. Adv. Healthc. Mater. 2014, 3, 106–114. [Google Scholar] [CrossRef]
- Liu, P.D.; Huang, Z.H.; Chen, Z.W.; Xu, R.Z.; Wu, H.; Zang, F.C.; Wang, C.L.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef]
- Miura, N.; Shinohara, Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem. Biophys. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Y.; Zhang, Y.; Wang, Q.; Huang, Z.; Ding, Q.; Guo, Z.; Zhou, X.; Zhu, L.; Gu, N. The cellular uptake and cytotoxic effect of silver nanoparticles on chronic myeloid leukemia cells. J. Biomed. Nanotechnol. 2014, 10, 669–678. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J. Nanomater. 2017, 2017, 5107485. [Google Scholar] [CrossRef] [PubMed]
- Swanner, J.; Fahrenholtz, C.D.; Tenvooren, I.; Bernish, B.W.; Sears, J.J.; Hooker, A.; Furdui, C.M.; Alli, E.; Li, W.; Donati, G.L.; et al. Silver nanoparticles selectively treat triple-negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB Bioadv. 2019, 1, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Fahrenholtz, C.J. Nanoparticle Compositions for Cancer Treatment. Patent WO2018231790A1, 20 December 2018. [Google Scholar]
- Swanner, J.; Mims, J.; Carroll, D.L.; Akman, S.A.; Furdui, C.M.; Torti, S.V.; Singh, R.N. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int. J. Nanomed. 2015, 10, 3937–3953. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Garg, S.; Waite, T.D. H2O2-Mediated Oxidation of Zero-Valent Silver and Resultant Interactions among Silver Nanoparticles, Silver Ions, and Reactive Oxygen Species. Langmuir 2012, 28, 10266–10275. [Google Scholar] [CrossRef]
- Waker, C.A. Metabolic Characterization of MPNST Cell Lines; Wright State University: Dayton, OH, USA, 2015. [Google Scholar]
- Frahm, S.; Mautner, V.F.; Brems, H.; Legius, E.; Debiec-Rychter, M.; Friedrich, R.E.; Knofel, W.T.; Peiper, M.; Kluwe, L. Genetic and phenotypic characterization of tumor cells derived from malignant peripheral nerve sheath tumors of neurofibromatosis type 1 patients. Neurobiol. Dis. 2004, 16, 85–91. [Google Scholar] [CrossRef]
- Gu, Y.H.; Cui, X.W.; Ren, J.Y.; Long, M.M.; Wang, W.; Wei, C.J.; Aimaier, R.; Li, Y.H.; Chung, M.H.; Gu, B.; et al. Selection of internal references for RT-qPCR assays in Neurofibromatosis type 1 (NF1) related Schwann cell lines. PLoS ONE 2021, 16, e0241821. [Google Scholar] [CrossRef]
- Carroll, S.L. Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol. 2012, 123, 321–348. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, V.K.; Subramaniyan, S.A.; Hwang, I. Molecular and Cellular Response of Co-cultured Cells toward Cobalt Chloride (CoCl2)-Induced Hypoxia. ACS Omega 2019, 4, 20882–20893. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Coert, J.H.; Flucke, U.E.; Slooff, W.M.; van de Sande, M.A.J.; van Noesel, M.M.; Grunhagen, D.J.; Wijnen, M.; Verhoef, C. Neurofibromatosis-associated malignant peripheral nerve sheath tumors in children have a worse prognosis: A nationwide cohort study. Pediatr. Blood Cancer 2020, 67, e28138. [Google Scholar] [CrossRef] [Green Version]
- Snyder, C.M.; Rohde, M.M.; Fahrenholtz, C.D.; Swanner, J.; Sloop, J.; Donati, G.L.; Furdui, C.M.; Singh, R. Low Doses of Silver Nanoparticles Selectively Induce Lipid Peroxidation and Proteotoxic Stress in Mesenchymal Subtypes of Triple-Negative Breast Cancer. Cancers 2021, 13, 4217. [Google Scholar] [CrossRef]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef]
- Holmila, R.; Wu, H.; Lee, J.; Tsang, A.W.; Singh, R.; Furdui, C.M. Integrated Redox Proteomic Analysis Highlights New Mechanisms of Sensitivity to Silver Nanoparticles. Mol. Cell Proteom. 2021, 20, 100073. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, S.J.; Sohn, Y.B.; Jin, H.S.; Han, J.H.; Kim, Y.B.; Yim, H.; Jeong, S.Y. NF1 deficiency causes Bcl-xL upregulation in Schwann cells derived from neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Int. J. Oncol. 2013, 42, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Moore, W.; Fattah, F.; Jiang, X.; Zheng, J.; Kurian, P.; Beg, M.S.; Khan, S.A. Activity and pharmacology of homemade silver nanoparticles in refractory metastatic head and neck squamous cell cancer. Head Neck 2019, 41, E11–E16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, J.F.; Brosius, S.N.; Znoyko, I.; Alers, V.A.; Jenkins, D.P.; Wilson, R.C.; Carroll, A.J.; Wolff, D.J.; Roth, K.A.; Carroll, S.L. Establishment and genomic characterization of a sporadic malignant peripheral nerve sheath tumor cell line. Sci. Rep. 2021, 11, 5690. [Google Scholar] [CrossRef] [PubMed]
- Rad, E.; Dodd, K.; Thomas, L.; Upadhyaya, M.; Tee, A. STAT3 and HIF1alpha Signaling Drives Oncogenic Cellular Phenotypes in Malignant Peripheral Nerve Sheath Tumors. Mol. Cancer Res. 2015, 13, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Brundage, M.E.; Tandon, P.; Eaves, D.W.; Williams, J.P.; Miller, S.J.; Hennigan, R.H.; Jegga, A.; Cripe, T.P.; Ratner, N. MAF mediates crosstalk between Ras-MAPK and mTOR signaling in NF1. Oncogene 2014, 33, 5626–5636. [Google Scholar] [CrossRef] [Green Version]
- Chaney, K.E.; Perrino, M.R.; Kershner, L.J.; Patel, A.V.; Wu, J.; Choi, K.; Rizvi, T.A.; Dombi, E.; Szabo, S.; Largaespada, D.A.; et al. Cdkn2a Loss in a Model of Neurofibroma Demonstrates Stepwise Tumor Progression to Atypical Neurofibroma and MPNST. Cancer Res. 2020, 80, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
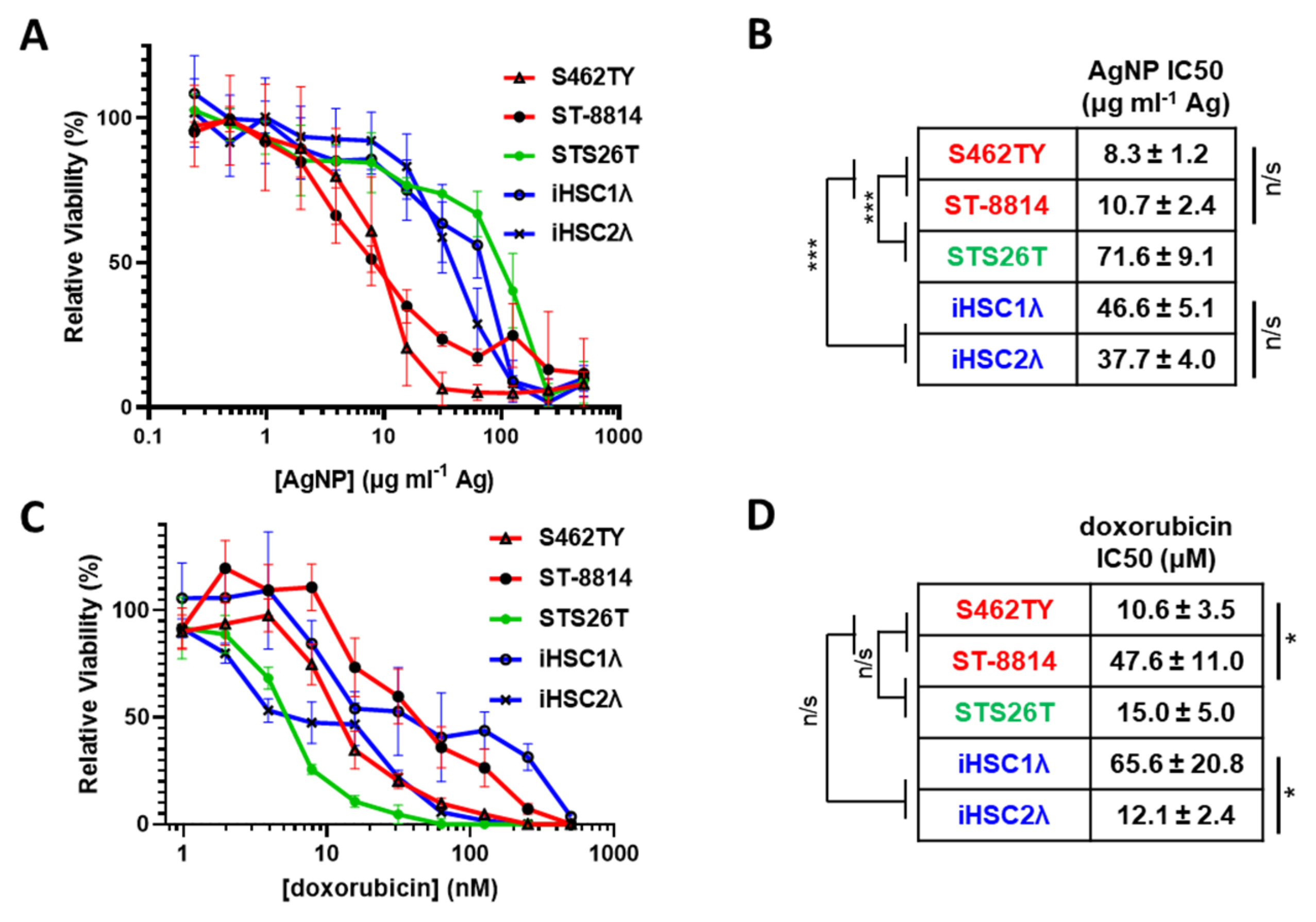
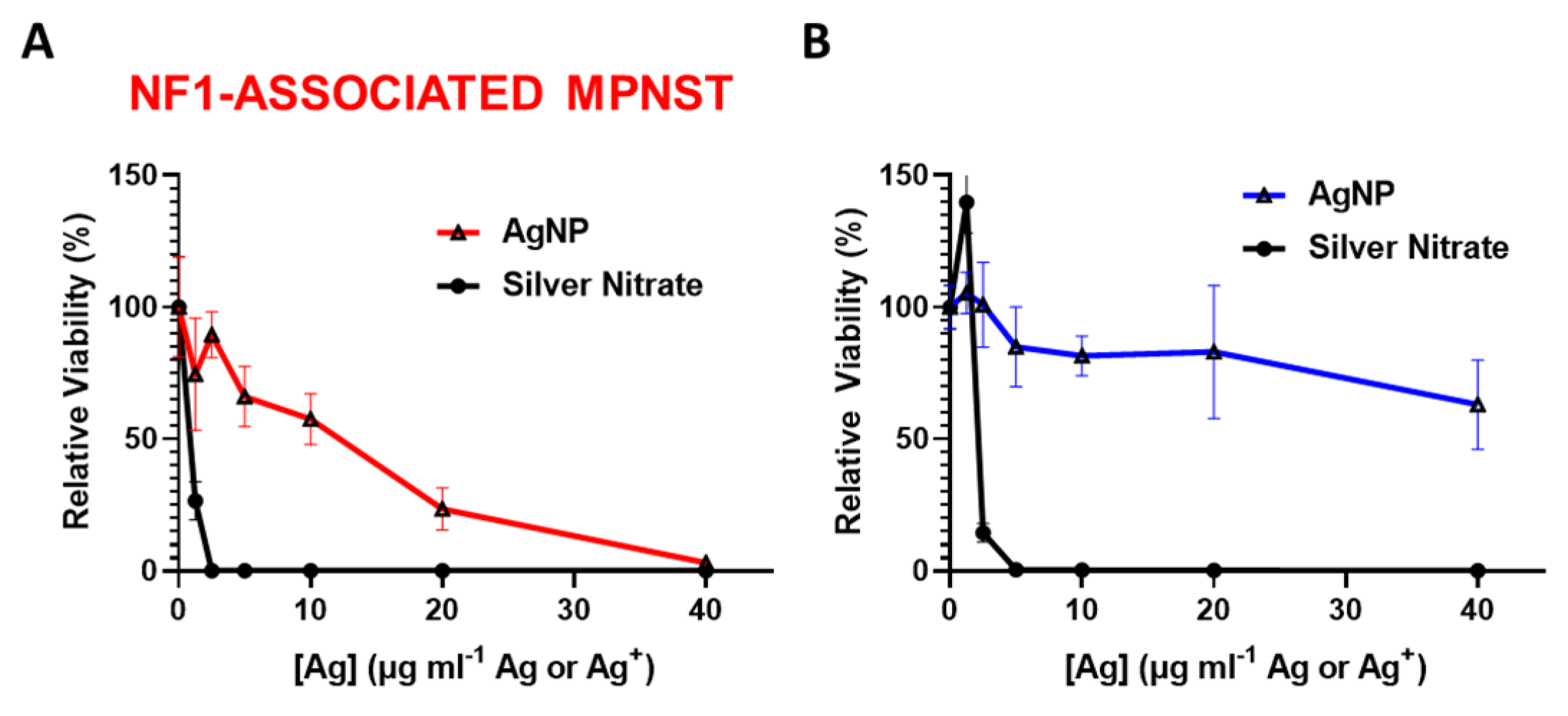
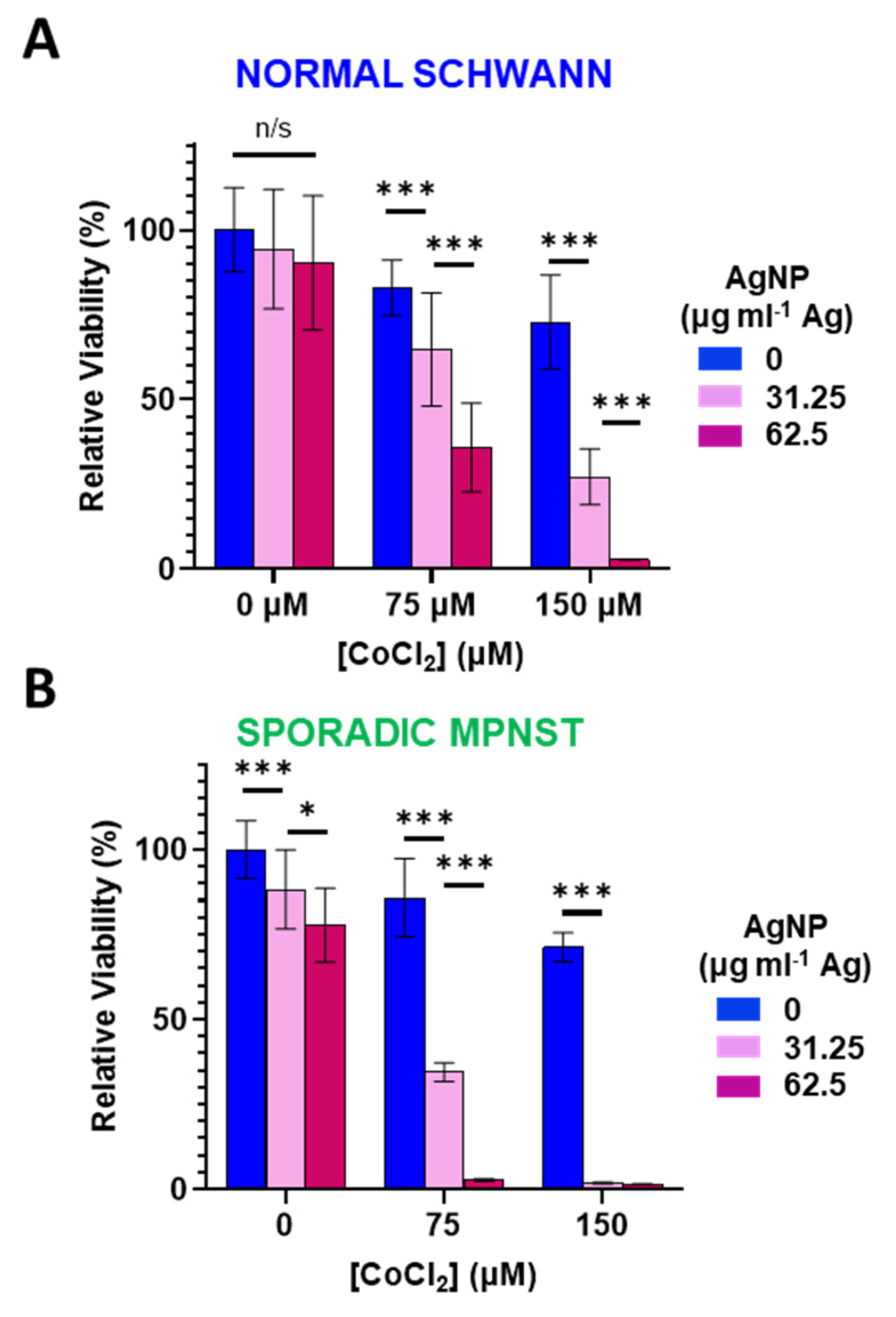
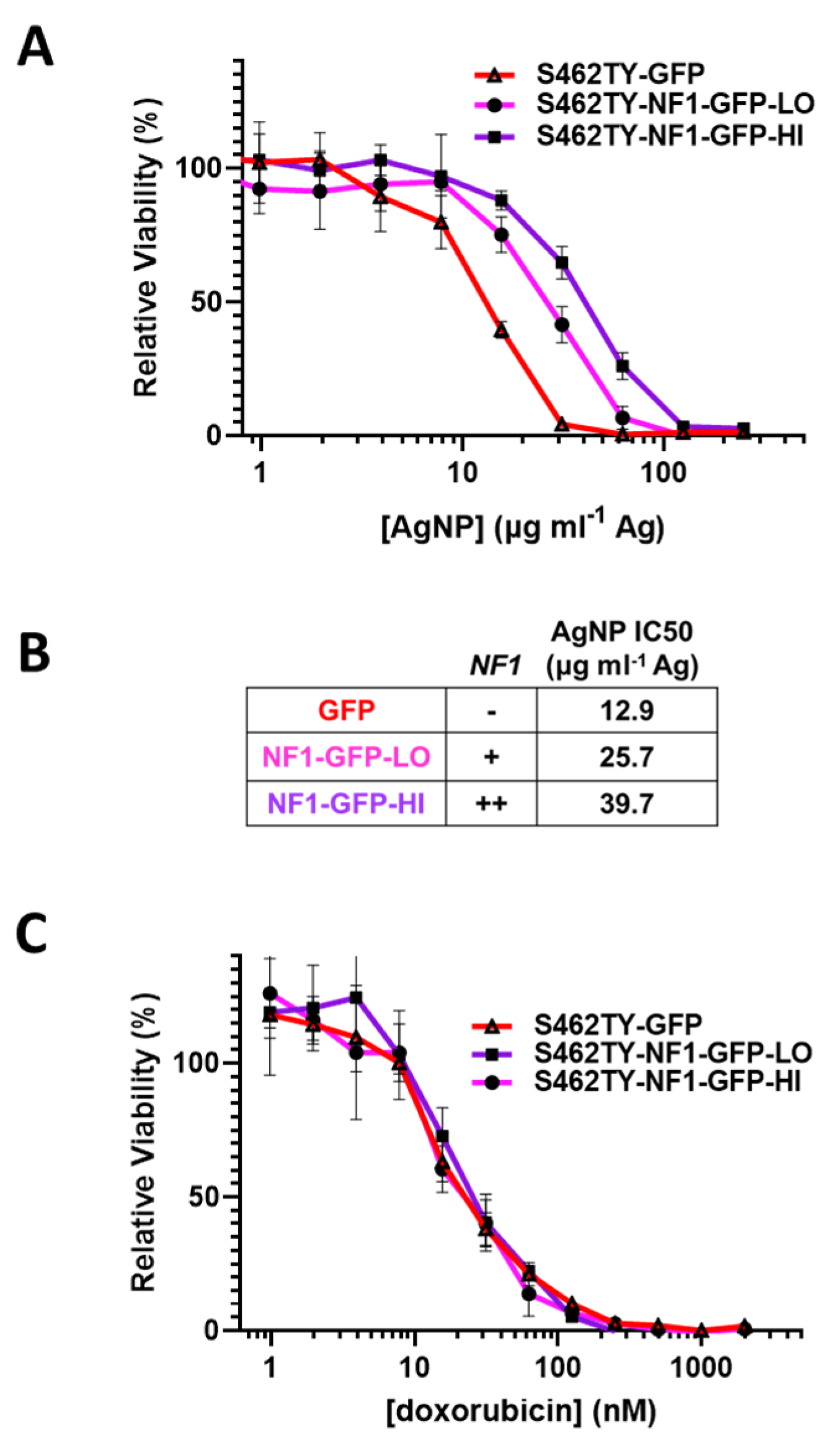
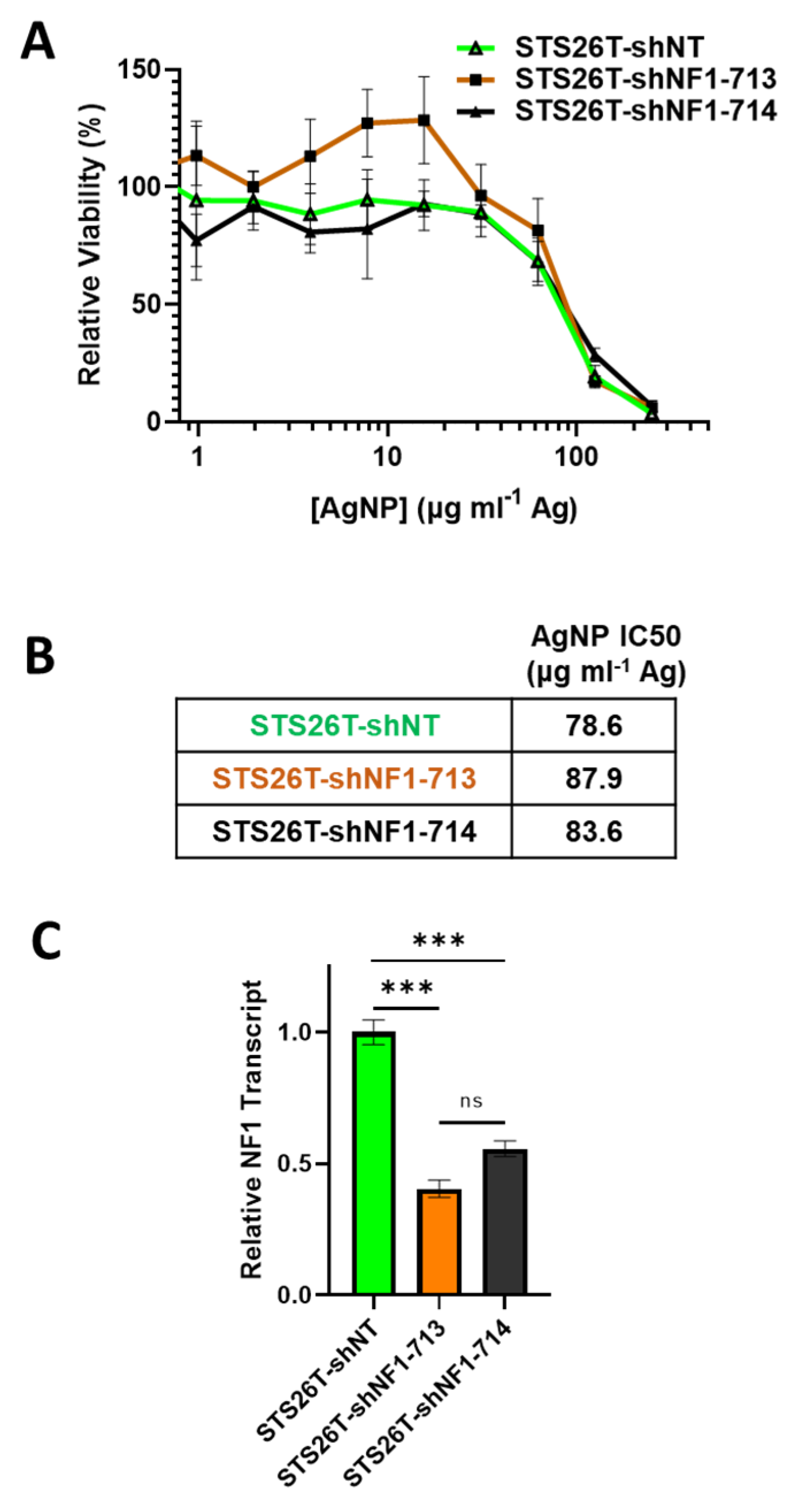
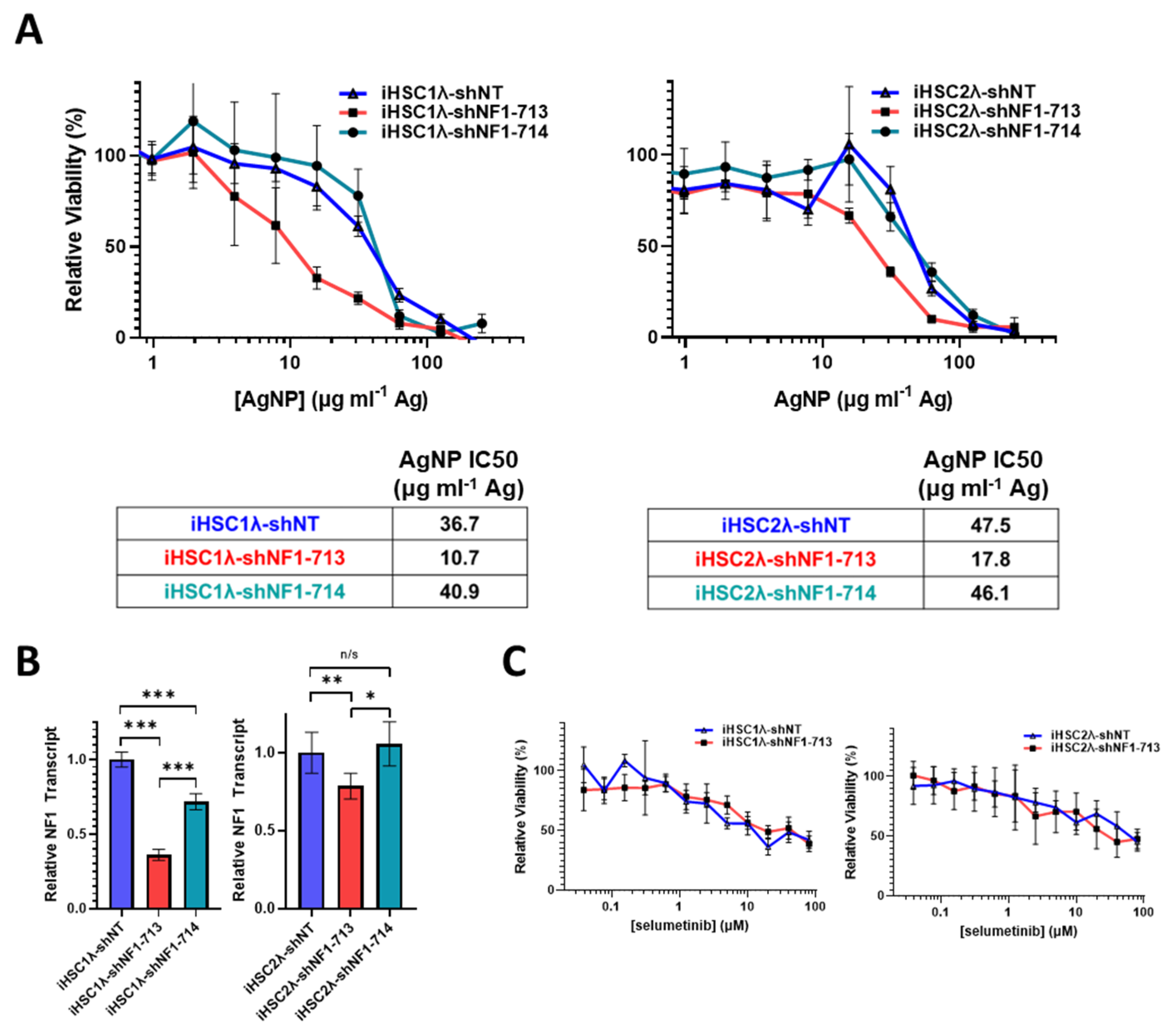
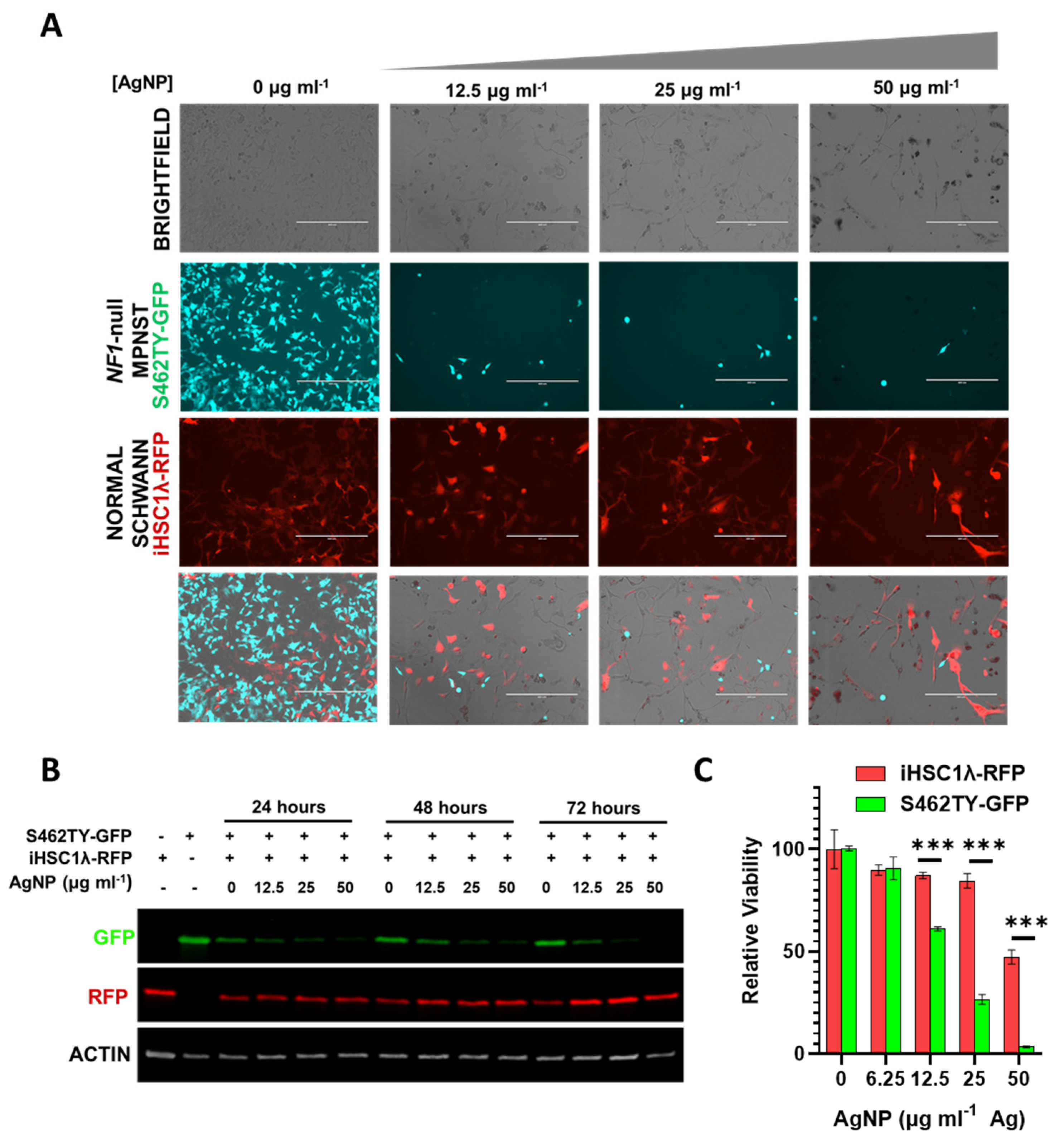
| Cell Line | NF1 Status | Subtype Classification |
|---|---|---|
| S462TY | −/− | NF1-associated MPNST |
| ST-8814 | −/− | NF1-associated MPNST |
| STS26T | +/+ | Sporadic MPNST |
| iHSC1λ | +/+ | Normal Schwann Cell |
| iHSC2λ | +/+ | Normal Schwann Cell |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alewine, G.; Knight, J.; Ghantae, A.; Mamrega, C.; Attiah, B.; Coover, R.A.; Fahrenholtz, C.D. Silver Nanoparticles Selectively Treat Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors in a Neurofibromin-Dependent Manner. J. Pers. Med. 2022, 12, 1080. https://doi.org/10.3390/jpm12071080
Alewine G, Knight J, Ghantae A, Mamrega C, Attiah B, Coover RA, Fahrenholtz CD. Silver Nanoparticles Selectively Treat Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors in a Neurofibromin-Dependent Manner. Journal of Personalized Medicine. 2022; 12(7):1080. https://doi.org/10.3390/jpm12071080
Chicago/Turabian StyleAlewine, Garrett, Jerrica Knight, Adithya Ghantae, Christina Mamrega, Bashnona Attiah, Robert A. Coover, and Cale D. Fahrenholtz. 2022. "Silver Nanoparticles Selectively Treat Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors in a Neurofibromin-Dependent Manner" Journal of Personalized Medicine 12, no. 7: 1080. https://doi.org/10.3390/jpm12071080
APA StyleAlewine, G., Knight, J., Ghantae, A., Mamrega, C., Attiah, B., Coover, R. A., & Fahrenholtz, C. D. (2022). Silver Nanoparticles Selectively Treat Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors in a Neurofibromin-Dependent Manner. Journal of Personalized Medicine, 12(7), 1080. https://doi.org/10.3390/jpm12071080








