Effects of Iloprost on Arterial Oxygenation and Lung Mechanics during One-Lung Ventilation in Supine-Positioned Patients: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
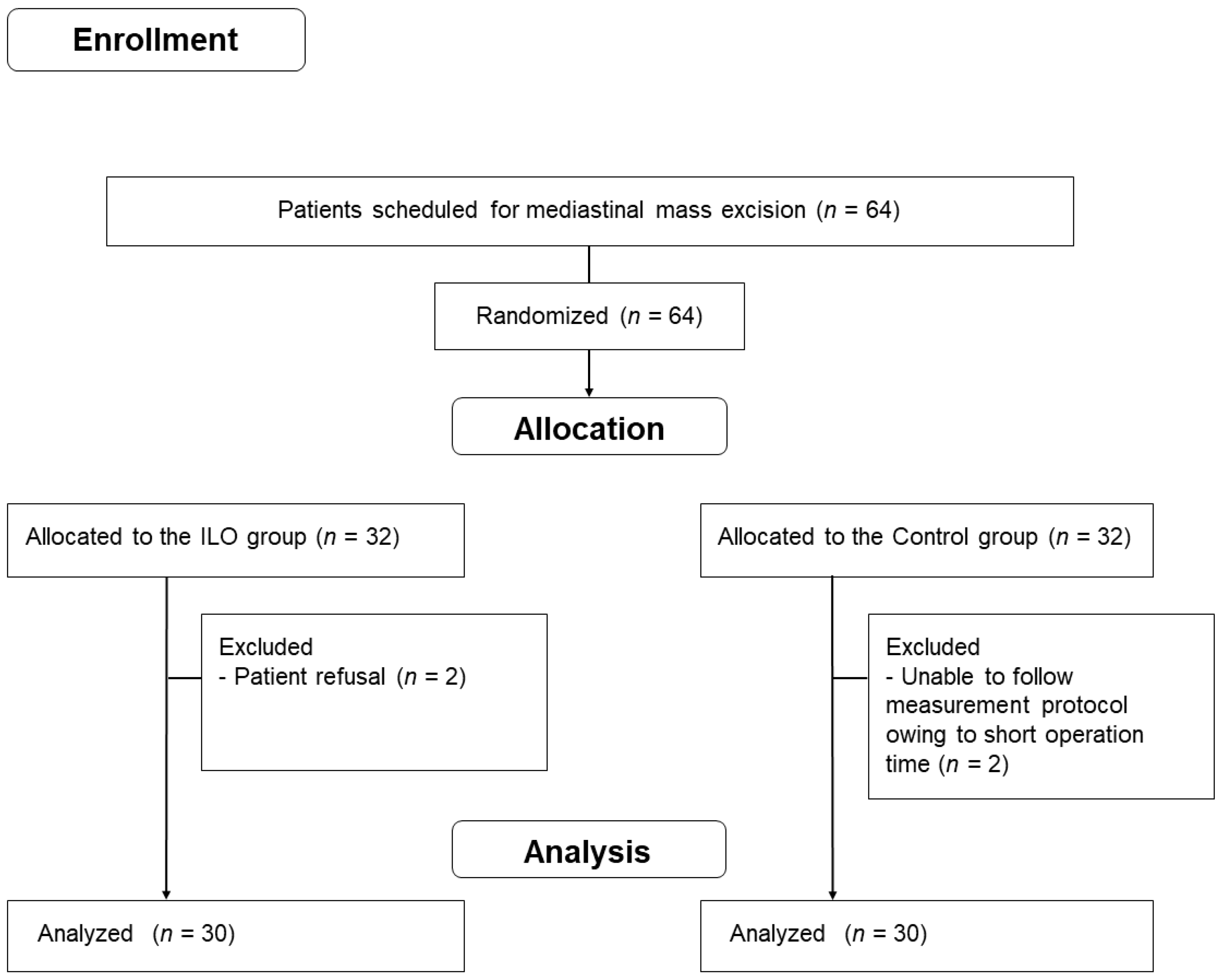
2.1. Study Population
2.2. Anesthetic Management
2.3. Study Design and Outcome Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos, J.H. Current techniques for perioperative lung isolation in adults. Anesthesiology 2002, 97, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.H.; Feider, A. Hypoxia During One-Lung Ventilation-A Review and Update. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Wittenstein, J.; Scharffenberg, M.; Ran, X.; Zhang, Y.; Keller, D.; Tauer, S.; Theilen, R.; Chai, Y.; Ferreira, J.; Muller, S.; et al. Effects of Body Position and Hypovolemia on the Regional Distribution of Pulmonary Perfusion During One-Lung Ventilation in Endotoxemic Pigs. Front. Physiol. 2021, 12, 717269. [Google Scholar] [CrossRef] [PubMed]
- Lumb, A.B.; Slinger, P. Hypoxic pulmonary vasoconstriction: Physiology and anesthetic implications. Anesthesiology 2015, 122, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, L.L.; D’Hollander, A.A.; Vermassen, F.E.; Deryck, F.; Wouters, P.F. Gravity is an important determinant of oxygenation during one-lung ventilation. Acta Anaesthesiol. Scand. 2010, 54, 744–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollin, A.; Mandel, F.; Grunenwald, E.; Mondoly, P.; Monteil, B.; Marcheix, B.; Maury, P. Hybrid surgical ablation for persistent or long standing persistent atrial fibrillation: A French single centre experience. Ann. Cardiol. Angeiol. 2020, 69, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sawheny, E.; Ellis, A.L.; Kinasewitz, G.T. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013, 144, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeon, J.; Huh, J.; Koo, J.; Yang, S.; Hwang, W. The Effects of Iloprost on Oxygenation During One-Lung Ventilation for Lung Surgery: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Lee, S.H.; Joe, Y.; Kim, T.; Shin, H.; Oh, Y.J. Effects of Inhaled Iloprost on Lung Mechanics and Myocardial Function During One-Lung Ventilation in Chronic Obstructive Pulmonary Disease Patients Combined with Poor Lung Oxygenation. Anesth. Analg. 2020, 130, 1407–1414. [Google Scholar] [CrossRef]
- Lee, K.; Oh, Y.J.; Kim, M.; Song, S.H.; Kim, N. Effects of Iloprost on Oxygenation during One-Lung Ventilation in Patients with Low Diffusing Capacity for Carbon Monoxide: A Randomized Controlled Study. J. Clin. Med. 2022, 11, 1542. [Google Scholar] [CrossRef]
- Hardman, J.G.; Aitkenhead, A.R. Estimation of alveolar deadspace fraction using arterial and end-tidal CO2: A factor analysis using a physiological simulation. Anaesth. Intensive Care 1999, 27, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olschewski, H.; Simonneau, G.; Galie, N.; Higenbottam, T.; Naeije, R.; Rubin, L.J.; Nikkho, S.; Speich, R.; Hoeper, M.M.; Behr, J.; et al. Inhaled iloprost for severe pulmonary hypertension. N. Engl. J. Med. 2002, 347, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Boeck, L.; Tamm, M.; Grendelmeier, P.; Stolz, D. Acute effects of aerosolized iloprost in COPD related pulmonary hypertension—A randomized controlled crossover trial. PLoS ONE 2012, 7, e52248. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.Z.; Zhao, Q.H.; Jiang, R.; Wu, W.H.; Gong, S.G.; He, J.; Liu, J.M.; Jing, Z.C. Hemodynamic and gas exchange effects of inhaled iloprost in patients with COPD and pulmonary hypertension. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3353–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Wang, H.; Yu, S.J.; Tu, G.W.; Luo, Z. Inhaled pulmonary vasodilators: A narrative review. Ann. Transl. Med. 2021, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Graeber, G.M.; Tanguilig, G.G.; Hobbs, G.; Murray, G.F. Effects of insufflation on hemodynamics during thoracoscopy. Ann. Thorac. Surg. 1993, 55, 1379–1382. [Google Scholar] [CrossRef]
- Brock, H.; Rieger, R.; Gabriel, C.; Polz, W.; Moosbauer, W.; Necek, S. Haemodynamic changes during thoracoscopic surgery the effects of one-lung ventilation compared with carbon dioxide insufflation. Anaesthesia 2000, 55, 10–16. [Google Scholar] [CrossRef]
| Control Group (n = 30) | ILO Group (n = 30) | p-Value | |
|---|---|---|---|
| Age (yrs) | 50 ± 12 | 53 ± 14 | 0.374 |
| Women (n) | 15 (50) | 16 (53) | 0.796 |
| Height (cm) | 164.4 ± 9.2 | 164.2 ± 11.1 | 0.923 |
| Weight (kg) | 67.9 ± 12.3 | 66.6 ± 13.9 | 0.705 |
| Body mass index (kg/m2) | 24.9 ± 3.0 | 24.5 ± 3.4 | 0.622 |
| ASA classification 2/3 (n) | 28 (93)/2 (7) | 26 (87)/4 (13) | 0.389 |
| Hypertension (n) | 10 (33) | 12 (40) | 0.592 |
| Diabetes mellitus (n) | 2 (7) | 5 (17) | 0.228 |
| Smoking history | |||
| Ex-smoker or current smoker (n) | 9 (30) | 14 (47) | 0.184 |
| Smoking index (pack × years) | 0 [0–11] | 0 [0–10] | 0.412 |
| Preoperative chest CT | |||
| Atelectasis (n) | 1 (3) | 4 (13) | 0.161 |
| Bronchiectasis (n) | 1 (3) | 3 (10) | 0.301 |
| Emphysema (n) | 0 (0) | 2 (7) | 0.150 |
| Bronchitis (n) | 3 (10) | 1 (3) | 0.301 |
| Preoperative spirometry | |||
| FEV1 (L) | 2.9 ± 0.8 | 2.6 ± 1.1 * | 0.037 |
| FEV1 (% predicted) | 92 [85–96] | 83 [75–94] | 0.368 |
| FVC (L) | 3.6 ± 1.0 | 3.4 ± 1.2 | 0.569 |
| FVC (% predicted) | 89 [83–98] | 88 [77–97] | 0.276 |
| FEV1/FVC (%) | 81 [76–84] | 77 [72–81] | 0.069 |
| Control Group (n = 30) | ILO Group (n = 30) | p-Value | |
|---|---|---|---|
| Approach direction (right/left) (n) | 16 (53)/14 (47) | 15 (50)/15 (50) | 0.796 |
| Anesthesia time (min) | 109 ± 32 | 116 ± 38 | 0.480 |
| Operation time (min) | 70 ± 29 | 76 ± 35 | 0.481 |
| OLV time (min) | 51 [42–66] | 49 [40–61] | 0.812 |
| FiO2 elevation (n) | 9 (30) | 1 (3) * | 0.006 |
| Hypoxia (n) | 1 (3) | 1 (3) | 1.000 |
| Hypotension (n) | 14 (47) | 13 (43) | 0.795 |
| Intake fluid (mL) | 702 ± 259 | 700 ± 302 | 0.982 |
| Urine output (mL) | 25 ± 45 | 43 ± 71 | 0.254 |
| Estimated blood loss (mL) | 35 ± 20 | 34 ± 18 | 0.738 |
| Control Group (n = 30) | ILO Group (n = 30) | p-Value | |
|---|---|---|---|
| Heart rate (beat/min) | 0.83 | ||
| T1 | 72 ± 10 | 78 ± 14 | |
| T2 | 74 ± 14 | 81 ± 12 | |
| Mean blood pressure (mmHg) | 0.95 | ||
| T1 | 77 ± 11 | 80 ± 11 | |
| T2 | 81 ± 12 | 85 ± 13 | |
| PaO2 (mmHg) | 0.77 | ||
| T1 | 253 ± 66 | 255 ± 68 | |
| T2 | 108 ± 37 * | 115 ± 45 * | |
| PaO2/FiO2 ratio (mmHg) | 0.29 | ||
| T1 | 422 ± 111 | 425 ± 113 | |
| T2 | 156 ± 42 * | 190 ± 75 * | |
| EtCO2 (mmHg) | 0.49 | ||
| T1 | 39 ± 5 | 40 ± 4 | |
| T2 | 40 ± 3 | 42 ± 5 | |
| PaCO2 (mmHg) | 0.04 | ||
| T1 | 43 ± 5 | 45 ± 5 | |
| T2 | 48 ± 5 * | 47 ± 6 | |
| Alveolar dead space | 0.002 | ||
| T1 | 12 ± 12 | 12 ± 9 | |
| T2 | 19 ± 6 * | 11 ± 5 | |
| Dynamic compliance (mL/cmH2O) | 0.41 | ||
| T1 | 28 ± 5 | 27 ± 6 | |
| T2 | 16 ± 3 * | 16 ± 5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kim, M.; Kim, N.; Kang, S.J.; Oh, Y.J. Effects of Iloprost on Arterial Oxygenation and Lung Mechanics during One-Lung Ventilation in Supine-Positioned Patients: A Randomized Controlled Study. J. Pers. Med. 2022, 12, 1054. https://doi.org/10.3390/jpm12071054
Lee K, Kim M, Kim N, Kang SJ, Oh YJ. Effects of Iloprost on Arterial Oxygenation and Lung Mechanics during One-Lung Ventilation in Supine-Positioned Patients: A Randomized Controlled Study. Journal of Personalized Medicine. 2022; 12(7):1054. https://doi.org/10.3390/jpm12071054
Chicago/Turabian StyleLee, Kyuho, Mina Kim, Namo Kim, Su Jeong Kang, and Young Jun Oh. 2022. "Effects of Iloprost on Arterial Oxygenation and Lung Mechanics during One-Lung Ventilation in Supine-Positioned Patients: A Randomized Controlled Study" Journal of Personalized Medicine 12, no. 7: 1054. https://doi.org/10.3390/jpm12071054
APA StyleLee, K., Kim, M., Kim, N., Kang, S. J., & Oh, Y. J. (2022). Effects of Iloprost on Arterial Oxygenation and Lung Mechanics during One-Lung Ventilation in Supine-Positioned Patients: A Randomized Controlled Study. Journal of Personalized Medicine, 12(7), 1054. https://doi.org/10.3390/jpm12071054






