Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases
Abstract
1. Introduction
2. Endogenous Levels of Relaxin-2 in Physiological Conditions
| Condition | Subjects (n) | Mean Relaxin-2 Concentration | Main Results | Reference |
|---|---|---|---|---|
| Healthy men | Men (1) | 91.5 ± 13.8 ng/mL | Relaxin-2 concentrations ↓ in the post-menopausal and male subjects in other conditions | [88] |
| Menstrual cycle | Periovulatory (1) | 142.9 ± 17.4 ng/mL | ||
| Follicular phase (1) | 112.8 ± 24.9 ng/mL | |||
| Pregnancy | Pregnant (1) | 128.7 ± 19.1 ng/mL | ||
| Menopause | Post-menopausal (1) | 46.5 ± 7.5 ng/mL | ||
| Spontaneously pregnancy | 5 weeks (4) | 315 (20–1200) pg/mL | Relaxin-2 levels ↑ during different weeks of pregnancy but start to ↓ in week 20 | [66] |
| 7 weeks (9) | 923 (230–1920) pg/mL | |||
| 11 weeks (20) | 1294 (538–3480) pg/mL | |||
| 14 weeks (18) | 1122 (400–2430) pg/mL | |||
| 20 weeks (19) | 555 (117–1712) pg/mL | |||
| 26 weeks (35) | 515 (134–1808) pg/mL | |||
| 30 weeks (30) | 568 (255–1774) pg/mL | |||
| 38 weeks (25) | 494 (212–1930) pg/mL | |||
| Pregnancy with superovulation | Singleton pregnancy (15) | 179–14,633 pg/mL | Relaxin-2 levels increase with conceptus number | [66] |
| Twin pregnancy (14) | 223–13,750 pg/mL | |||
| Triplet pregnancy (28) | 850–21,700 pg/mL | |||
| Quadruplet pregnancy (10) | 1030–18,700 pg/mL | |||
| Quintuplet pregnancy (10) | 4820–28,800 pg/mL | |||
| Women with normal ovarian function | Basal follicular (8) | 13 ± 5.0 pg/mL * | Relaxin-2 levels in the luteal phase were significantly ↑ | [64] |
| Luteal (8) | 41 ± 11 pg/mL * | |||
| Healthy men | Men (9) | 8.3 ± 0.8 pg/mL * | Relaxin-2 levels were ↑ in women compared with men | [71] |
| Healthy women | Women in reproductive age (18) | 15.3 ± 1.3 pg/mL * | ||
| Menopausal women (23) | 14.9 ± 0.9 pg/mL * | |||
| Menstrual cycle | Days 1–4 (20) | 82.1 ± 23.8 pg/mL * | Relaxin-2 were ↑ in the luteal phase | [65] |
| Days 12–14 (20) | 86.2 ± 25.5 pg/mL * | |||
| Days 20–23 (20) | 94.1 ± 27.1 pg/mL * | |||
| Pregnancy | Prepregnancy (13) | 52.88 ± 29.66 pg/mL | Relaxin-2 levels at each time point during pregnancy were ↑ except at 26 weeks of pregnancy Relaxin-2 ↓ at 36 weeks’ gestation and in postpartum when compared with pregnancy state | [67] |
| 6 weeks (12) | 1949.91 ± 895.62 pg/mL | |||
| 10 weeks (11) | 1713.08 ± 516.70 pg/mL | |||
| 16 weeks (12) | 1345.82 ± 751.56 pg/mL | |||
| 26 weeks (13) | 1493.66 ± 2552.82 pg/mL | |||
| 36 weeks (12) | 859.93 ± 376.28 pg/mL | |||
| Postpartum (11) | 53.18 ± 69.71 pg/mL |
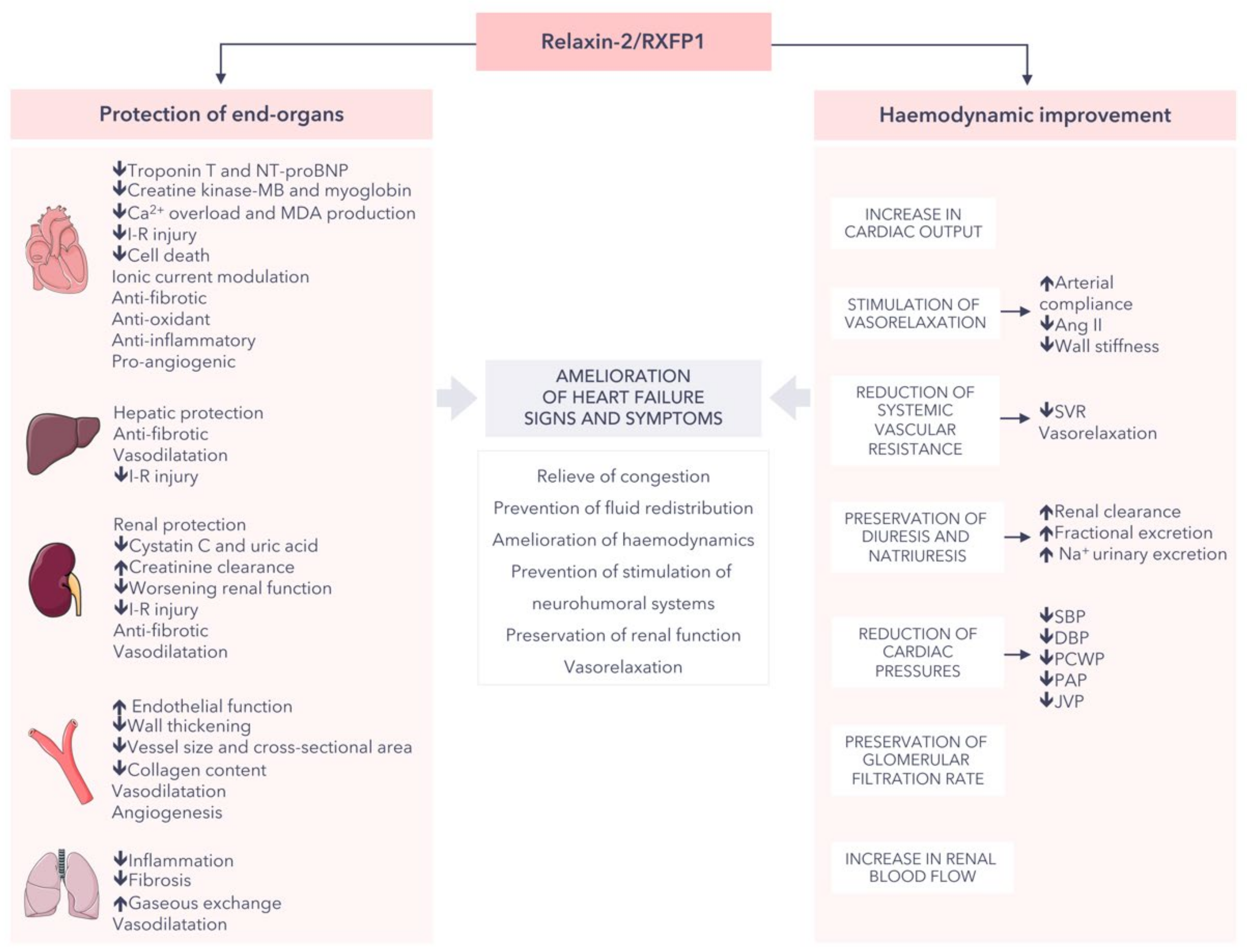
3. Relaxin-2 as a Biomarker in Cardiovascular Disease
3.1. Heart Failure
3.2. Atrial Fibrillation
3.3. Ischemic Heart Disease
3.4. Myocardial Infarction
3.5. Aortic Valve Disease
3.6. Hypertension
3.7. Atherosclerosis
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 1 April 2022).
- Twerenbold, R.; Jaffe, A.; Reichlin, T.; Reiter, M.; Mueller, C. High-Sensitive Troponin T Measurements: What Do We Gain and What Are the Challenges? Eur. Heart J. 2012, 33, 579–586. [Google Scholar] [CrossRef]
- Haybar, H.; Shokuhian, M.; Bagheri, M.; Davari, N.; Saki, N. Involvement of Circulating Inflammatory Factors in Prognosis and Risk of Cardiovascular Disease. J. Mol. Cell. Cardiol. 2019, 132, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.M. Future Biomarkers in Cardiology: My Favourites. Eur. Hear. J. Suppl. 2018, 20, G37–G44. [Google Scholar] [CrossRef]
- Hisaw, F.L. Experimental Relaxation of the Pubic Ligament of the Guinea Pig. Exp. Biol. Med. 1926, 23, 661–663. [Google Scholar] [CrossRef]
- Casten, G.G.; Boucek, R.J. Use of Relaxin in the Treatment of Scleroderma. J. Am. Med. Assoc. 1958, 166, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zarrow, M.X.; Holmstrom, E.G.; Salhanick, H.A. The Concentration of Relaxin in the Blood Serum and Other Tissues of Women during Pregnancy. J. Clin. Endocrinol. Metab. 1955, 15, 22–27. [Google Scholar] [CrossRef]
- MacLennan, A.H. The Role of Relaxin in Human Reproduction. Clin. Reprod. Fertil. 1983, 2, 77–95. [Google Scholar] [PubMed]
- Chen, S.A.; Perlman, A.J.; Spanski, N.; Peterson, C.M.; Sanders, S.W.; Jaffe, R.; Martin, M.; Yalcinkaya, T.; Cefalo, R.C.; Chescheir, N.C.; et al. The Pharmacokinetics of Recombinant Human Relaxin in Nonpregnant Women After Intravenous, Intravaginal, and Intracervical Administration. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1993, 10, 834–838. [Google Scholar]
- Fei, D.T.W.; Gross, M.C.; Lofgren, J.L.; Mora-Worms, M.; Chen, A.B. Cyclic AMP Response to Recombinant Human Relaxin by Cultured Human Endometrial Cells—A Specific and High Throughput in Vitro Bioassay. Biochem. Biophys. Res. Commun. 1990, 170, 214–222. [Google Scholar] [CrossRef]
- Goldsmith, L.T.; Weiss, G. Relaxin in Human Pregnancy. Ann. N. Y. Acad. Sci. 2009, 1160, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bathgate, R.A.D.; Halls, M.L.; van der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R.J. Relaxin Family Peptides and Their Receptors. Physiol. Rev. 2013, 93, 405–480. [Google Scholar] [CrossRef] [PubMed]
- Valkovic, A.L.; Bathgate, R.A.; Samuel, C.S.; Kocan, M. Understanding Relaxin Signalling at the Cellular Level. Mol. Cell. Endocrinol. 2019, 487, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E. Serelaxin in Clinical Development: Past, Present and Future. Br. J. Pharmacol. 2017, 174, 921–932. [Google Scholar] [CrossRef]
- Smith, M.C.; Danielson, L.A.; Conrad, K.P.; Davison, J.M. Influence of Recombinant Human Relaxin on Renal Hemodynamics in Healthy Volunteers. J. Am. Soc. Nephrol. 2006, 17, 3192–3197. [Google Scholar] [CrossRef]
- Dahlke, M.; Ng, D.; Yamaguchi, M.; Machineni, S.; Berger, S.; Canadi, J.; Rajman, I.; Lloyd, P.; Pang, Y. Safety and Tolerability of Serelaxin, a Recombinant Human Relaxin-2 in Development for the Treatment of Acute Heart Failure, in Healthy Japanese Volunteers and a Comparison of Pharmacokinetics and Pharmacodynamics in Healthy Japanese and Caucasian Populat. J. Clin. Pharmacol. 2015, 55, 415–422. [Google Scholar] [CrossRef]
- Weiss, G.; Teichman, S.; Stewart, D.; Nader, D.; Wood, S.; Breining, P.; Unemori, E. Recombinant Human Relaxin versus Placebo for Cervical Ripening: A Double-Blind Randomised Trial in Pregnant Women Scheduled for Induction of Labour. BMC Pregnancy Childbirth 2016, 16, 260. [Google Scholar] [CrossRef]
- Bell, R.J.; Permezel, M.; MacLennan, A.; Hughes, C.; Healy, D.; Brennecke, S. A Randomized, Double-Blind, Placebo-Controlled Trial of the Safety of Vaginal Recombinant Human Relaxin for Cervical Ripening. Obstet. Gynecol. 1993, 82, 328–333. [Google Scholar]
- Unemori, E.; Sibai, B.; Teichman, S.L. Scientific Rationale and Design of a Phase I Safety Study of Relaxin in Women with Severe Preeclampsia. Ann. N. Y. Acad. Sci. 2009, 1160, 381–384. [Google Scholar] [CrossRef]
- Dschietzig, T.; Teichman, S.; Unemori, E.; Wood, S.; Boehmer, J.; Richter, C.; Baumann, G.; Stangl, K. Intravenous Recombinant Human Relaxin in Compensated Heart Failure: A Safety, Tolerability, and Pharmacodynamic Trial. J. Card. Fail. 2009, 15, 182–190. [Google Scholar] [CrossRef]
- Voors, A.A.; Dahlke, M.; Meyer, S.; Stepinska, J.; Gottlieb, S.S.; Jones, A.; Zhang, Y.; Laurent, D.; Slart, R.H.J.A.; Navis, G.J. Renal Hemodynamic Effects of Serelaxin in Patients with Chronic Heart Failure a Randomized, Placebo-Controlled Study. Circ. Hear. Fail. 2014, 7, 994–1002. [Google Scholar] [CrossRef]
- Teerlink, J.; Saini, R.; Gullestad, L.; Descotes, J.; Masior, T.; Wang, Y.; Pak, J.; Pang, Y.; Unemori, E.; Severin, T. RELAX-REPEAT: A Multicenter, Prospective, Randomized, Double- Blind Study Evaluating the Safety and Tolerability of Repeat Doses of Serelaxin in Patients with Chronic Heart Failure. J. Card. Fail. 2016, 22, S14–S15. [Google Scholar] [CrossRef][Green Version]
- Teerlink, J.R.; Metra, M.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Weatherley, B.D.; Marmor, A.; Katz, A.; Grzybowski, J.; Unemori, E.; et al. Relaxin for the Treatment of Patients with Acute Heart Failure (Pre-RELAX-AHF): A Multicentre, Randomised, Placebo-Controlled, Parallel-Group, Dose-Finding Phase IIb Study. Lancet 2009, 373, 1429–1439. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F.; et al. Serelaxin, Recombinant Human Relaxin-2, for Treatment of Acute Heart Failure (RELAX-AHF): A Randomised, Placebo-Controlled Trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Sato, N.; Lam, C.S.P.; Teerlink, J.R.; Greenberg, B.H.; Tsutsui, H.; Oh, B.H.; Zhang, J.; Lefkowitz, M.; Hua, T.A.; Holbro, T.; et al. Evaluating the Efficacy, Safety, and Tolerability of Serelaxin When Added to Standard Therapy in Asian Patients With Acute Heart Failure: Design and Rationale of RELAX-AHF-ASIA Trial. J. Card. Fail. 2017, 23, 63–71. [Google Scholar] [CrossRef]
- Maggioni, A.P.; López-Sendón, J.; Nielsen, O.W.; Hallén, J.; Aalamian-Mattheis, M.; Wang, Y.; Ertl, G. Efficacy and Safety of Serelaxin When Added to Standard of Care in Patients with Acute Heart Failure: Results from a PROBE Study, RELAX-AHF-EU. Eur. J. Heart Fail. 2019, 21, 322–333. [Google Scholar] [CrossRef]
- Seibold, J.R.; Clements, P.J.; Furst, D.E.; Mayes, M.D.; McCloskey, D.A.; Moreland, L.W.; White, B.; Wigley, F.M.; Rocco, S.; Erikson, M.; et al. Safety and Pharmacokinetics of Recombinant Human Relaxin in Systemic Sclerosis. J. Rheumatol. 1998, 25, 302–307. [Google Scholar]
- Seibold, J.R.; Korn, J.H.; Simms, R.; Clements, P.J.; Moreland, L.W.; Mayes, M.D.; Furst, D.E.; Rothfield, N.; Steen, V.; Weisman, M.; et al. Recombinant Human Relaxin in the Treatment of Scleroderma. A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2000, 132, 871–879. [Google Scholar] [CrossRef]
- Khanna, D.; Clements, P.J.; Furst, D.E.; Korn, J.H.; Ellman, M.; Rothfield, N.; Wigley, F.M.; Moreland, L.W.; Silver, R.; Kim, Y.H.; et al. Recombinant Human Relaxin in the Treatment of Systemic Sclerosis with Diffuse Cutaneous Involvement: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 2009, 60, 1102–1111. [Google Scholar] [CrossRef]
- Kobalava, Z.; Villevalde, S.; Kotovskaya, Y.; Hinrichsen, H.; Petersen-Sylla, M.; Zaehringer, A.; Pang, Y.; Rajman, I.; Canadi, J.; Dahlke, M.; et al. Pharmacokinetics of Serelaxin in Patients with Hepatic Impairment: A Single-Dose, Open-Label, Parallel Group Study. Br. J. Clin. Pharmacol. 2015, 79, 937–945. [Google Scholar] [CrossRef]
- Dahlke, M.; Halabi, A.; Canadi, J.; Tsubouchi, C.; Machineni, S.; Pang, Y. Pharmacokinetics of Serelaxin in Patients with Severe Renal Impairment or End-Stage Renal Disease Requiring Hemodialysis: A Single-Dose, Open-Label, Parallel-Group Study. J. Clin. Pharmacol. 2016, 56, 474–483. [Google Scholar] [CrossRef]
- Gifford, F.J.; Dunne, P.D.J.; Weir, G.; Ireland, H.; Graham, C.; Tuck, S.; Hayes, P.C.; Fallowfield, J.A. A Phase 2 Randomised Controlled Trial of Serelaxin to Lower Portal Pressure in Cirrhosis (STOPP). Trials 2020, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Romero, G.; Salama, G. Cardioprotective Actions of Relaxin. Mol. Cell. Endocrinol. 2019, 487, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Du, X.-J.; Dschietzig, T.B.; Summers, R.J. The Actions of Relaxin on the Human Cardiovascular System. Br. J. Pharmacol. 2017, 174, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Pini, A.; Sassoli, C.; Squecco, R.; Francini, F.; Formigli, L.; Bani, D. Relaxin Promotes Growth and Maturation of Mouse Neonatal Cardiomyocytes in Vitro: Clues for Cardiac Regeneration. J. Cell. Mol. Med. 2012, 16, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Moore, X.; Tan, S.; Lo, C.; Fang, L.; Su, Y.-D.; Gao, X.-M.; Woodcock, E.A.; Summers, R.J.; Tregear, G.W.; Bathgate, R.A.D.; et al. Relaxin Antagonizes Hypertrophy and Apoptosis in Neonatal Rat Cardiomyocytes. Endocrinology 2007, 148, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.E.; Wood, P.; Kulpa, J.; Yang, F.H.; Summerlee, A.J.; Pyle, W.G. Relaxin Alters Cardiac Myofilament Function through a PKC-Dependent Pathway. Am. J. Physiol. Circ. Physiol. 2009, 297, H29–H36. [Google Scholar] [CrossRef]
- Boccalini, G.; Sassoli, C.; Formigli, L.; Bani, D.; Nistri, S. Relaxin Protects Cardiac Muscle Cells from Hypoxia/Reoxygenation Injury: Involvement of the Notch-1 Pathway. FASEB J. 2015, 29, 239–249. [Google Scholar] [CrossRef]
- Aragón-Herrera, A.; Feijóo-Bandín, S.; Rodríguez-Penas, D.; Roselló-Lletí, E.; Portolés, M.; Rivera, M.; Bigazzi, M.; Bani, D.; Gualillo, O.; González-Juanatey, J.R.; et al. Relaxin Activates AMPK-AKT Signaling and Increases Glucose Uptake by Cultured Cardiomyocytes. Endocrine 2018, 60, 103–111. [Google Scholar] [CrossRef]
- Aragón-Herrera, A.; Feijóo-Bandín, S.; Abella, V.; Álvarez, L.; Roselló-Lletí, E.; Portolés, M.; Tarazón, E.; Bigazzi, M.; Bani, D.; Gualillo, O.; et al. Serelaxin (Recombinant Human Relaxin-2) Treatment Affects the Endogenous Synthesis of Long Chain Poly-Unsaturated Fatty Acids and Induces Substantial Alterations of Lipidome and Metabolome Profiles in Rat Cardiac Tissue. Pharmacol. Res. 2019, 144, 51–65. [Google Scholar] [CrossRef]
- Samuel, C.S.; Unemori, E.N.; Mookerjee, I.; Bathgate, R.A.D.; Layfield, S.L.; Mak, J.; Tregear, G.W.; Du, X.J. Relaxin Modulates Cardiac Fibroblast Proliferation, Differentiation, and Collagen Production and Reverses Cardiac Fibrosis in Vivo. Endocrinology 2004, 145, 4125–4133. [Google Scholar] [CrossRef]
- Wang, C.; Pinar, A.A.; Widdop, R.E.; Hossain, M.A.; Bathgate, R.A.D.; Denton, K.M.; Kemp-Harper, B.K.; Samuel, C.S. The Anti-fibrotic Actions of Relaxin Are Mediated through AT 2 R-associated Protein Phosphatases via RXFP1-AT 2 R Functional Crosstalk in Human Cardiac Myofibroblasts. FASEB J. 2020, 34, 8217–8233. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Pini, A.; Tani, A.; Nistri, S.; Nosi, D.; Zecchi-Orlandini, S.; Bani, D.; Formigli, L. Relaxin Prevents Cardiac Fibroblast-Myofibroblast Transition via Notch-1-Mediated Inhibition of TGF-β/Smad3 Signaling. PLoS ONE 2013, 8, e63896. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Boccalini, G.; Baccari, M.C.; Becatti, M.; Garella, R.; Fiorillo, C.; Calosi, L.; Bani, D.; Nistri, S. Protection from Cigarette Smoke-Induced Vascular Injury by Recombinant Human Relaxin-2 (Serelaxin). J. Cell. Mol. Med. 2016, 20, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Leo, C.H.; Parry, L.J. Serelaxin (Recombinant Human Relaxin-2) Prevents High Glucose-Induced Endothelial Dysfunction by Ameliorating Prostacyclin Production in the Mouse Aorta. Pharmacol. Res. 2016, 107, 220–228. [Google Scholar] [CrossRef]
- Dschietzig, T.; Brecht, A.; Bartsch, C.; Baumann, G.; Stangl, K.; Alexiou, K. Relaxin Improves TNF-α-Induced Endothelial Dysfunction: The Role of Glucocorticoid Receptor and Phosphatidylinositol 3-Kinase Signalling. Cardiovasc. Res. 2012, 95, 97–107. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Voors, A.A.; Ponikowski, P.; Pang, P.S.; Greenberg, B.H.; Filippatos, G.; Felker, G.M.; Davison, B.A.; Cotter, G.; Gimpelewicz, C.; et al. Serelaxin in Addition to Standard Therapy in Acute Heart Failure: Rationale and Design of the RELAX-AHF-2 Study. Eur. J. Heart Fail. 2017, 19, 800–809. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Voors, A.A.; et al. Effects of Serelaxin in Patients with Acute Heart Failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kocan, M.; Yao, S.T.; Royce, S.G.; Nair, V.B.; Siwek, C.; Patil, N.A.; Harrison, I.P.; Rosengren, K.J.; Selemidis, S.; et al. A Single-Chain Derivative of the Relaxin Hormone Is a Functionally Selective Agonist of the G Protein-Coupled Receptor, RXFP1. Chem. Sci. 2016, 7, 3805–3819. [Google Scholar] [CrossRef]
- Agoulnik, A.I.; Agoulnik, I.U.; Hu, X.; Marugan, J. Synthetic Non-Peptide Low Molecular Weight Agonists of the Relaxin Receptor 1. Br. J. Pharmacol. 2017, 174, 977–989. [Google Scholar] [CrossRef]
- Mardhian, D.F.; Storm, G.; Bansal, R.; Prakash, J. Nano-Targeted Relaxin Impairs Fibrosis and Tumor Growth in Pancreatic Cancer and Improves the Efficacy of Gemcitabine in Vivo. J. Control. Release 2018, 290, 1–10. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Y.; Xu, L.; An, S.; Tang, Y.; Zhou, X.; Li, J.; Liu, R.; Huang, L. Relaxin Gene Delivery Mitigates Liver Metastasis and Synergizes with Check Point Therapy. Nat. Commun. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Petersen, L.K.; Skajaa, K.; Uldbjerg, N. Serum Relaxin as a Potential Marker for Preterm Labour. Br. J. Obstet. Gynaecol. 1992, 99, 292–295. [Google Scholar] [CrossRef]
- Post Uiterweer, E.D.; Koster, M.P.H.; Jeyabalan, A.; Kuc, S.; Siljee, J.E.; Stewart, D.R.; Conrad, K.P.; Franx, A. Circulating Pregnancy Hormone Relaxin as a First Trimester Biomarker for Preeclampsia. Pregnancy Hypertens. 2020, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Skurnick, J.H.; Goldsmith, L.T.; Emmi, A.; Weiss, G. Human Chorionic Gonadotropin and Relaxin Concentrations in Early Ectopic and Normal Pregnancies. Obstet. Gynecol. 1990, 75, 779–783. [Google Scholar] [PubMed]
- Rehfeldt, M.; Eklund, E.; Struck, J.; Sparwasser, A.; O’Brien, B.; Palomaki, G.E.; Köhrle, J.; Bergmann, A.; Lambert-Messerlian, G. Relaxin-2 Connecting Peptide (pro-RLX2) Levels in Second Trimester Serum Samples to Predict Preeclampsia. Pregnancy Hypertens. 2018, 11, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Gkrozou, F.; Pappa, C.; Tsonis, O.; Dimitriou, E.; Paschopoulos, M. Relaxin as a Potential Diagnostic Biomarker for Ovarian Cancer- A Prospective Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 99–104. [Google Scholar] [CrossRef]
- Vogel, I.; Goepfert, A.R.; Møller, H.J.; Cliver, S.; Thorsen, P.; Andrews, W.W. Early Mid-Trimester Serum Relaxin, Soluble CD163, and Cervical Length in Women at High Risk for Preterm Delivery. Am. J. Obstet. Gynecol. 2006, 195, 208–214. [Google Scholar] [CrossRef]
- Bramham, K.; Seed, P.T.; Lightstone, L.; Nelson-Piercy, C.; Gill, C.; Webster, P.; Poston, L.; Chappell, L.C. Diagnostic and Predictive Biomarkers for Pre-Eclampsia in Patients with Established Hypertension and Chronic Kidney Disease. Kidney Int. 2016, 89, 874–885. [Google Scholar] [CrossRef]
- Bigazzi, M.; Bani, D.; Sacchi, T.B. Relaxin: A Possible Future Preventive Therapy for Cardiovascular Disease in Postmenopausal Women and Men? Climacteric 2001, 4, 137–143. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, E.M.; Carriere, B.T.; Sorensen, L.; Segaloff, A.; Schwabe, C.; Steinetz, B.G. Plasma Immunoreactive Relaxin Levels in Pregnant and Nonpregnant Women. J. Clin. Endocrinol. Metab. 1978, 47, 1106–1110. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, Q.; Lohstroh, P.N.; Overstreet, J.W.; Lasley, B.L. Hormonal Characteristics in the Early Luteal Phase of Conceptive and Nonconceptive Menstrual Cycles. J. Soc. Gynecol. Investig. 2003, 10, 27–31. [Google Scholar] [CrossRef]
- Stewart, D.R.; Celniker, A.C.; Taylor, C.A.; Cragun, J.R.; Lasley, B.L. Relaxin in the Peri-Implantation Period. J. Clin. Endocrinol. Metab. 1990, 70, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Davison, J.; Conrad, K.; Danielson, L. Renal Hemodynamic Effects of Relaxin in Humans. Ann. N. Y. Acad. Sci. 2005, 1041, 163–172. [Google Scholar] [CrossRef]
- Pearson, S.J.; Burgess, K.E.; Onambélé, G.L. Serum Relaxin Levels Affect the in Vivo Properties of Some but Not All Tendons in Normally Menstruating Young Women. Exp. Physiol. 2011, 96, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.R.; Abbas, A.A.; Allman, A.C.J.; Nicolaides, K.H.; Lightman, S.L. The Regulation of Plasma Relaxin Levels during Human Pregnancy. J. Endocrinol. 1994, 142, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Ogueh, O.; Clough, A.; Hancock, M.; Johnson, M.R. A Longitudinal Study of the Control of Renal and Uterine Hemodynamic Changes of Pregnancy. Hypertens. Pregnancy 2011, 30, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Wathen, N.C.; Perry, L.A.; Gunn, L.; Campbell, D.J.; Chard, T. Relaxin Levels in Amniotic Fluid, Extraembryonic Coelomic Fluid and Maternal Serum in Early Human Pregnancy. Early Hum. Dev. 1995, 43, 71–74. [Google Scholar] [CrossRef]
- Lafayette, R.A.; Hladunewich, M.A.; Derby, G.; Blouch, K.; Druzin, M.L.; Myers, B.D. Serum Relaxin Levels and Kidney Function in Late Pregnancy with or without Preeclampsia. Clin. Nephrol. 2011, 75, 226–232. [Google Scholar] [CrossRef]
- Dschietzig, T.; Richter, C.; Bartsch, C.; Laule, M.; Armbruster, F.P.; Baumann, G.; Stangl, K. The Pregnancy Hormone Relaxin Is a Player in Human Heart Failure. FASEB J. 2001, 15, 2187–2195. [Google Scholar] [CrossRef]
- Giordano, N.; Papakostas, P.; Lucani, B.; Amendola, A.; Cipolli, F.; Agate, V.M.; Battisti, E.; Martini, G.; Nuti, R. Serum Relaxin in Systemic Sclerosis. J. Rheumatol. 2005, 32, 2164–2166. [Google Scholar]
- Ivell, R.; Agoulnik, A.I.; Anand-Ivell, R. Relaxin-like Peptides in Male Reproduction—A Human Perspective. Br. J. Pharmacol. 2017, 174, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Nose-Ogura, S.; Yoshino, O.; Yamada-Nomoto, K.; Nakamura, M.; Harada, M.; Dohi, M.; Okuwaki, T.; Osuga, Y.; Kawahara, T.; Saito, S. Oral Contraceptive Therapy Reduces Serum Relaxin-2 in Elite Female Athletes. J. Obstet. Gynaecol. Res. 2017, 43, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Castillo, T.N.; Korotkova, T.A.; Kennedy, A.C.; Kim, H.J.; Stewart, D.R. Trends in Serum Relaxin Concentration among Elite Collegiate Female Athletes. Int. J. Womens. Health 2011, 3, 19–24. [Google Scholar]
- Wreje, U.; Kristiansson, P.; Aberg, H.; Bystrom, B.; Von Schoultz, B. Serum Levels of Relaxin during the Menstrual Cycle and Oral Contraceptive Use. Gynecol. Obstet. Investig. 1995, 39, 197–200. [Google Scholar] [CrossRef]
- Johnson, M.R.; Carter, G.; Grint, C.; Lightman, S.L. Relationship between Ovarian Steroids, Gonadotrophins and Relaxin during the Menstrual Cycle. Acta Endocrinol. 1993, 129, 121–125. [Google Scholar] [CrossRef]
- Bryant-Greenwood, G.D.; Schwabe, C. Human Relaxins: Chemistry and Biology. Endocr. Rev. 1994, 15, 5–26. [Google Scholar]
- Pehrsson, M.; Westberg, L.; Landén, M.; Ekman, A. Stable Serum Levels of Relaxin throughout the Menstrual Cycle: A Preliminary Comparison of Women with Premenstrual Dysphoria and Controls. Arch. Womens. Ment. Health 2007, 10, 147–153. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Tissue Expression of RLN2-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000107014-RLN2/tissue (accessed on 9 April 2022).
- Szepietowska, B.; Gorska, M.; Szelachowska, M. Plasma Relaxin Concentration Is Related to Beta-Cell Function and Insulin Sensitivity in Women with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2008, 79, e1–e3. [Google Scholar] [CrossRef]
- Binder, C.; Simon, A.; Binder, L.; Hagemann, T.; Schulz, M.; Emons, G.; Trümper, L.; Einspanier, A. Elevated Concentrations of Serum Relaxin Are Associated with Metastatic Disease in Breast Cancer Patients. Breast Cancer Res. Treat. 2004, 87, 157–166. [Google Scholar] [CrossRef]
- Ren, P.; Yu, Z.-T.; Xiu, L.; Wang, M.; Liu, H.-M. Elevated Serum Levels of Human Relaxin-2 in Patients with Esophageal Squamous Cell Carcinoma. World J. Gastroenterol. 2013, 19, 2412–2418. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Castillo, T.N.; Braun, H.J.; Ridley, B.A.; Kennedy, A.C.; Golish, S.R. Prospective Correlation between Serum Relaxin Concentration and Anterior Cruciate Ligament Tears among Elite Collegiate Female Athletes. Am. J. Sports Med. 2011, 39, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Cameron, K.L.; Clifton, K.B.; Svoboda, S.J.; Wolf, J.M. Association between Serum Relaxin and Subsequent Shoulder Instability. Orthopedics 2016, 39, e724–e728. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M.; Williams, A.E.; Delaronde, S.; Leger, R.; Clifton, K.B.; King, K.B. Relationship of Serum Relaxin to Generalized and Trapezial-Metacarpal Joint Laxity. J. Hand Surg. Am. 2013, 38, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Garvin, R.; Burns, A. Serum Relaxin Levels in Subjects with Multiple Sclerosis. Ital. J. Anat. Embryol. 2016, 121, 51–59. [Google Scholar]
- Hocher, B.; Ziebig, R.; Krause, R.; Asmus, G.; Neumayer, H.H.; Liefeldt, L.; Stasch, J.P. Relaxin Is an Independent Risk Factor Predicting Death in Male Patients with End-Stage Kidney Disease. Circulation 2004, 109, 2266–2268. [Google Scholar] [CrossRef][Green Version]
- Bryant, G.D.; Sassin, J.F.; Weitzman, E.D.; Kapen, S.; Frantz, A. Relaxin Immunoactivity in Human Plasma during a 24 Hr Period. J. Reprod. Fertil. 1976, 48, 389–392. [Google Scholar] [CrossRef]
- Ma, J.; Niu, M.; Yang, W.; Zang, L.; Xi, Y. Role of Relaxin-2 in Human Primary Osteosarcoma. Cancer Cell Int. 2013, 13, 59. [Google Scholar] [CrossRef]
- Damp, J.; Givertz, M.M.; Semigran, M.; Alharethi, R.; Ewald, G.; Felker, G.M.; Bozkurt, B.; Boehmer, J.; Haythe, J.; Skopicki, H.; et al. Relaxin-2 and Soluble Flt1 Levels in Peripartum Cardiomyopathy. Results of the Multicenter IPAC Study. JACC Hear. Fail. 2016, 4, 380–388. [Google Scholar] [CrossRef]
- Pintalhao, M.; Castro-Chaves, P.; Vasques-Novoa, F.; Gonçalves, F.; Mendonça, L.; Fontes-Carvalho, R.; Lourenço, P.; Almeida, P.; Leite-Moreira, A.; Bettencourt, P. Relaxin Serum Levels in Acute Heart Failure Are Associated with Pulmonary Hypertension and Right Heart Overload. Eur. J. Heart Fail. 2017, 19, 218–225. [Google Scholar] [CrossRef]
- Bani, D. Recombinant Human H2 Relaxin (Serelaxin) as a Cardiovascular Drug: Aiming at the Right Target. Drug Discov. Today 2020, 25, 1239–1244. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.; Ruilope, L.M. Serelaxin for the Treatment of Acute Heart Failure: A Review with a Focus on End-Organ Protection. Eur. Hear. J. Cardiovasc. Pharmacother. 2016, 2, 119–130. [Google Scholar] [CrossRef][Green Version]
- Fisher, C.; Al-Benna, S.; Kirk, A.; Morton, J.J.; McMurray, J.J.V. Transcardiac and Transpulmonary Gradients in the Putative New Cardiovascular Hormone Relaxin. Heart 2003, 89, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Berry, C.; Blue, L.; Morton, J.J.; McMurray, J. N-Terminal pro B Type Natriuretic Peptide, but Not the New Putative Cardiac Hormone Relaxin, Predicts Prognosis in Patients with Chronic Heart Failure. Heart 2003, 89, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Y.; Li, L.; Zhang, S. H2 Relaxin Expression and Its Effect on Clinical Outcomes in Patients with Chronic Heart Failure. Int. J. Clin. Exp. Med. 2015, 8, 4420–4424. [Google Scholar] [PubMed]
- Han, L.; Luo, J.; Bai, S.; Jia, Y.; Chen, X.; Zhao, Y.; Chen, L.; Zhu, X.; Li, Y.; Jiang, Y.; et al. Combined Assessment of Relaxin and B-Type Natriuretic Peptide Improves Diagnostic Value in Patients With Congestive Heart Failure. Am. J. Med. Sci. 2017, 354, 480–485. [Google Scholar] [CrossRef]
- Kupari, M.; Mikkola, T.S.; Turto, H.; Lommi, J. Is the Pregnancy Hormone Relaxin an Important Player in Human Heart Failure? Eur. J. Heart Fail. 2005, 7, 195–198. [Google Scholar] [CrossRef][Green Version]
- Krüger, S.; Graf, J.; Merx, M.W.; Stickel, T.; Kunz, D.; Hanrath, P.; Janssens, U. Relaxin Kinetics during Dynamic Exercise in Patients with Chronic Heart Failure. Eur. J. Intern. Med. 2004, 15, 54–56. [Google Scholar] [CrossRef]
- Heringlake, M.; Kox, T.; Poeling, J.; Klaus, S.; Hanke, T.; Franz, N.; Eberhardt, F.; Heinze, H.; Armbruster, F.P.; Bahlmann, L. The Effects of Physical Exercise on Plasma Levels of Relaxin, NTproANP, and NTproBNP in Patients with Ischemic Heart Disease. Eur. J. Med. Res. 2009, 14, 106–112. [Google Scholar] [CrossRef]
- Mazurek, J.A.; Horne, B.D.; Kelesidis, I.; Salamon, J.N.; Zolty, R. Relaxin Levels in Pulmonary Hypertension: A Comparison between Pulmonary Arterial Hypertension and Diastolic Heart Failure-Induced Pulmonary Hypertension. J. Heart Lung Transplant. 2013, 32, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Emmens, J.E.; Ter Maaten, J.M.; Voors, A.A. Are Circulating Relaxin Levels Related to Pulmonary Hypertension in Patients with Heart Failure? Eur. J. Heart Fail. 2017, 19, 958–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miró, Ò.; Herrero-Puente, P.; Prieto, B.; García-García, M.; García-Hernández, P.; Martín-Sánchez, F.J.; Jacob, J.; Ríos, J.; Romero, R.; Gil, V.; et al. The Subset of Patients with Acute Heart Failure Able to Secrete Relaxin-2 at Pregnancy Concentrations Could Have a Longer Survival: A Pilot Study. Biomarkers 2018, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Shah, S.J.; Gomberg-Maitland, M.; Collander, B.; Vallakati, A.; Shroff, P.; Rich, S. Clinical Characteristics of Pulmonary Hypertension in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Hear. Fail. 2011, 4, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Dschietzig, T.; Richter, C.; Bartsch, C.; Böhme, C.; Heinze, D.; Ott, F.; Zartnack, F.; Baumann, G.; Stangl, K. Flow-Induced Pressure Differentially Regulates Endothelin-1, Urotensin II, Adrenomedullin, and Relaxin in Pulmonary Vascular Endothelium. Biochem. Biophys. Res. Commun. 2001, 289, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; MacLean, M.; Morecroft, I.; Seed, A.; Johnston, F.; Hillier, C.; McMurray, J. Is the Pregnancy Hormone Relaxin Also a Vasodilator Peptide Secreted by the Heart? Circulation 2002, 106, 292–295. [Google Scholar] [CrossRef]
- Shao, D.; Park, J.E.S.; Wort, S.J. The Role of Endothelin-1 in the Pathogenesis of Pulmonary Arterial Hypertension. Pharmacol. Res. 2011, 63, 504–511. [Google Scholar] [CrossRef]
- Jankowich, M.; Choudhary, G. Endothelin-1 Levels and Cardiovascular Events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Beiert, T.; Knappe, V.; Tiyerili, V.; Stöckigt, F.; Effelsberg, V.; Linhart, M.; Steinmetz, M.; Klein, S.; Schierwagen, R.; Trebicka, J.; et al. Chronic Lower-Dose Relaxin Administration Protects from Arrhythmia in Experimental Myocardial Infarction Due to Anti-Inflammatory and Anti-Fibrotic Properties. Int. J. Cardiol. 2018, 250, 21–28. [Google Scholar] [CrossRef]
- Parikh, A.; Patel, D.; McTiernan, C.F.; Xiang, W.; Haney, J.; Yang, L.; Lin, B.; Kaplan, A.D.; Bett, G.C.L.; Rasmusson, R.L.; et al. Relaxin Suppresses Atrial Fibrillation by Reversing Fibrosis and Myocyte Hypertrophy and Increasing Conduction Velocity and Sodium Current in Spontaneously Hypertensive Rat Hearts. Circ. Res. 2013, 113, 313–321. [Google Scholar] [CrossRef]
- Henry, B.L.; Gabris, B.; Li, Q.; Martin, B.; Giannini, M.; Parikh, A.; Patel, D.; Haney, J.; Schwartzman, D.S.; Shroff, S.G.; et al. Relaxin Suppresses Atrial Fibrillation in Aged Rats by Reversing Fibrosis and Upregulating Na+ Channels. Hear. Rhythm 2016, 13, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Piedras-Rentería, E.S.; Sherwood, O.D.; Best, P.M. Effects of Relaxin on Rat Atrial Myocytes. I. Inhibition of I(to) via PKA-Dependent Phosphorylation. Am. J. Physiol. 1997, 272, H1791–H1797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mas, J.; Lax, A.; Asensio-Lopez, M.C.; Lencina, M.; Fernandez-del Palacio, M.J.; Soriano-Filiu, A.; de Boer, R.A.; Pascual-Figal, D.A. Early Anti-Inflammatory and Pro-Angiogenic Myocardial Effects of Intravenous Serelaxin Infusion for 72 H in an Experimental Rat Model of Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Gabris-Weber, B.A.; Reddy, R.; Romero, G.; Chattopadhyay, A.; Salama, G. Relaxin Reverses Inflammatory and Immune Signals in Aged Hearts. PLoS ONE 2018, 13, e0190935. [Google Scholar] [CrossRef]
- Nistri, S.; Fiorillo, C.; Becatti, M.; Bani, D. Human Relaxin-2 (Serelaxin) Attenuates Oxidative Stress in Cardiac Muscle Cells Exposed In Vitro to Hypoxia-Reoxygenation. Evidence for the Involvement of Reduced Glutathione Up-Regulation. Antioxidants 2020, 9, 774. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, X.; Gao, Z.; Zheng, G.; Lin, J.; Su, L.; Huang, Z.; Li, H.; Huang, W. Relaxin Level in Patients With Atrial Fibrillation and Association with Heart Failure Occurrence. Medicine 2016, 95, e3664. [Google Scholar] [CrossRef]
- Qu, X.; Chen, L.; Sun, L.; Chen, C.; Gao, Z.; Huang, W.; Zhou, H. Serum Relaxin Level Predicts Recurrence of Atrial Fibrillation after Radiofrequency Catheter Ablation. Heart Vessels 2019, 34, 1543–1551. [Google Scholar] [CrossRef]
- Masini, E.; Bani, D.; Bello, M.G.; Bigazzi, M.; Mannaioni, P.F.; Sacchi, T.B. Relaxin Counteracts Myocardial Damage Induced by Ischemia-Reperfusion in Isolated Guinea Pig Hearts: Evidence for an Involvement of Nitric Oxide. Endocrinology 1997, 138, 4713–4720. [Google Scholar] [CrossRef]
- Bani, D.; Masini, E.; Bello, M.G.; Bigazzi, M.; Bani Sacchi, T. Relaxin Protects against Myocardial Injury Caused by Ischemia and Reperfusion in Rat Heart. Am. J. Pathol. 1998, 152, 1367–1376. [Google Scholar]
- Perna, A.-M.; Masini, E.; Nistri, S.; Bani Sacchi, T.; Bigazzi, M.; Bani, D. Human Recombinant Relaxin Reduces Heart Injury and Improves Ventricular Performance in a Swine Model of Acute Myocardial Infarction. Ann. N. Y. Acad. Sci. 2005, 1041, 431–433. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.F.; Geng, B.; Pan, C.S.; Zhao, J.; Chen, L.; Yang, J.; Chang, J.K.; Tang, C.S. Effect of Relaxin on Myocardial Ischemia Injury Induced by Isoproterenol. Peptides 2005, 26, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Clark, C.L. Evidence for a Novel Source of Relaxin: Atrial Cardiocytes. J. Endocrinol. 1994, 143, R5–R8. [Google Scholar] [CrossRef] [PubMed]
- Osheroff, P.L.; Ho, W.H. Expression of Relaxin MRNA and Relaxin Receptors in Postnatal and Adult Rat Brains and Hearts. Localization and Developmental Patterns. J. Biol. Chem. 1993, 268, 15193–15199. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Nakabayashi, K.; Nishi, S.; Kumagai, J.; Kudo, M.; Sherwood, O.D.; Hsueh, A.J.W. Activation of Orphan Receptors by the Hormone Relaxin. Science 2002, 295, 671–674. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Grodstein, F.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Trends in the Incidence of Coronary Heart Disease and Changes in Diet and Lifestyle in Women. N. Engl. J. Med. 2000, 343, 530–537. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Yu, S.; Niu, H.; Gong, X.; Miao, X. Serum Relaxin Levels as a Novel Biomarker for Detection of Acute Myocardial Infarction. Int. J. Clin. Exp. Med. 2015, 8, 16937–16940. [Google Scholar]
- Kapelouzou, A.; Tsourelis, L.; Kaklamanis, L.; Degiannis, D.; Kogerakis, N.; Cokkinos, D.V. Serum and Tissue Biomarkers in Aortic Stenosis. Glob. Cardiol. Sci. Pract. 2015, 2015, 49. [Google Scholar] [CrossRef]
- Gedikli, O.; Yilmaz, H.; Kiris, A.; Karaman, K.; Ozturk, S.; Baykan, M.; Ucar, U.; Durmus, I.; Karahan, C.; Celik, S. Circulating Levels of Relaxin and Its Relation to Cardiovascular Function in Patients with Hypertension. Blood Press. 2009, 18, 68–73. [Google Scholar] [CrossRef]
- Sanidas, E.; Tsakalis, K.; Papadopoulos, D.P.; Zerva, K.; Velliou, M.; Perrea, D.; Mantzourani, M.; Iliopoulos, D.; Barbetseas, J. The Impact of Apelin and Relaxin Plasma Levels in Masked Hypertension and White Coat Hypertension. J. Clin. Hypertens. 2019, 21, 48–52. [Google Scholar] [CrossRef]
- Papadopoulos, D.P.; Makris, T.; Perrea, D.; Zerva, K.; Tsioufis, C.; Faselis, C.; Papademetriou, V. Apelin and Relaxin Plasma Levels in Young Healthy Offspring of Patients with Essential Hypertension. J. Clin. Hypertens. 2014, 16, 198–201. [Google Scholar] [CrossRef]
- Papadopoulos, D.P.; Mourouzis, I.; Faselis, C.; Perrea, D.; Makris, T.; Tsioufis, C.; Papademetriou, V. Masked Hypertension and Atherogenesis: The Impact of Apelin and Relaxin Plasma Levels. J. Clin. Hypertens. 2013, 15, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Kapelouzou, A.; Tsilimigras, D.I.; Patelis, N.; Kouvelos, G.; Schizas, D.; Karavokyros, I.; Georgopoulos, S. Associations between Serum Relaxin 2, Aneurysm Formation/Size and Severity of Atherosclerosis: A Preliminary Prospective Analysis Article. Acta Pharmacol. Sin. 2018, 39, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Cinci, L.; Perna, A.M.; Masini, E.; Mastroianni, R.; Bani, D. Relaxin Induces Mast Cell Inhibition and Reduces Ventricular Arrhythmias in a Swine Model of Acute Myocardial Infarction. Pharmacol. Res. 2008, 57, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Nistri, S.; Nanni, C.; Gelsomino, S.; Pini, A.; Cinci, L.; Maiani, M.; Zecchi-Orlandini, S.; Lorusso, R.; Fanti, S.; et al. Functional and Histopathological Improvement of the Post-Infarcted Rat Heart upon Myoblast Cell Grafting and Relaxin Therapy. J. Cell. Mol. Med. 2009, 13, 3437–3448. [Google Scholar] [CrossRef]
- Samuel, C.S.; Cendrawan, S.; Gao, X.M.; Ming, Z.; Zhao, C.; Kiriazis, H.; Xu, Q.; Tregear, G.W.; Bathgate, R.A.D.; Du, X.J. Relaxin Remodels Fibrotic Healing Following Myocardial Infarction. Lab. Investig. 2011, 91, 675–690. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, H.; Yang, Q.; Sun, Y. Effects of Relaxin on Cardiac Fibrosis, Apoptosis, and Tachyarrhythmia in Rats with Myocardial Infarction. Biomed. Pharmacother. 2016, 84, 348–355. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Nistri, S.; Chiappini, L.; Sassoli, C.; Bani, D. Relaxin Inhibits Lipopolysaccharide-Induced Adhesion of Neutrophils to Coronary Endothelial Cells by a Nitric Oxide-Mediated Mechanism. FASEB J. 2003, 17, 2109–2111. [Google Scholar] [CrossRef]
- Masini, E.; Nistri, S.; Vannacci, A.; Bani Sacchi, T.; Novelli, A.; Bani, D. Relaxin Inhibits the Activation of Human Neutrophils: Involvement of the Nitric Oxide Pathway. Endocrinology 2004, 145, 1106–1112. [Google Scholar] [CrossRef]
- Brecht, A.; Bartsch, C.; Baumann, G.; Stangl, K.; Dschietzig, T. Relaxin Inhibits Early Steps in Vascular Inflammation. Regul. Pept. 2011, 166, 76–82. [Google Scholar] [CrossRef]
- Kraler, S.; Blaser, M.C.; Aikawa, E.; Camici, G.G.; Lüscher, T.F. Calcific Aortic Valve Disease: From Molecular and Cellular Mechanisms to Medical Therapy. Eur. Heart J. 2022, 43, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Pioli, M.R.; de Faria, A.P. Pro-Inflammatory Cytokines and Resistant Hypertension: Potential for Novel Treatments? Curr. Hypertens. Rep. 2019, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, A.E.; Spyropoulos, F.; Michael, Z.; Joung, K.E.; Briana, D.D.; Malamitsi-Puchner, A.; Mantzoros, C.S.; Christou, H. Adipokines and Metabolic Regulators in Human and Experimental Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Lekgabe, E.D.; Kiriazis, H.; Zhao, C.; Xu, Q.; Moore, X.L.; Su, Y.; Bathgate, R.A.D.; Du, X.J.; Samuel, C.S. Relaxin Reverses Cardiac and Renal Fibrosis in Spontaneously Hypertensive Rats. Hypertension 2005, 46, 412–418. [Google Scholar] [CrossRef]
- Conrad, K.P. Unveiling the Vasodilatory Actions and Mechanisms of Relaxin. Hypertension 2010, 56, 2–9. [Google Scholar] [CrossRef]
- Xu, Q.; Chakravorty, A.; Bathgate, R.A.D.; Dart, A.M.; Du, X.J. Relaxin Therapy Reverses Large Artery Remodeling and Improves Arterial Compliance in Senescent Spontaneously Hypertensive Rats. Hypertension 2010, 55, 1260–1266. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Sasser, J.M.; Cunningham, M.W.; Baylis, C. Serelaxin Reduces Oxidative Stress and Asymmetric Dimethylarginine in Angiotensin II-Induced Hypertension. Am. J. Physiol.-Ren. Physiol. 2014, 307, F1355–F1362. [Google Scholar] [CrossRef]
- Wang, D.; Luo, Y.; Myakala, K.; Orlicky, D.J.; Dobrinskikh, E.; Wang, X.; Levi, M. Serelaxin Improves Cardiac and Renal Function in DOCA-Salt Hypertensive Rats. Sci. Rep. 2017, 7, 9793. [Google Scholar] [CrossRef]
- Tientcheu, D.; Ayers, C.; Das, S.R.; McGuire, D.K.; De Lemos, J.A.; Khera, A.; Kaplan, N.; Victor, R.; Vongpatanasin, W. Target Organ Complications and Cardiovascular Events Associated with Masked Hypertension and White-Coat Hypertension: Analysis from the Dallas Heart Study. J. Am. Coll. Cardiol. 2015, 66, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Lagou, S.; Zeniodi, M.E.; Boubouchairopoulou, N.; Stergiou, G.S. Association of Central Versus Brachial Blood Pressure With Target-Organ Damage: Systematic Review and Meta-Analysis. Hypertension 2016, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Christou, H.; Khalil, R.A. Mechanisms of Pulmonary Vascular Dysfunction in Pulmonary Hypertension and Implications for Novel Therapies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H702–H724. [Google Scholar] [CrossRef]
- Ponikowski, P.; Mitrovic, V.; Ruda, M.; Fernandez, A.; Voors, A.A.; Vishnevsky, A.; Cotter, G.; Milo, O.; Laessing, U.; Zhang, Y.; et al. A Randomized, Double-Blind, Placebo-Controlled, Multicentre Study to Assess Haemodynamic Effects of Serelaxin in Patients with Acute Heart Failure. Eur. Heart J. 2014, 35, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A.; Novak, J.; Danielson, L.A.; Kerchner, L.J.; Opett, S.L.; Conrad, K.P. Essential Role for Vascular Gelatinase Activity in Relaxin-Induced Renal Vasodilation, Hyperfiltration, and Reduced Myogenic Reactivity of Small Arteries. Circ. Res. 2003, 93, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A.; Kerchner, L.J.; Fisher, M.C.; McGuane, J.T.; Doty, K.D.; Conrad, K.P. Matrix Metalloproteinase-2 Activity, Protein, MRNA, and Tissue Inhibitors in Small Arteries from Pregnant and Relaxin-Treated Nonpregnant Rats. J. Appl. Physiol. 2006, 100, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A.; Novak, J.; Doty, K.D.; Matthews, J.; Fisher, M.C.; Kerchner, L.J.; Conrad, K.P. Vascular Matrix Metalloproteinase-9 Mediates the Inhibition of Myogenic Reactivity in Small Arteries Isolated from Rats after Short-Term Administration of Relaxin. Endocrinology 2007, 148, 189–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix Metalloproteinases Promote Arterial Remodeling in Aging, Hypertension, and Atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E.N.; Lewis, M.; Constant, J.; Arnold, G.; Grove, B.H.; Normand, J.; Deshpande, U.; Salles, A.; Pickford, L.B.; Erikson, M.E.; et al. Relaxin Induces Vascular Endothelial Growth Factor Expression and Angiogenesis Selectively at Wound Sites. Wound Repair Regen. 2000, 8, 361–370. [Google Scholar] [CrossRef]
- Bitto, A.; Irrera, N.; Minutoli, L.; Caló, M.; Cascio, P.L.; Caccia, P.; Pizzino, G.; Pallio, G.; Micali, A.; Vaccaro, M.; et al. Relaxin Improves Multiple Markers of Wound Healing and Ameliorates the Disturbed Healing Pattern of Genetically Diabetic Mice. Clin. Sci. 2013, 125, 575–585. [Google Scholar] [CrossRef]
- Debrah, D.O.; Conrad, K.P.; Jeyabalan, A.; Danielson, L.A.; Shroff, S.G. Relaxin Increases Cardiac Output and Reduces Systemic Arterial Load in Hypertensive Rats. Hypertension 2005, 46, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Debrah, D.O.; Novak, J.; Matthews, J.E.; Ramirez, R.J.; Shroff, S.G.; Conrad, K.P. Relaxin Is Essential for Systemic Vasodilation and Increased Global Arterial Compliance during Early Pregnancy in Conscious Rats. Endocrinology 2006, 147, 5126–5131. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Leo, C.H.; Girling, J.E.; Tare, M.; Beard, S.; Hannan, N.J.; Parry, L.J. Relaxin Treatment Reduces Angiotensin II-Induced Vasoconstriction in Pregnancy and Protects against Endothelial Dysfunction. Biol. Reprod. 2017, 96, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Tiyerili, V.; Beiert, T.; Schatten, H.; Camara, B.; Jehle, J.; Schrickel, J.W.; Nickenig, G.; Andrié, R.P. Anti-Atherosclerotic Effects of Serelaxin in Apolipoprotein E-Deficient Mice. Atherosclerosis 2016, 251, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory Cytokines in Atherosclerosis: Current Therapeutic Approaches. Eur. Heart J. 2016, 37, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Benderly, M.; Esper, R.J.; Behar, S.; Boyko, V.; Adler, Y.; Tanne, D.; Matas, Z.; Tenenbaum, A. Interleukin-6 and the Risk of Future Cardiovascular Events in Patients With Angina Pectoris and/or Healed Myocardial Infarction. Am. J. Cardiol. 2006, 98, 14–18. [Google Scholar] [CrossRef]
- Nijm, J.; Wikby, A.; Tompa, A.; Olsson, A.G.; Jonasson, L. Circulating Levels of Proinflammatory Cytokines and Neutrophil-Platelet Aggregates in Patients with Coronary Artery Disease. Am. J. Cardiol. 2005, 95, 452–456. [Google Scholar] [CrossRef]
- Papoutsis, K.; Kapelouzou, A.; Georgiopoulos, G.; Kontogiannis, C.; Kourek, C.; Mylonas, K.S.; Patelis, N.; Cokkinos, D.V.; Karavokyros, I.; Georgopoulos, S. Tissue-Specific Relaxin-2 Is Differentially Associated with the Presence/Size of an Arterial Aneurysm and the Severity of Atherosclerotic Disease in Humans. Acta Pharmacol. Sin. 2020, 41, 745–752. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Koroleva, E.A.; Khapaev, R.S.; Korbut, A.I.; Lykov, A.P. Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers. J. Clin. Med. 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, M.; Sparwasser, A.; Funk, E.; Köhrle, J.; Bergmann, A. Quantification of Relaxin-2 Connecting Peptide (Pro-RLX2) in Human Blood Samples. J. Appl. Lab. Med. 2017, 2, 322–334. [Google Scholar] [CrossRef]
- Martínez Solano, J.; Santos Mateo, J.J.; Sánchez-Más, J.; Sánchez, J.; Asensio López, M.C.; Pascual Figal, D. Relaxin Concentrations in Acute Heart Failure Patients: Kinetics and Clinical Determinants. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 1230–1232. [Google Scholar] [CrossRef]
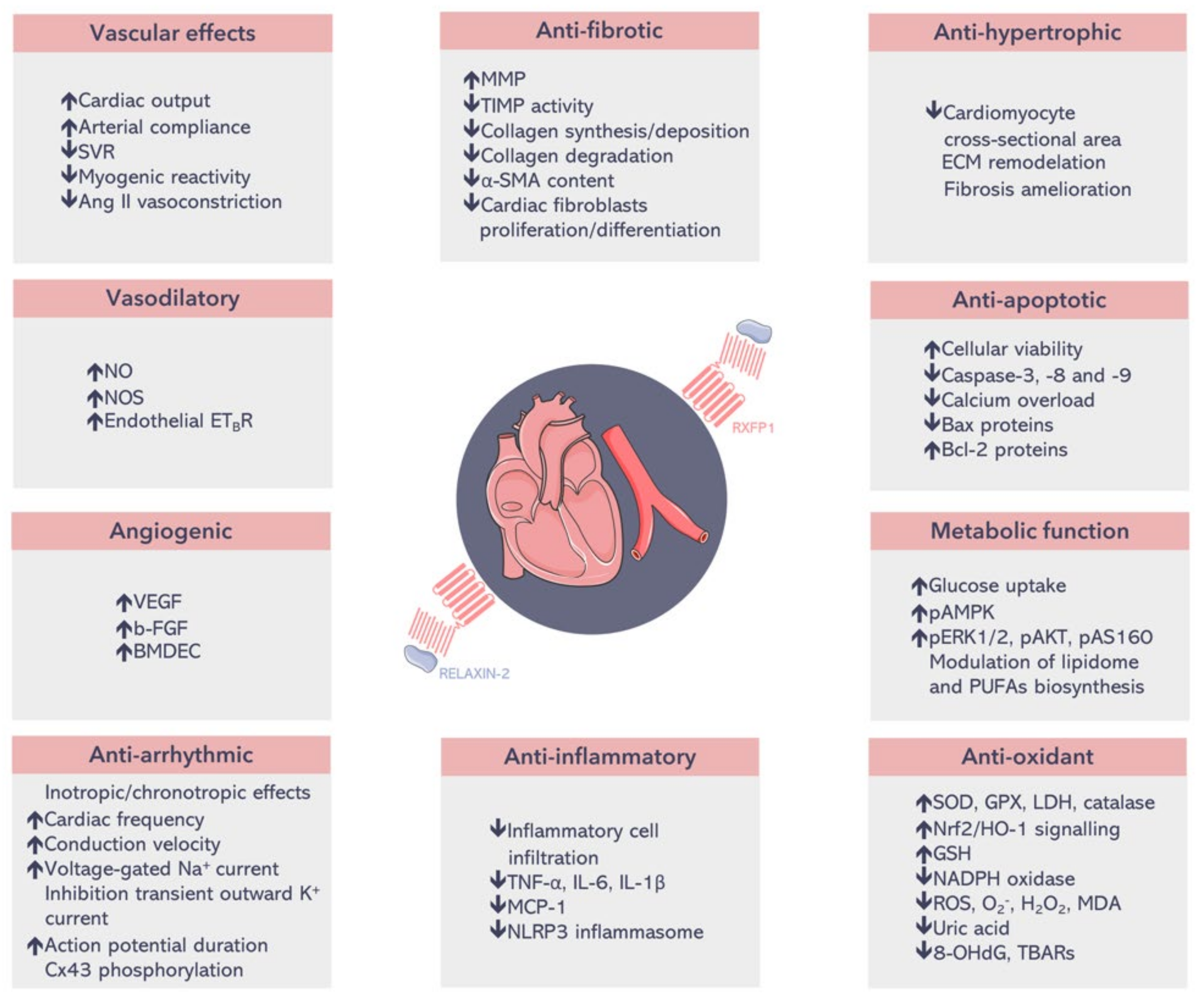
| Heart Failure | ||||||
|---|---|---|---|---|---|---|
| Condition | Patients (n) | Controls (n) | Relaxin-2 Levels in Controls | Relaxin-2 Levels in Patients | Main Results | Reference |
| CHF | Severe CHF-NHYA class IV (9♂:5♀) | Subjects undergoing cardiac catheterization and with no structural CVD (9♂:4♀) | <5 pg/mL | 18.9 ± 3.4 pg/mL | ↑ Relaxin-2 plasma levels in patients ↑ Relaxin-2 synthesis by failing myocardium | [70] |
| Moderate CHF-NHYA class II (9♂:4♀) | 7.9 ± 1.2 pg/mL | |||||
| CHF | Chronic HFrEF (51♂:36♀) | No controls | <2 pg/mL considered normal | 89 pg/mL | ↑ Relaxin-2 levels when compared with controls No relation between relaxin-2 and outcome | [97] |
| CHF | Chronic HFrEF-NYHA classes II to III (35) | Healthy subjects (10) | Rest: 19 ± 13 pg/mL | Rest: 72 ± 177 pg/mL | Relaxin-2 plasma levels did not discriminate between patients with and without CHF | [101] |
| Peak exercise: 31 ± 31 pg/mL | Peak exercise: 59 ± 125 pg/mL | |||||
| AVS and HF | AVS free of HF (88) | Subjects undergoing invasive electrophysiologic study (6♂:5♀) | 42 pg/mL | 32 pg/mL | No changes in relaxin-2 plasma levels between groups Relaxin-2 secretion by the failing and extraction by the non-failing myocardium | [100] |
| AVS with HF (41) | 28 pg/mL | |||||
| AVS with HFpEF (25) | 40 pg/mL | |||||
| AVS with HFrEF (16) | 18 pg/mL | |||||
| IHD and HF | HF secondary to IHD (35♂:5♀) | No controls | - | 20 pg/mL | ↓ Relaxin-2 levels during recovery and positively correlated with cardiac output and inversely correlated with natriuretic peptides | [102] |
| HFpEF and PAH | HFpEF (14) | Subjects free of significant cardiovascular or systemic disease (18) | 13.4 pg/mL * | 12.9 pg/mL * | ↑ Relaxin-2 levels in patients with PAH | [103] |
| HFpEF-PAH (33) | 12.8 pg/mL * | |||||
| PAH (31) | 46.6 pg/mL * | |||||
| CHF | CHF-NYHA classes I to IV (81♂:34♀) | Patients without CHF (20♂:11♀) | 0.390 pg/mL | 0.593 pg/mL | ↑ Relaxin-2 plasma levels in patients Relaxin-2 can predict severe cardiovascular events in CHF within 180 days after discharge | [98] |
| PPCM | First quartile (0–11 days) (25♀) | No controls | - | 97 ± 203 pg/mL * | ↑ Relaxin-2 levels are associated with faster myocardial recovery | [90] |
| Second quartile (12–24 days) (27♀) | 10 ± 31 pg/mL * | |||||
| Third quartile (25–51 days) (25♀) | 4 ± 2 pg/mL * | |||||
| Fourth quartile (52–95 days) (23♀) | 5 ± 7 pg/mL * | |||||
| CHF | 81 decompensated CHF (41♂:40♀) | 36 (16♂:20♀) | 36.7 pg/mL | 67.1 pg/mL | ↑ Relaxin-2 levels in patients Nonlinear correlation between relaxin-2 and NYHA cardiac function | [99] |
| HFpEF and PH | HFpEF and PH (15♂:36♀) | No controls | - | 82.3 pg/mL | No correlation between relaxin-2 levels and PAP and right heart overload | [104] |
| AHF | AHF-HFrEF (74) | No controls | - | 33.0 pg/mL * | Relaxin-2 levels are associated with right heart overload and pulmonary hypertension | [91] |
| AHF-HFpEF (43) | 28.5 pg/mL * |
| Atrial Fibrillation | ||||||
|---|---|---|---|---|---|---|
| Condition | Patients (n) | Controls (n) | Relaxin-2 Levels in Controls | Relaxin-2 Levels in Patients | Main Results | Reference |
| AF | Paroxysmal AF (46♂:34♀) | Patients with sinus rhythm (75♂:41♀) | 170.21 ± 85.45 ng/L * | 244.95 ± 83.55 ng/L * | ↑ Relaxin-2 levels with the development of AF Relaxin-2 is associated with fibrosis-related biomarkers | [118] |
| Persistent AF (73♂:42♀) | 269.47 ± 77.24 ng/L * | |||||
| AF | Patients with AF recurrence (33♂:20♀) | No controls | - | 382.21 ± 149.89 ng/L * | ↑ Relaxin-2 level in patients with than without AF recurrence | [119] |
| Patients without AF recurrence (124♂:71♀) | 275.42 ± 108.70 ng/L * | |||||
| Paroxysmal AF (83♂:44♀) | 293.51 ± 124.06 ng/L * | |||||
| Persistent AF (76♂:45♀) | 303.21 ± 128.80 ng/L * | |||||
| Ischemic Heart Disease | ||||||
|---|---|---|---|---|---|---|
| Condition | Patients (n) | Controls (n) | Relaxin-2 Levels in Controls | Relaxin-2 Levels in Patients | Main Results | Reference |
| IHD with HF | IHD with high degree and low degree of HF (35♂:5♀) | No controls | - | 20 pg/mL | ↓ Relaxin-2 levels in patients with IHD than patients with preserved myocardial function | [102] |
| Myocardial Infarction | ||||||
| AMI | Patients who presented AMI for the first time (63♂:17♀) | Healthy subjects (61♂:19♀) | 9.2 ± 2.3 ng/mL * | 27.4 ± 6.3 ng/mL * | ↑ Relaxin-2 levels in AMI patients compared with controls | [128] |
| Aortic Valve Disease | ||||||
| CAVS | Patients scheduled to undergo surgery for severe CAVS (60) | Healthy subjects (20) | 0.5 ± 0.08 ng/mL * | 0.02 ± 0.005 ng/mL * | ↓ Relaxin-2 levels in patients with CAVS compared with controls Relaxin-2 levels are correlated with several calcification markers | [129] |
| Hypertension | ||||||
| HT | Never treated patients with HT (40) | Normotensive individuals (42) | 49.7 ± 39.8 pg/mL * | 36.5 ± 7.3 pg/mL * | ↓ Relaxin-2 levels in HT compared with controls | [130] |
| Masked HT and white coat HT | Masked HT | No controls | - | 35.2 ± 6.7 pg/mL | ↓ Relaxin-2 levels in patients with masked HT | [131] |
| White coat HT | 46.8 ± 23.6 pg/mL | |||||
| Arterial aneurysm (16♂) | 49.39 ± 8.62 pg/mL * | |||||
| Patients undergoing TAB (4♂:2♀) | 15.86 ± 4.29 pg/mL * | |||||
| Healthy offspring of patients with essential HT | Healthy offspring of HT patients (24♂:22♀) | Healthy offspring of healthy parents (28♂:22♀) | 10 ± 5 pg/mL | 6 ± 3 pg/mL | ↓ Relaxin-2 levels in healthy offspring of HT parents | [132] |
| Masked HT | Patients with masked HT (8♂:16♀) | Healthy normotensives (52♂:54♀) | 56.8 ± 13.6 pg/mL | 35.2 ± 6.7 pg/mL | ↓ Relaxin-2 levels in masked HT compared with controls | [133] |
| PAH and HFpEF | PAH (31) | Subjects free of significant cardiovascular or systemic disease (18) | 13.4 pg/mL * | 46.6 pg/mL * | ↑ Relaxin-2 levels in PAH compared with healthy controls Relaxin-2 positively associated with PVR and RV dysfunction | [103] |
| HFpEF-PAH (33) | 12.8 pg/mL * | |||||
| HFpEF (14) | 12.9 pg/mL * | |||||
| Atherosclerosis | ||||||
| ATH and arterial aneurysm | ATH patients (16♂:5♀) | Healthy subjects (7♂:3♀) | 10.32 ± 1.35 pg/mL * | 16.22 ± 4.70 pg/mL * | ↑ Relaxin-2 levels in arterial aneurysm patients ↑ Relaxin-2 levels at early stages of ATH | [134] |
| Arterial aneurysm (16♂) | 49.39 ± 8.62 pg/mL * | |||||
| Patients undergoing TAB (4♂:2♀) | 15.86 ± 4.29 pg/mL * | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Herrera, A.; Feijóo-Bandín, S.; Anido-Varela, L.; Moraña-Fernández, S.; Roselló-Lletí, E.; Portolés, M.; Tarazón, E.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases. J. Pers. Med. 2022, 12, 1021. https://doi.org/10.3390/jpm12071021
Aragón-Herrera A, Feijóo-Bandín S, Anido-Varela L, Moraña-Fernández S, Roselló-Lletí E, Portolés M, Tarazón E, Gualillo O, González-Juanatey JR, Lago F. Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases. Journal of Personalized Medicine. 2022; 12(7):1021. https://doi.org/10.3390/jpm12071021
Chicago/Turabian StyleAragón-Herrera, Alana, Sandra Feijóo-Bandín, Laura Anido-Varela, Sandra Moraña-Fernández, Esther Roselló-Lletí, Manuel Portolés, Estefanía Tarazón, Oreste Gualillo, José Ramón González-Juanatey, and Francisca Lago. 2022. "Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases" Journal of Personalized Medicine 12, no. 7: 1021. https://doi.org/10.3390/jpm12071021
APA StyleAragón-Herrera, A., Feijóo-Bandín, S., Anido-Varela, L., Moraña-Fernández, S., Roselló-Lletí, E., Portolés, M., Tarazón, E., Gualillo, O., González-Juanatey, J. R., & Lago, F. (2022). Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases. Journal of Personalized Medicine, 12(7), 1021. https://doi.org/10.3390/jpm12071021







