Evaluation of a Proportional–Integral–Derivative Controller for Hemorrhage Resuscitation Using a Hardware-in-Loop Test Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. PID Controller Design and Tuning
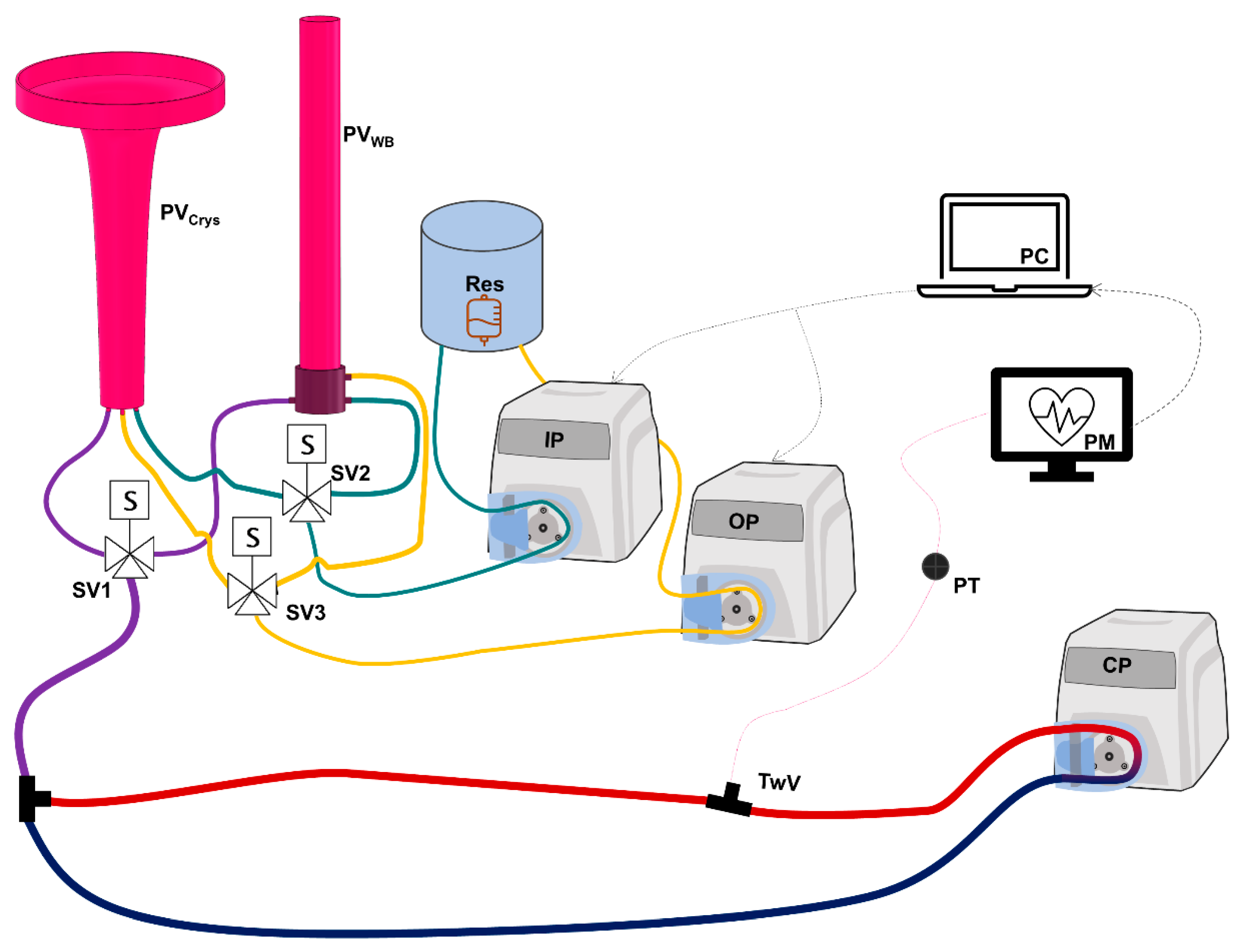
2.2. Hardware-in-Loop Automated Testbed for Resuscitation Controllers
2.2.1. Overview of HATRC Outflow Logic
2.2.2. Determination of Hemostasis Factors for Use with HATRC
2.3. Hemorrhage Testing Scenarios: Overview and Rationale
2.3.1. Compressible Hemorrhage Scenarios
2.3.2. Non-Compressible Hemorrhage Scenarios
2.3.3. Infusate Swapover Scenario
2.3.4. Coagulopathy Scenarios
2.4. Control Systems Performance Evaluation
3. Results
3.1. Compressible Hemorrhage Scenarios
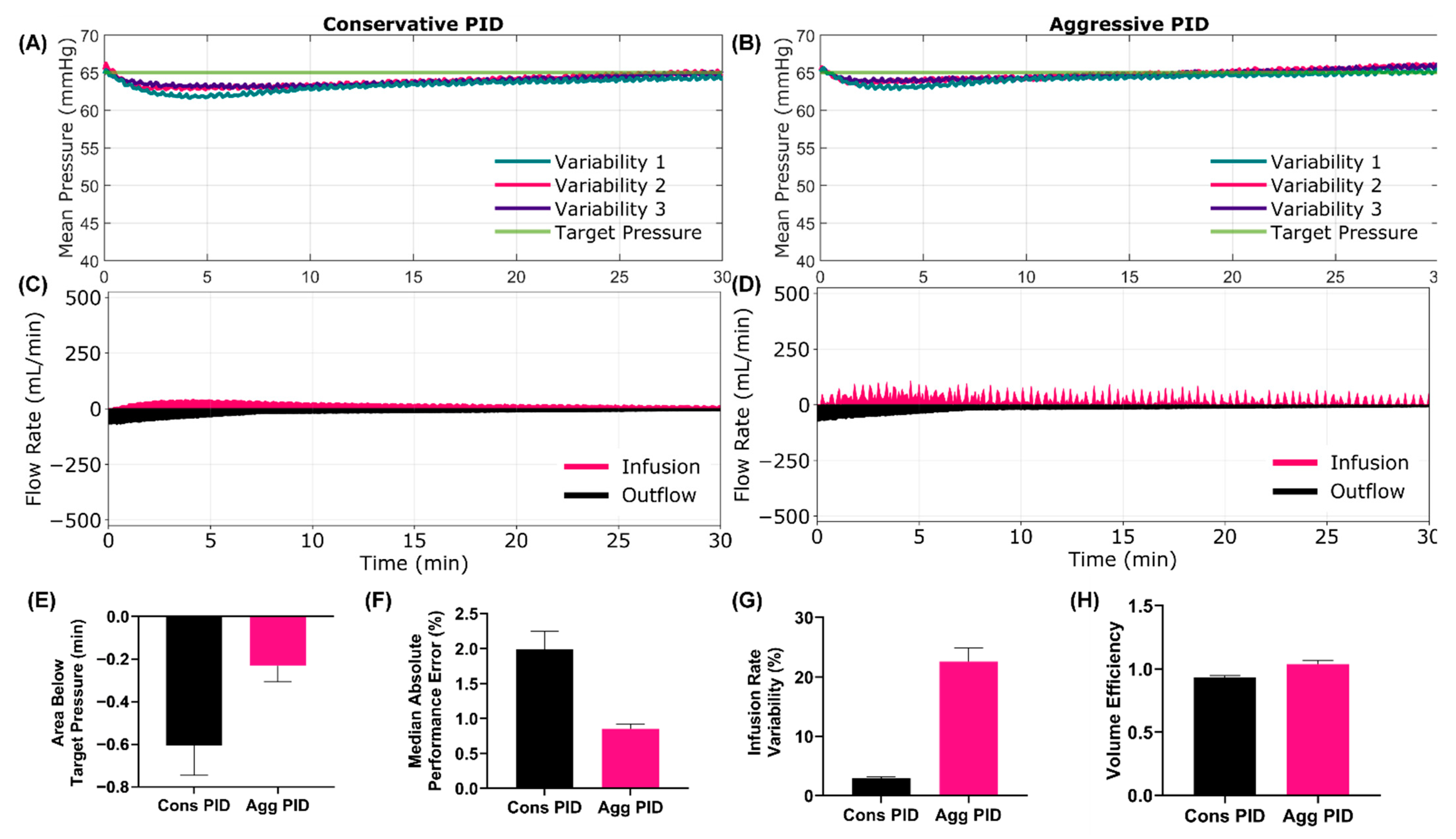
3.1.1. Scenario 1 Results
3.1.2. Scenario 2 Results
3.2. Non-Compressible, Internal Hemorrhage Scenarios
3.2.1. Scenario 3 Results
3.2.2. Scenario 4 Results
3.2.3. Scenario 5 Results
3.2.4. Scenario 6 Results
3.2.5. Scenario 7 Results
3.2.6. Scenario 8 Results
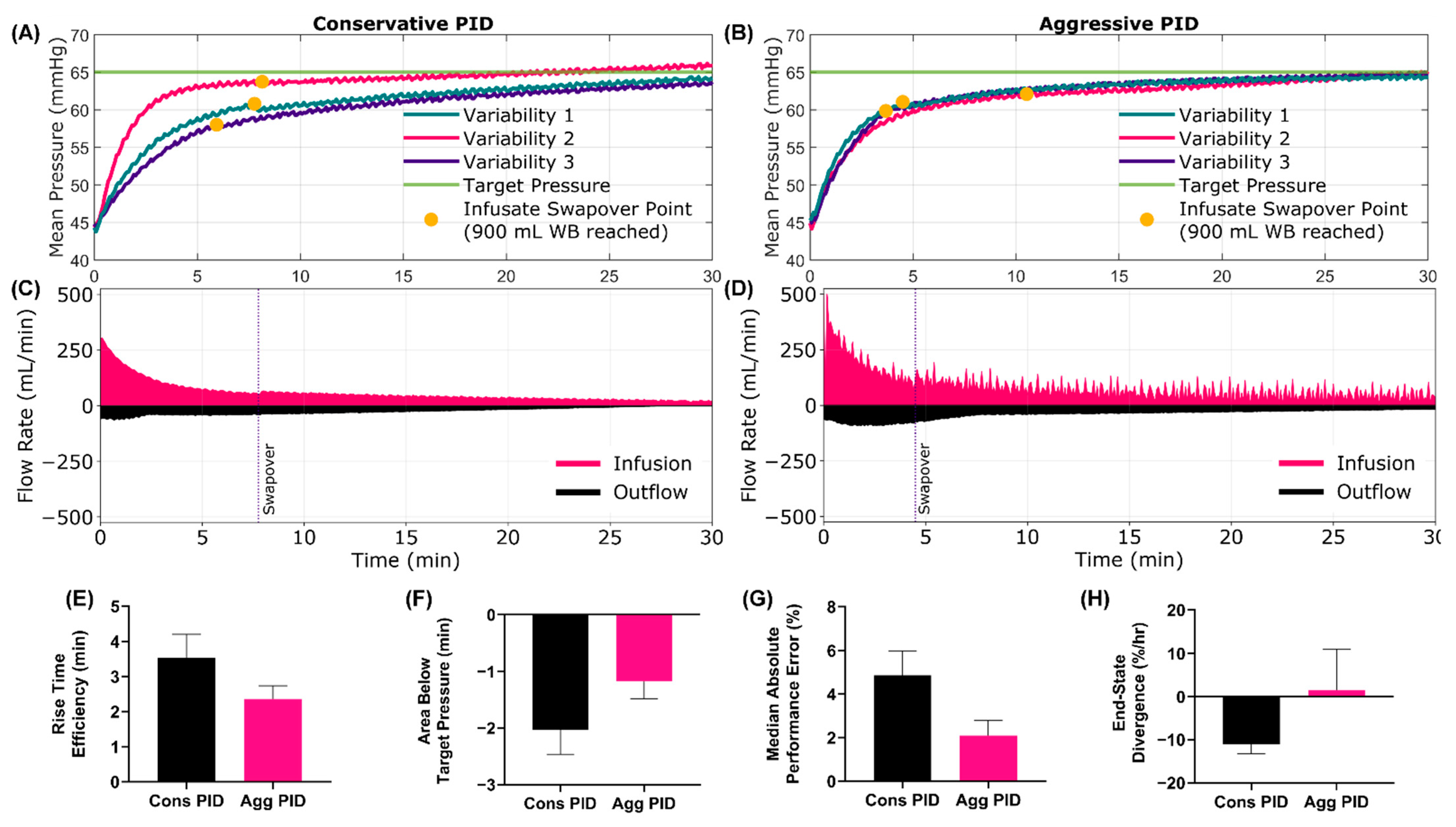
3.3. Swapover Scenario 9 Results
3.4. Coagulopathy Scenarios
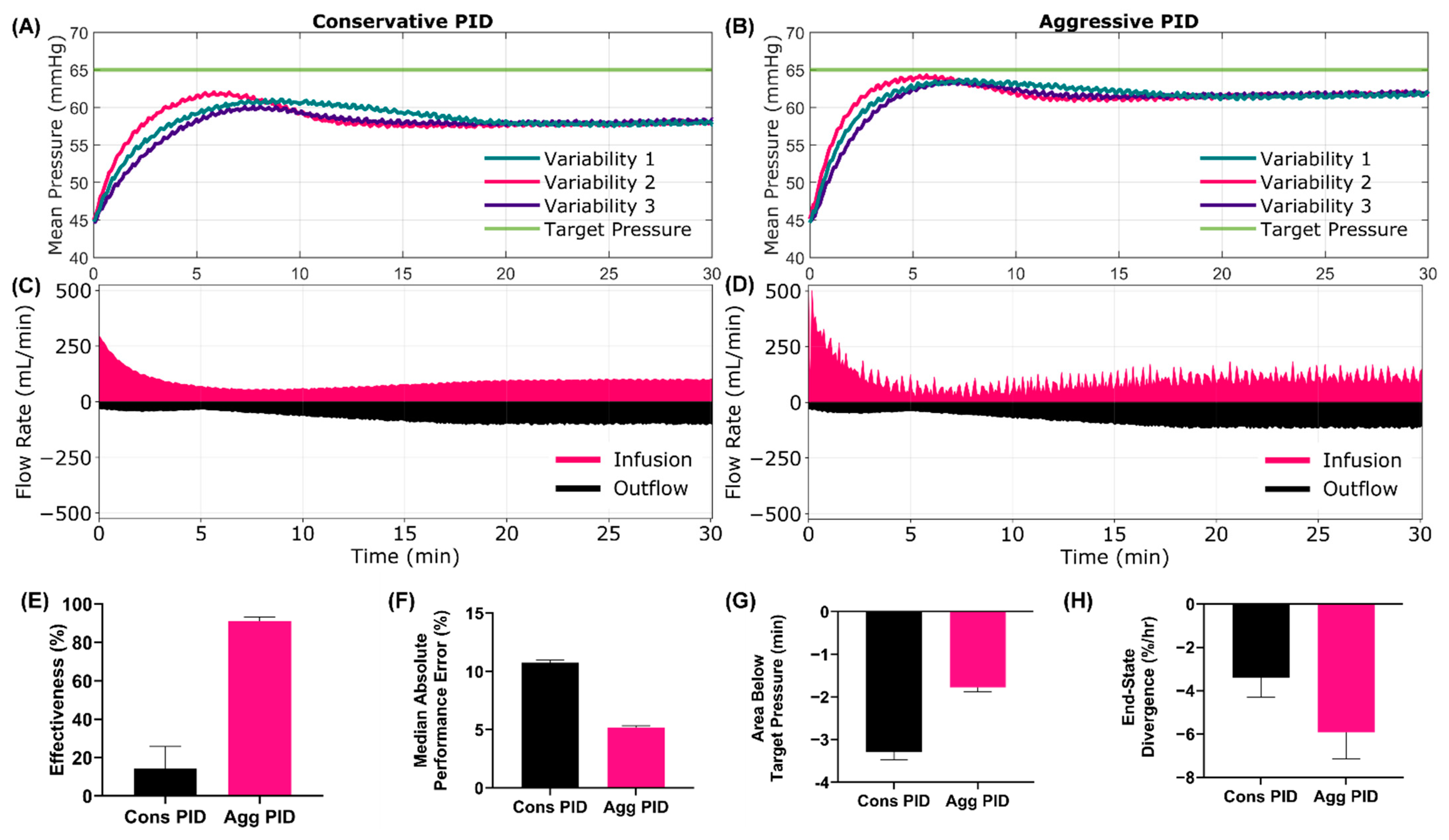
3.4.1. Scenario 10 Results
3.4.2. Scenario 11 Results
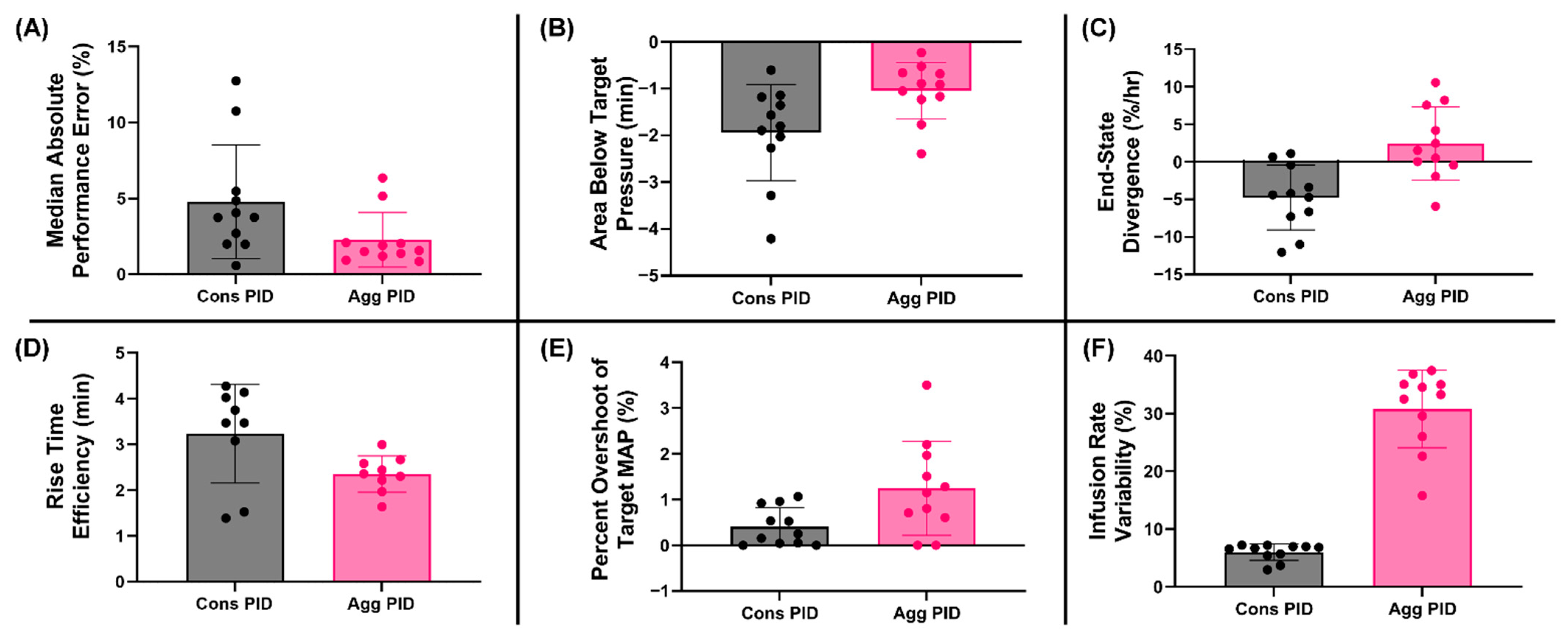
3.5. Summary Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
DoD Disclaimer
References
- Kleber, C.; Giesecke, M.T.; Tsokos, M.; Haas, N.P.; Buschmann, C.T. Trauma-Related Preventable Deaths in Berlin 2010: Need to Change Prehospital Management Strategies and Trauma Management Education. World J. Surg. 2013, 37, 1154–1161. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Mabry, R.L.; Seguin, P.; Cantrell, J.; Tops, T.; Uribe, P.; Mallett, O.; Zubko, T.; Oetjen-Gerdes, L.; Rasmussen, T.E.; et al. Death on the Battlefield (2001–2011): Implications for the Future of Combat Casualty Care. J. Trauma Acute Care Surg. 2012, 73, S431–S437. [Google Scholar] [CrossRef]
- White, N.J.; Ward, K.R.; Pati, S.; Strandenes, G.; Cap, A.P. Hemorrhagic Blood Failure: Oxygen Debt, Coagulopathy and Endothelial Damage. J. Trauma Acute Care Surg. 2017, 82, S41–S49. [Google Scholar] [CrossRef]
- Gurney, J.M.; Spinella, P.C. Blood Transfusion Management in the Severely Bleeding Military Patient. Curr. Opin. Anaesthesiol. 2018, 31, 207–214. [Google Scholar] [CrossRef]
- Jenkins, D.H.; Rappold, J.F.; Badloe, J.F.; Berséus, O.; Blackbourne, L.; Brohi, K.H.; Butler, F.K.; Cap, A.P.; Cohen, M.J.; Davenport, R.; et al. THOR Position Paper on Remote Damage Control Resuscitation: Definitions, Current Practice and Knowledge Gaps. Shock 2014, 41, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Nimri, R.; Kovatchev, B.; Phillip, M. Decision Support Systems and Closed-Loop. Diabetes Technol. Ther. 2021, 23, S-69. [Google Scholar] [CrossRef]
- von Platen, P.; Pomprapa, A.; Lachmann, B.; Leonhardt, S. The Dawn of Physiological Closed-Loop Ventilation—A Review. Crit. Care 2020, 24, 121. [Google Scholar] [CrossRef] [Green Version]
- Salinas, J.; Drew, G.; Gallagher, J.; Cancio, L.C.; Wolf, S.E.; Wade, C.E.; Holcomb, J.B.; Herndon, D.N.; Kramer, G.C. Closed-Loop and Decision-Assist Resuscitation of Burn Patients. J. Trauma Inj. Infect. Crit. Care 2008, 64, S321–S332. [Google Scholar] [CrossRef] [Green Version]
- Gholami, B.; Bailey, J.M.; Haddad, W.M.; Tannenbaum, A.R. Clinical Decision Support and Closed-Loop Control for Cardiopulmonary Management and Intensive Care Unit Sedation Using Expert Systems. IEEE Trans. Control Syst. Technol. 2012, 20, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Marques, N.R.; Ford, B.J.; Khan, M.N.; Kinsky, M.; Deyo, D.J.; Mileski, W.J.; Ying, H.; Kramer, G.C. Automated Closed-Loop Resuscitation of Multiple Hemorrhages: A Comparison between Fuzzy Logic and Decision Table Controllers in a Sheep Model. Disaster Mil. Med. 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Mirinejad, H.; Parvinian, B.; Ricks, M.; Zhang, Y.; Weininger, S.; Hahn, J.-O.; Scully, C.G. Evaluation of Fluid Resuscitation Control Algorithms via a Hardware-in-the-Loop Test Bed. IEEE Trans. Biomed. Eng. 2020, 67, 471–481. [Google Scholar] [CrossRef]
- Snider, E.J.; Vega, S.J.; Ross, E.; Berard, D.; Hernandez-Torres, S.I.; Salinas, J.; Boice, E.N. Supervisory Algorithm for Autonomous Hemodynamic Management Systems. Sensors 2022, 22, 529. [Google Scholar] [CrossRef]
- Rinehart, J.; Alexander, B.; Manach, Y.L.; Hofer, C.K.; Tavernier, B.; Kain, Z.N.; Cannesson, M. Evaluation of a Novel Closed-Loop Fluid-Administration System Based on Dynamic Predictors of Fluid Responsiveness: An in Silico Simulation Study. Crit. Care 2011, 15, R278. [Google Scholar] [CrossRef] [Green Version]
- Snider, E.J.; Berard, D.; Vega, S.J.; Hernandez-Torres, S.; Avital, G.; Boice, E.N. Hardware in Loop Automated Testbed for Evaluating Hemorrhagic Shock Fluid Resuscitation Controllers. Sci. Rep. 2022; submitted. [Google Scholar]
- Berard, D.; Vega, S.J.; Torres, S.I.H.; Polykratis, I.A.; Salinas, J.; Ross, E.; Avital, G.; Boice, E.N.; Snider, E.J. Development of the PhysioVessel: A Customizable Platform for Simulating Physiological Fluid Resuscitation. Biomed. Phys. Eng. Express 2022, 8, 035017. [Google Scholar] [CrossRef]
- Hoskins, S.L.; Elgjo, G.I.; Lu, J.; Ying, H.; Grady, J.J.; Herndon, D.N.; Kramer, G.C. Closed-Loop Resuscitation of Burn Shock. J. Burn Care Res. 2006, 27, 377–385. [Google Scholar] [CrossRef]
- Bennett, S. Development of the PID Controller. IEEE Control. Syst. Mag. 1993, 13, 58–62. [Google Scholar] [CrossRef]
- Ho, M.-T.; Lin, C.-Y. PID Controller Design for Robust Performance. IEEE Trans. Autom. Control. 2003, 48, 1404–1409. [Google Scholar] [CrossRef]
- Yoo, C.K.; Cho, J.H.; Kwak, H.J.; Choi, S.K.; Chun, H.D.; Lee, I. Closed-Loop Identification and Control Application for Dissolved Oxygen Concentration in a Full-Scale Coke Wastewater Treatment Plant. Water Sci. Technol. 2001, 43, 207–214. [Google Scholar] [CrossRef]
- Cap, A.P.; Pidcoke, H.F.; Spinella, P.; Strandenes, G.; Borgman, M.A.; Schreiber, M.; Holcomb, J.; Tien, H.C.-N.; Beckett, A.N.; Doughty, H.; et al. Damage Control Resuscitation. Mil. Med. 2018, 183, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Sondeen, J.L.; Coppes, V.G.; Holcomb, J.B. Blood Pressure at Which Rebleeding Occurs after Resuscitation in Swine with Aortic Injury. J. Trauma 2003, 54, S110–S117. [Google Scholar] [CrossRef]
- Varvel, J.R.; Donoho, D.L.; Shafer, S.L. Measuring the Predictive Performance of Computer-Controlled Infusion Pumps. J. Pharm. Biopharm. 1992, 20, 63–94. [Google Scholar] [CrossRef]
- 14:00–17:00 IEC 60601-1-10:2007. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/41/44104.html (accessed on 25 March 2022).

| Duration (min) | Starting MAP | Initial Hemorrhage Rate | Hemostasis? | Coagulopathy? | Infusate Type | Infusate Swapover? | |
| Compressible Hemorrhage Scenarios | |||||||
| Scenario 1a | 30 | 45 mmHg | None | NA | No | WB | No |
| Scenario 1b | 30 | End MAP 1a | Fast, 120 s Followed by None | NA | No | WB | No |
| Scenario 2 | 30 | 45 mmHg | None | NA | No | Crystalloid | No |
| Non-Compressible Hemorrhage Scenarios | |||||||
| Scenario 3 | 30 | 65 mmHg | Slow | Yes | No | WB | No |
| Scenario 4 | 30 | 45 mmHg | Slow | Yes | No | WB | No |
| Scenario 5 | 30 | 45 mmHg | Slow | Yes | No | Crystalloid | No |
| Scenario 6 | 30 | 65 mmHg | Fast | Yes | No | WB | No |
| Scenario 7 | 30 | 45 mmHg | Fast | Yes | No | WB | No |
| Scenario 8 | 30 | 45 mmHg | Fast | Yes | No | Crystalloid | No |
| Infusate Swapover Scenarios | |||||||
| Scenario 9 | 30 | 45 mmHg | Fast | Yes | No | WB → Crystalloid | Yes, at 900 mL WB |
| Coagulopathy Scenarios | |||||||
| Scenario 10a | 5 | 45 mmHg | Slow | Yes | No | WB | No |
| Scenario 10b | 25 | End MAP of 10a | Fast | No | Yes, increasing hemorrhage rate until Fast | WB | No |
| Scenario 11 | 30 | 45 mmHg | Fast | No | Yes, hemorrhage rate held at Fast | Crystalloid | No |
| Testing Metric | Description | Ref. |
|---|---|---|
| Median performance error (MDPE) | Median performance error (% error from target pressure) across the test scenario | [12,23] |
| Median absolute performance error (MDAPE) | Median of the absolute value of the performance errors across the test scenario | [23] |
| Steady-state overshoot | Maximum MAP reached after reaching steady-state relative to the steady-state MAP | [12,23] |
| Steady-state undershoot | Minimum MAP reached after reaching steady-state relative to the steady-state MAP | |
| Target overshoot | Maximum MAP reached relative to the target MAP | [15] |
| Effectiveness | Percentage of time MAP was within +/− 5 mmHg of the target pressure | [11] |
| Wobble | Median of the absolute value for the difference between performance error and MDPE across the test scenario | [12,23] |
| End-state divergence | Slope of MDAPE vs. time for the final 10% of each testing scenario. (Note: The calculation was limited to this time range to evaluate the final controller trends at the end of each scenario) | [12,15] |
| Rise time efficiency | Amount of time required for MAP to reach 90% of steady-state MAP | [11,24] |
| Volume efficiency | Ratio between volume infused and volume lost | [11] |
| Median infusion rate | Median of the infusion rates across the test scenario | [15] |
| Mean infusion rate | Arithmetic mean of the infusion rates across the test scenario | [15] |
| Area above target pressure | Area above the target pressure while below the MAP vs. time curve for each test scenario, relative to target MAP | [15] |
| Area below target pressure | Area below the target pressure while above the MAP vs. time curve for each test scenario, relative to target MAP, representing a “hypotensive burden”. | [15] |
| Area to rise time | Area below target pressure until rise time was reached (90% of the target pressure), relative to target MAP. | [15] |
| Infusion rate variability | Average of the 2-min binned standard deviations of the infusion rate relative to the mean across each scenario |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snider, E.J.; Berard, D.; Vega, S.J.; Avital, G.; Boice, E.N. Evaluation of a Proportional–Integral–Derivative Controller for Hemorrhage Resuscitation Using a Hardware-in-Loop Test Platform. J. Pers. Med. 2022, 12, 979. https://doi.org/10.3390/jpm12060979
Snider EJ, Berard D, Vega SJ, Avital G, Boice EN. Evaluation of a Proportional–Integral–Derivative Controller for Hemorrhage Resuscitation Using a Hardware-in-Loop Test Platform. Journal of Personalized Medicine. 2022; 12(6):979. https://doi.org/10.3390/jpm12060979
Chicago/Turabian StyleSnider, Eric J., David Berard, Saul J. Vega, Guy Avital, and Emily N. Boice. 2022. "Evaluation of a Proportional–Integral–Derivative Controller for Hemorrhage Resuscitation Using a Hardware-in-Loop Test Platform" Journal of Personalized Medicine 12, no. 6: 979. https://doi.org/10.3390/jpm12060979
APA StyleSnider, E. J., Berard, D., Vega, S. J., Avital, G., & Boice, E. N. (2022). Evaluation of a Proportional–Integral–Derivative Controller for Hemorrhage Resuscitation Using a Hardware-in-Loop Test Platform. Journal of Personalized Medicine, 12(6), 979. https://doi.org/10.3390/jpm12060979






