Abstract
In the last decades, both animal and human studies have neglected female subjects with the aim of evading a theorized intricacy of feminine hormonal status. However, clinical experience proves that pharmacological response may vary between the two sexes since pathophysiological dissimilarities between men and women significantly influence the pharmacokinetics and pharmacodynamics of drugs. Sex-related differences in central nervous system (CNS) medication are particularly challenging to assess due to the complexity of disease manifestation, drugs’ intricate mechanisms of action, and lack of trustworthy means of evaluating the clinical response to medication. Although many studies showed contrary results, it appears to be a general tendency towards a certain sex-related difference in each pharmacological class. Broadly, opioids seem to produce better analgesia in women especially when they are administered for a prolonged period of time. On the other hand, respiratory and gastrointestinal adverse drug reactions (ADRs) following morphine therapy are more prevalent among female patients. Regarding antidepressants, studies suggest that males might respond better to tricyclic antidepressants (TCAs), whereas females prefer selective serotonin reuptake inhibitors (SSRI), probably due to their tolerance to particular ADRs. In general, studies missed spotting any significant sex-related differences in the therapeutic effect of antiepileptic drugs (AED), but ADRs have sex variations in conjunction with sex hormones’ metabolism. On the subject of antipsychotic therapy, women appear to have a superior response to this pharmacological class, although there are also studies claiming the opposite. However, it seems that reported sex-related differences regarding ADRs are steadier: women are more at risk of developing various side effects, such as metabolic dysfunctions, cardiovascular disorders, and hyperprolactinemia. Taking all of the above into account, it seems that response to CNS drugs might be occasionally influenced by sex as a biological variable. Nonetheless, although for each pharmacological class, studies generally converge to a certain pattern, opposite outcomes are standing in the way of a clear consensus. Hence, the fact that so many studies are yielding conflicting results emphasizes once again the need to address sex-related differences in pharmacological response to drugs.
1. Introduction
Differences between the two sexes exist, and, from a medical point of view, they have a significant impact on prevalence, incidence, and severity of a wide range of diseases and conditions. Accordingly, physiologic differences between the two sexes affect drug activity with dissimilarities within pharmacokinetics, pharmacodynamics, and pharmacotoxicity. However, most drugs are prescribed to women and men at the same dose, although therapeutic effectiveness varies (Table 1) [1]. In research, women and non-human female mammals have often been underrepresented, especially in previous decades. The reasoning behind it is the assumption that results from males readily apply to females, or the concern that hormonal cycles negatively influence the homogeneity of study populations and complicate experimental designs [2]. Moreover, the risk of ADRs such as teratogenicity or toxicity may outweigh other considerations, and, thus, females of child-bearing potential, pregnant, or breastfeeding are sometimes advised by healthcare professionals against enrolling in such studies (Table 2) [3].
Pharmacokinetics in women is influenced by several factors, such as: lower body weight, higher percentage of body fat, slower gastrointestinal motility, higher gastric pH, decreased intestinal enzymatic activity, and slower glomerular filtration rate [4]. With regard to medication, drugs in women usually have a larger volume of distribution, and the free fraction is also increased. Female sex hormones alter hepatic enzyme activity, which can result in decreased elimination and accumulation for some drugs. However, the way estrogen and progesterone affect pharmacokinetics of drugs is hard to predict and assess, with studies yielding conflicting results [5]. Differences in pharmacodynamics occur when the same plasma concentration of a drug does not cause the same pharmacological response between the sexes. Unlike pharmacokinetic differences, pharmacodynamic disparities are more difficult to assess, as pharmacological effects are not easily measurable [6]. However, there are certain examples in the literature where such differences are obvious. To name a few, women are more likely to experience QT interval prolongation following therapy, while males show a greater sensitivity to propofol’s anesthetic effect [7]. Another example would be that verapamil has a lower bioavailability and increased clearance in male patients compared to females [8]. Regarding pharmacotoxicology, women are significantly more likely to be hospitalized secondary to an ADR, as they have a nearly two-fold greater risk than men for exhibiting side effects across many drug classes [9]. For instance, women appear to be at a higher risk for ADRs following treatment with thyroid hormones, phychoanaleptics, and TNF-α inhibitors [10]. Additionally, women are more at risk of admission using thiazide diuretics causing hypokalemia and anticoagulants causing rectal bleeding, whereas males have higher rates of hematuria and subdural hemorrhage following treatment with anticoagulants [11].
The purpose of this narrative review is to explore to what extent the response to CNS medication is influenced by sex as a biological variable in: drug delivery, pharmacokinetic response, drug efficacy, and ADRs. The aim is to identify and summarize how the observed sex-related differences are corelated with: a disease’s pathophysiology, epidemiology, drugs’ underlying mechanisms of action, a patient’s habits/medication, or any other interfering factor.
2. Materials and Methods
2.1. Literature Search Strategy
From January 2021 to June 2021, we searched five electronic databases (PubMED, Web of Science, Elsevier’s Scopus, Google Scholar, and PsychINFO) for papers analyzing treatment response to various CNS diseases. We intended to identify relevant articles from 1980 to 2021 and limited our analysis to articles written in English.
Searches were conducted separately in each database, and the records were exported to citation software after removing the duplicates. Search terms were utilized in several combinations, taking into account the possibility of encountering various suffixes (including plural terminations), hereby represented by the * character. The primary search terms were: analgesia, opioid *, depression OR depressive, antidepressant *, epilepsy, anticonvulsant * OR antiepileptic *, psychosis OR schizophrenia, antipsychotic *, sex OR gender difference *, male * OR men, female * OR women (e.g., (depress * OR antidepressant *) AND (gender OR sex) AND (difference * or dissimilarity * OR distinction)). For each distinct pharmacological class, a manual search was performed on the same electronic databases in order to look into additional distinctive keywords and broaden the results range (e.g., morphine, ISRSs, atypical antipsychotics, etc.).
Afterwards, we searched reference lists of identified articles to ensure the capture of significant literature missed during the primary search. Then, we checked relevant review papers for supplementary references. Lastly, with the aim of elucidating the various results obtained in studies, for each of the diseases tackled in the present review, we consulted articles approaching sex-related differences in epidemiology, pathophysiology, prognostic, and co-morbidities.
2.2. Selection Criteria
This narrative review analyzed articles that met the following inclusion criteria:
- -
- Human studies (clinical trials, experimental studies, case reports, reviews, meta-analysis);
- -
- Studies in adult patients (subjects > 18 years);
- -
- Studies that investigated sex differences.
When any of these criteria were not met, papers were eliminated from the analysis. We also excluded studies that:
- -
- Investigated animal populations;
- -
- Failed to analyze outcomes and differentiate between men and women;
- -
- Were methodologically flawed;
- -
- Provided insufficient details or irrelevant outcomes.
2.3. Selection Process
Electronic database searches, alongside supplemental searching strategies, yielded a total number of 1943 records (Figure 1). After removing the duplicates, 632 studies remained for further examination. A first selection was performed based on titles and short abstract screening, narrowing down the range to 217 records. All articles that examined sex differences were retained, even if this was not the primary focus of the study, as long as the search terms were mentioned in the publication title, abstract, or subject headings. Then, two reviewers (V.B. and L.D.) independently restricted the analysis to those articles that met the inclusion criteria. The reviewers reached consensus and performed the final selection procedure including inspection of overall study quality and risk of bias. This resulted in a total number of 143 eligible articles, including 59 original papers and 84 reviews and meta-analyses. All remaining papers were read in full.
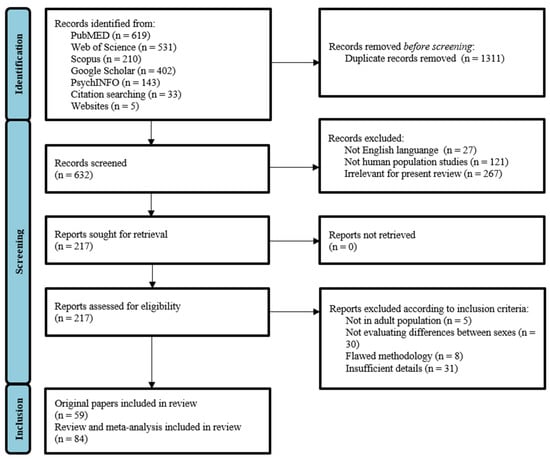
Figure 1.
Flow chart of studies identified and selected for this review.
2.4. Data Extraction
The results of the pertinent articles were closely evaluated by two reviewers (V.B. and L.D.). Each selected paper was assessed, and the following variables were extracted: study design, number of patients, disease, medication under study, dose, duration of treatment, response to treatment (efficacy and/or ADRs). Any disagreements or queries during the writing of the article were settled by contacting the two reviewers (V.B. and L.D.) and reaching a consensus.
3. Opioids
It is well known that pain sensitivity varies among individuals; therefore, assessing and treating postoperative pain requires a personalized approach, making it difficult to follow protocols strictly. An effective analgesia needs to take into account various factors, such as weight, height, age, body mass index, sex, type of surgery, surgery site, preoperative pain, and medication [12]. It is established that the majority of chronic pain syndromes occur more often in women (chronic fatigue syndrome, fibromyalgia, interstitial cystitis, temporomandibular disorder, headache, migraine, low back pain, neck pain, and osteoarthritis) [13]. Likewise, studies indicate a greater pain prevalence among women and determined that women seem to show greater sensitivity to the majority of experimentally induced pain methods [14,15,16]. However, the differences in pain perception observed within most studies may not be statistically significant and are not always consistent, as suggested by a series of review papers [17,18,19,20]. Pain perception seems to be linked to sex hormones, since testosterone was found to decrease pain sensitivity; thus, a low testosterone state is incriminated in a wide range of chronic pain conditions. Nevertheless, female hormones have both pro- and antinociceptive properties, making the effects of estrogen and progesterone on pain more difficult to evaluate [21]. Regarding opioid addiction, it is established that the desire for opioids is considerably higher among women, and they are at an increased risk of abusing opioids through initial prescription painkiller use [22].
Most pain-related animal studies only include male subjects; few are focused on females, and just a small number are explicitly designed to test for sex differences [23]. Animal studies reveal that, generally, opioid analgesia is more effective in males compared with females. It is known that adult female rodents have a lower percentage of body fat than males, whereas the situation is opposite in humans. These sex-related differences may affect the distribution of highly lipophilic drugs, having a substantial impact on drugs’ potency, efficacy, and duration of action [23,24,25]. However, results in human subjects are not as consistent as those in rodents.
Although recent years were marked with significantly increased research regarding sex differences in pain, studies on gender differences in opioids concluded a mixture of different results. Most papers focus on μ agonists, especially morphine, as a main treatment for pain alleviation, since it is perhaps the most clinically significant opioid [26]. Existing data regarding sex differences in response to morphine are highly inconsistent, and a general assumption is difficult to make. It appears that discrepancies in the sex-related response to morphine analgesia might virtually depend on the pain model and/or drug dose/regimen used.
To begin with, some clinical studies investigating the difference in the postoperative morphine requirements determined that male opioid consumption was higher than the one observed in females through patient-controlled analgesia (PCA) [12,27,28]. This means that women self-administer significantly less morphine than men [29]. In addition, further research showed that women experience greater morphine potency, as well as a slower speed of onset and offset of the analgesic effect. However, no sex differences in plasma concentrations of morphine and its major metabolites (morphine-3 and 6-glucuronide) were observed. Those findings suggest that gender differences in opioid analgesia are not related to morphine’s pharmacokinetics [25,30]. On the other hand, when analyzing the immediate postoperative analgesia, following intravenous titration of morphine, it appears that women require a higher dosage than men [31,32]. This might be explained by the slower onset of morphine in women, who experience later analgesia. Contrarywise, other research papers reported similar analgesic effects of morphine in both sexes. These studies used experimental pain models and evaluated the response to intramuscular morphine by measuring its plasma levels as well as subjective experience, performance, and physiological effects [33,34]. However, the results on elderly patients appear steadier, with no significant differences in the analgesic effect of morphine being observed in most studies [31,35].
Regarding ADRs, there is considerable, as well as consistent, evidence that sex influences the intensity and frequency of morphine’s side effects. Findings indicate that females have a substantially higher risk of developing nausea and vomiting than men following opioid analgesia [36]. These observations might be related to the higher frequency of post-operative nausea and vomiting among women than men [37,38]. However, the same results were also obtained in a study of narcotic-induced emesis in the emergency department, which strengthens the initial conjecture [39]. Furthermore, additional studies reported greater morphine-induced respiratory depression in females than in males [40,41] as well as an increased feeling of disorientation and sluggishness [42]. In addition, preliminary results suggest some cardiovascular differences between the two sexes following IV morphine administration: women experienced a lower heart rate, but only men developed hypertension and had an attenuated cardiovascular response to ischemic pain. However, the observed differences were small, and only one low morphine dose was tested; therefore, further investigations are needed [34].
Leaving morphine aside and analyzing opioids as a whole, the same conclusion can be drawn—distinct investigations reveal various results. It appears that earlier studies tend to deduce that opioids are better analgesics for females [43,44,45]. However, it is hypothesized that this may happen because older studies did not correct for the body weight differences between the two sexes [46]. For instance, the mixed µ-k-opioid agonist-antagonist nalbuphine, butorphanol, and pentazocine produced significantly better postoperative analgesia in women than in men [47]. In contrast, other studies using experimental pain models showed that pentazocine produced analgesia of similar magnitude in men and women [48,49]. Regarding µ-opioids, a similar analgesic response in the two sexes is achieved after the administration of alfentanil, as well as morphine’s active metabolite, morphine-6-glucuronide [50,51]. Considering PCA studies on µ-opioids, it generally appears that opioid consumption is higher in men than in women. However, most studies actually assess opioid consumption rather than pain relief. Therefore, PCA poses problems in terms of reliability, as it can be influenced by other factors than just postoperative pain, such as: expectations, baseline pain sensitivity, fear of addiction, and the occurrence of unpleasant ARDs, such as nausea and vomiting [52].
The general verdict regarding the diverse outcomes of studies is that results may be influenced by procedural and subject variables. Broadly, opioids seem to produce better analgesia in women, especially when they are administered for a few days, as the onset of action is delayed in this category of patients. However, the various responses to pharmacological pain interventions appear inconsistent and dependent on treatment type, genotype, gonadal steroid hormone state of subjects, and characteristics of the pain and the provider [14,16,26,53].
4. Antidepressants
Epidemiological studies demonstrate that in children the rates of depression are equivalent between the sexes, but adolescence makes women more than twice as likely to suffer from depression than males. Females also experience more atypical symptoms than men, show greater severity, earlier age of onset, and increased duration of depressive episodes [54,55]. Atypical symptoms are often associated with greater depression severity and more overall comorbidity, and because of that women are also more likely to report comorbid anxiety [56].
Both the pharmacokinetics and pharmacodynamics of antidepressants seem to be affected by physiological and molecular differences between the two sexes. The majority of antidepressants are weak bases, so they are more effectively absorbed from women’s gastrointestinal tract since females secrete less gastric acid and have a more basic environment [57].
In terms of physiological differences, two studies highlighted that patients with lower body weight, generally women, responded preferentially to the SSRI fluoxetine [58,59]. Additionally, the magnitude of the effect of body weight on antidepressant activity seems to depend on gender. A study of the response to a number of SSRIs reported differences between sexes related to body mass index. Specifically, obese men failed to respond to any of the tested SSRIs better than a placebo, while obese women showed superior response when compared to a placebo [60]. Moreover, antidepressants are mostly strongly lipophilic; therefore, their volume of distribution is higher in women than in men. This might explain why trazodone and bupropion have an increased half-life in women with this effect being further highlighted in elderly females [61].
Plasmatic level of tryptophan is more reduced in females than in males, which leads to a deficiency in serotonin and, consequently, to depression [62,63]. It is suggested that estrogen can affect the production of serotonin as well as the expression and binding of serotonin to transporter receptors within its pathway, processes that have a direct impact on SSRIs’ efficacy [56]. This might explain why women in their reproductive period are more responsive to SSRIs. Moreover, it appears that female patients produce less cortisol and more tryptophan when exposed to SSRIs [64].
Moving on to proteins and enzymatic activity, it looks as though women may have a lower activity of P-gp, a drug-efflux pump found in the gut and brain, which lowers the uptake of substances. Animal studies showed that the inhibition of P-gp may amplify the uptake of various antidepressants, such as nortriptyline, amitriptyline doxepin, venlafaxine, citalopram, and paroxetine [65,66]. A review analyzing gender differences in antidepressants found that plasma levels of TCAs imipramine, nortriptyline, and desipramine are reportedly higher in women, consistent with lower P-gp activity at absorption and lower activity of CYP1A2 and CYP2C19 enzymes. Therefore, antidepressants may have higher serum levels in women due to decreased expression and inhibition of many of the phase I enzymes necessary for the metabolism of these drugs [56]. In addition, some SSRIs, such as fluoxetine, fluvoxamine, or, to a lesser extent, paroxetine and sertraline, are known to be able to inhibit certain CYP enzymes involved in the metabolism of co-administered drugs [67]. For example, it is not uncommon to see benzodiazepines prescribed alongside SSRIs for a variety of reasons: (1) reduction in SSRI-induced anxiety which occurs early in the course of therapy; (2) improvement in adherence to antidepressant therapy, (3) control of comorbid anxiety associated with atypical symptoms. As stated previously, females are more likely to associate anxiety and atypical symptoms; therefore, they could benefit more from this association [68,69]. Fluoxetine was reported to decrease the metabolic clearance of benzodiazepines such as diazepam and alprazolam through inhibition of CYP-2C19 (diazepam) and CYP-3A4 (diazepam, alprazolam). As a consequence, fluoxetine might increase benzodiazepines’ plasmatic concentration with the risk of exacerbating their effects [70,71]. The same can be said of fluvoxamine, which was shown to impair the elimination of alprazolam and diazepam by interfering with the same CYP enzymes [72].
Recent reviews and clinical studies reported that females experience an increased efficacy of pro-serotoninergic antidepressants, while men respond better to pro-noradrenergic drugs [55,56,73,74,75]. Generally, women are more likely to respond favorably to fluoxetine and citalopram, whereas men prefer the tetracyclic antidepressant maprotiline [76,77]. Female patients are also more likely to respond favorably to sertraline than to imipramine, whereas the opposite might be said for men, although to a lesser extent [78,79]. Other papers suggested that women showed a superior response to SSRIs than to serotonin and norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine. They also responded better responses to citalopram than to the selective noradrenergic reuptake inhibitor reboxetine [75,80]. This can happen because women have the tendency to display more somatic symptoms associated with atypical depression, therefore responding preferentially to SSRIs [81]. Further research revealed that younger women generally have a better response to SSRIs than women aged 50+ years [78,79,82]. Atypical depression symptoms can be also cured using monoamine oxidase inhibitors, which were reported to produce a larger response in women than in men, although other findings suggest no sex response difference within the class [83,84]. On the other hand, there are also studies that report no difference in efficacy in men and women for antidepressants such as fluoxetine, clomipramine, citalopram, paroxetine, and moclobemide [84,85,86]. Furthermore, there are not many studies concerning newer non-SSRI antidepressants, but few reported that there was not any sex difference in response to venlafaxine, bupropion, or sertraline [87].
Another aspect worth mentioning is the use of augmentation therapy—drugs that have other primary indications that are thought to enhance the effect of antidepressants. For example, folic acid supplementation enhances the effect of fluoxetine in female patients [88], while the triiodothyronine hormone accelerates the response to TCA, and this effect is more pronounced in women [89]. As previously stated, females also benefit more from the association with benzodiazepines [68]. More than that, hormone replacement therapy was found to eliminate a poor SSRI response in older women; therefore, estradiol might augment SSRIs’ effectiveness [82].
With regard to adverse drug reactions, women seem to have a lower tolerance for imipramine than sertraline, imipramine being responsible for increased instances of constipation, sweating, dry mouth, and tremor. For this reason, women were almost three times more likely to cease treatment with imipramine than sertraline, whereas men showed similar drop-out rates [78,79]. The fact that women are more likely to experience adverse drug reactions to tricyclic antidepressants is probably linked to pharmacokinetic studies which concluded that women have higher plasma levels of these drugs than men. Another hypothesis is that women and men have a different tolerance of particular adverse reactions, with the anticholinergic effects (dry mouth, constipation, sedation, sweating, and tremor) associated with TCAs possibly being more acceptable to men than the ADRs associated with SSRIs [56]. The activation of the 5-HT2 receptors impairs all stages of the sexual response. Therefore, although SSRIs induce less ADRs than TCAs, they deteriorate the sexual function precisely through: impairment in desire and arousal, inhibition of orgasm, delayed ejaculation, and male impotence [90,91,92]. Apart from inhibiting the reuptake of noradrenaline and serotonin, TCAs are also known to antagonize postsynaptic α1-adrenoceptors, histamine (H1) receptors, muscarinic cholinergic receptors, and serotonin (5-HT2) receptors, resulting in a series of ADRs including sedation [93]. Because of its sedating effects, this class of antidepressants was used in the past as the first-choice agent in the treatment of comorbid anxiety or insomnia, which, as mentioned above, is more frequent in females [94,95].
As previously stated, most studies show that male patients respond better to TCAs, whereas females prefer SSRIs. This tendency seems to be especially dictated by the tolerance to particular ADRs, with anticholinergic effects being less desirable in women, and sexual dysfunction being displeasing to men. However, as with opioids, conflicting outcomes restrict the freedom of drawing a generally valid conclusion.
5. Anticonvulsants
Epilepsy—one of the biggest causes of chronic neurological morbidity—has a specific sex incidence with males being relatively more susceptible to seizures than females [96,97,98]. Although women are not as frequently affected by epilepsy as men, they experience more complex and refractory seizure syndromes. This might be explained by the effects that hormones have on seizures, with numerous women stating that their seizure manifestation goes through different stages at puberty, the menstrual cycle, and menopause [96]. Closely linked to menstrual cycle periodicity is hormonal contraception, which seems not only to interact with anticonvulsants, but also to exacerbate the seizures. While estrogen seems to exert an excitatory effect by increasing glutamate and decreasing GABA, progesterone appears to be doing the opposite, which leads to the conclusion that the first hormone promotes seizures, whereas the other one reduces their frequency [98,99,100].
There are not many reports on sex differences in the anti-seizure effect of anticonvulsants. The results reported by clinical trials either do not address gender dissimilarities, or findings are not significant. Sex-related differences in the pharmacokinetics of antiepileptic drugs (AEDs) are reported occasionally, but they lack in consistency and clinical significance. Although it is expected that estrogens accelerate the rate at which drugs are being glucuronidated, studies hardly show any relevant changes in the plasma concentration of anticonvulsants in women compared to men [101,102]. However, despite the fact that variation in endogenous hormones during a regular menstrual cycle has no significant impact on serum concentrations of AEDs, a study conducted in 2009 revealed that women who take oral contraceptives have a substantially reduced concentration of lamotrigine and valproic acid in the serum [101,103].These findings suggest that drug interactions may occur with medications that are used specifically in one of the two sexes. Moreover, additional research papers indicate that certain AEDs, mostly enzyme inducing drugs (carbamazepine, phenytoin, phenobarbital, primidone, and also lamotrigine, topiramate, felbamate, oxcarbazepine, eslicarbazepine acetate, and rufinamide) reduce the contraceptive efficacy of: the combined oral contraceptive pill, combined contraceptive patch, vaginal ring, progestogen-only pill (minipill), progestogen implant, and postcoital contraceptives [104,105]. AEDs’ serum concentration can be affected by another medication with gender-specific indications—tamoxifen—a drug used in the treatment of breast cancer. This molecule seems to increase plasma levels of phenytoin and possibly to cause clinical signs of phenytoin toxicity [106]. Another drug interaction relevant to the male gender is tadalafil which, according to the Summary of Product Characteristics, seems to be affected by CYP3A4 inducers, such as phenobarbital, phenytoin, and carbamazepine [107].
One recent review analyzing the well-known pharmacokinetic changes during pregnancy revealed important drops in the serum concentration of several anticonvulsants. The most prominent effect appears to occur with lamotrigine, which can be explained by its elimination route through glucuronidation [108,109]. Oxcarbazepine, a prodrug following the same metabolic route as lamotrigine, behaves in a similar manner, having plasma levels reduced by 30–40% [110]. Antiepileptic drugs cleared by other routes seem to be as well considerably affected during the gestation period. As stated by Tomson et al., levetiracetam plasma levels declined during the first two trimesters and dropped even more sharply in the third one compared to baseline [111]. Following the same pattern, pregnant women experience decreased levels of phenytoin, phenobarbital, topiramate, and valproate, while other drugs such as carbamazepine seem to be inconsistently affected by pregnancy [108].
Certain ADRs of AEDs seem to be considerably influenced by gender, most of them consisting of changes within sex hormones’ metabolism [101]. In women, AEDs such as carbamazepine, phenytoin, and phenobarbital appear to be responsible for a variety of sex hormones’ abnormalities, including low levels of total serum testosterone, free androgen index, dehydroepiandrosterone sulfate, and estradiol, alongside increased levels of sex-hormone-binding-globulin. These drugs produced sexual dysfunction and low arousal scores as well as a concerning health problem for postmenopausal women—alteration in bone metabolism [112,113]. In males, enzyme inducing anticonvulsants determined a drop in androgen levels and a series of sexual dysfunctions, such as reduced fertility, reduced sperm counts, and morphological sperm abnormalities [113,114,115]. Valproic acid, one of the non-enzyme inducing AEDs, was incriminated in producing gender-related side effects, especially among women, increasing the incidence of polycystic ovary syndrome, hyperinsulinism, hyperandrogenism, hypothalamic amenorrhea, and functional hyperprolactinemia apart from the acknowledged effects on offspring [116].
Generally, studies did not find any significant sex-related difference in response to AEDs. The results reported by clinical trials either do not address gender dissimilarities, or findings are not statistically significant. Anticonvulsants can lead to various changes within sex hormones’ metabolism, which is particularly why many of them determine such ADRs. However, since AEDs have a multitude of mechanisms of action, a universal conclusion over sex-related disparities cannot be drawn, with different drugs exhibiting a different ADRs’ profile.
6. Antipsychotics
There are a few sex differences that affect drug response in neuro-psychiatric disorders, such as schizophrenia. First of all, women receive a later diagnosis since the onset of the disease is delayed [117]. Deficit symptoms are less prevalent in women with schizophrenia, and they experience less severe symptoms, fewer hospitalizations, and shorter admissions [118,119]. Therapeutic adherence is higher in women, and they have better outcomes due to lifestyle, social support, the advantage of later onset, and relative hormonal protection. Moreover, associated pathologies are more common among females, such as pain conditions, allergies, mood problems, sleep disturbances, endocrine disturbances, eating disorders, and personality disorders. Therefore, they require additional medication and are more prone to drug interactions. Conversely, men smoke more and are more likely to abuse multiple street drugs and alcohol. It is established that smoking status strongly affects the metabolism of certain drugs. Therefore, all the factors mentioned above lead to a poorer prognosis [120,121].
On the subject of antipsychotics’ pharmacokinetics, the volume of distribution of these lipophilic drugs is greater in women than in men, leading to prolonged half-life and to accumulation over time [120]. Women eliminate antipsychotics slower than do men due to their decreased glomerular filtration rate, renal tubular secretion, and reabsorption, all contributing to drug accumulation within the body [122]. For instance, the summary of product characteristics of olanzapine shows a different elimination profile in female versus male subjects, with women having a prolonged mean elimination half-life (36.7 versus 32.3 h) and a reduced clearance [123].

Table 1.
Sex-related differences in therapeutic effectiveness.
Table 1.
Sex-related differences in therapeutic effectiveness.
| Pharmacological Class/Drug | Effectiveness | Comments and Conclusion | Reference | |
|---|---|---|---|---|
| Results | Consistency | |||
| Opioids | F > M | C | Mixed µ-k-opioid agonist-antagonists and pure µ-agonists appear to be slightly more effective in women judging by the consumption of opioids in the two sexes. | [43,44,45,46,47,48,49,50,51] |
| Morphine | F > M | B | The majority of studies indicate that immediate postoperative analgesia is less effective in women since they experience a slower speed of onset. Contrarywise, PCA shows that female patients self-administer significantly less morphine than males. | [12,25,27,28,29,30,31,32,33,34,35] |
| TCAs | M > F | C | Males report an increased efficacy of pro-noradrenergic drugs probably due to their lower tolerance to sexual dysfunctions associated with SSRIs. | [55,56,73,74,75,76,77,78,79] |
| SSRIs | F > M | C | Women respond better to pro-serotoninergic drugs because anticholinergic ADRs associated with TCAs might be less desirable to these patients. | [55,56,73,74,75,76,77,78,79] |
| Anticonvulsants | M = F | B | There are few studies available, which either do not address gender dissimilarities, or their findings are not statistically significant. | [101,102] |
| Antipsychotics | F > M | C | Antipsychotic response seems to be higher in females, but this may simply indicate that, compared to males, they are at an earlier stage of illness. | [124,125,126,127,128,129,130] |
Legend: B—Indicated by many studies, although there are some stating the opposite; C—conflicting results, but there is a tendency towards the indicated direction; F—Females; M—Males; PCA—patient-controlled analgesia.
The clinical dissimilarities between antipsychotic drugs are mostly in the areas of safety and tolerability [124]. As stated by a systematic review of gender differences in schizophrenia treatment restricted to randomized controlled trials and meta-analyses, there is evidence that points to an inferior antipsychotic response in men, who require a higher dosage in order to achieve an equivalent drop in psychotic symptoms [125]. However, when comparing male/female results in drug trials, it is essential to take into account that response to antipsychotics is nearly always better during early episodes of schizophrenia than it is after multiple episodes. Therefore, women’s superior response to therapy may simply indicate the fact that, compared to men, they are at an earlier stage of illness [126].
Neuroleptic-naive women show a better response to antipsychotic treatment than neuroleptic-naive men. Moreover, males have a reduced likelihood of response to antipsychotic treatment at 1 year compared with females; yet, there are no sex differences in risk of relapse [122]. Gender appears to be a significant predictor of response to antipsychotic treatment with women responding better and requiring lower doses than men [127,128]. One study following long-term antipsychotic treatment response and serum levels suggests that males require twice the dose of antipsychotic compared with females [129]. In contrast, another paper showed that oral haloperidol, risperidone, and olanzapine are all equally effective, but men respond better to acute treatment than women do, both during the initial 2 h period as well as over the 5-day course [130].
Schizophrenic patients are reported to have a higher prevalence of smoking than the general population. Certain antipsychotics, namely clozapine and olanzapine, are highly influenced by smoke constituents in cigarettes. Polycyclic aromatic hydrocarbons are inducers of the CYP1A2 hepatic enzyme [131]. The main metabolic pathway for clozapine and olanzapine is N-demethylation, a reaction catalyzed by the cytochrome isoenzyme in question. Thus, enzyme induction results in accelerated metabolism of these two drugs, lower plasma levels, and, thereupon, lower efficacy and therapy refractoriness [132]. On the other hand, substantial reduction in the number of smoked cigarettes or total smoking cessation results in increased plasma levels of olanzapine/clozapine and can have deleterious consequences. Hence, patients undergoing treatment who are advised by health-care professionals to quit smoking or who are forced to do so upon hospital admission may be at a high risk of developing serious ADRs [133]. As previously stated, men are more prone to tobacco consumption than women, so male patients should be monitored even more strictly especially regarding their smoking cessation plans [120].

Table 2.
Sex-related differences in ADRs.
Table 2.
Sex-related differences in ADRs.
| Pharmacological Class/Drug | ADRs’ Frequency/Intensity | Comments and Conclusion | Reference | ||
|---|---|---|---|---|---|
| Results | Consistency | Type of ADRs | |||
| Morphine | F > M | A | Nausea, vomiting, respiratory depression | Gastrointestinal and respiratory ADRs are considerably more frequent in women. There are hints that cardiovascular ADRs are also influenced by sex, but the available data are scarce. | [36,37,38,39,40,41,42] |
| TCAs | F > M | B | Dry mouth, constipation, sedation, sweating, and tremor | Pharmacokinetic studies revealed that women have higher plasma levels of TCAs than men, therefore being more sensitive to side effects. | [56,78,79] |
| SSRIs | M > F | B | Sexual dysfunction | SSRIs deteriorate the sexual function precisely through: impairment in desire and arousal, inhibition of orgasm, delayed ejaculation, and male impotence. | [90,91,92] |
| Anticonvulsants | - | I | Sex-hormone-related ADRs | Generally, AEDs can lead to changes within sex hormones’ metabolism. However, since these drugs have a multitude of mechanisms of action, a general conclusion over sex-related differences cannot be drawn. | [101,112,113,114,115,116] |
| Valproic acid | F > M | B | Polycystic ovary syndrome, hyperinsulinism, hyperandrogenism, hypothalamic amenorrhea | Valproic acid has been incriminated in producing gender-related side effects, especially among women, increasing the incidence of the mentioned ADRs, apart from the acknowledged effects on offsprings. | [116] |
| CarbamazepinePhenytoinPhenobarbital | F > M | B | Alteration in bone metabolism | They increase the levels of sex-hormone-binding-globulin and decrease the levels of total serum testosterone, free androgen index, dehydroepiandosterone sulfate, and estradiol. | [112,113] |
| Antipsychotics | F > M | B | metabolic dysfunctions, cardiovascular disorders, hyperprolactinemia | Females exhibit lower fasting plasma glucose levels, elevated waist circumference and waist-to-hip ratio, prolonged QTc interval, and reduced bone density due to hyperprolactinemia. | [124,134,135,136,137] |
| M > F | C | acute dystonic reactions, tardive dystonia, akathisia | Males are generally more prone to developing extrapyramidal side effects. | [122] | |
| M = F | B | Sexual dysfunction | ADRs are due to dopaminergic antagonists (females) or drugs having α1-antiadrenergic/anticholinergic properties (males). | [138,139,140,141,142,143] | |
Legend: A—Indicated by the majority of studies; B—Indicated by many studies, although there are some stating the opposite; C—conflicting results, but there is a tendency towards the indicated direction; F—Females; M—Males; I—Inconsistent Results.
Regarding adverse drug reactions (ADRs), females suffering from schizophrenia seem to be diagnosed with metabolic diseases at higher rates than males. A thorough study divided antipsychotic drugs in 3 groups according to their metabolic risk: high—clozapine and olanzapine; moderate—quetiapine, risperidone, paliperidone, and iloperidone; low—all other antipsychotics. According to the results, women had lower fasting plasma glucose levels but an elevated waist circumference and waist-to-hip ratio compared to men. Surprisingly, glucose levels were elevated even within the low-risk group, although medication in this category is not associated with this kind of side effect. Additionally, men have higher diastolic and systolic blood pressure than women, but values seen in both sexes fell within the normal range. On the other hand, although men have a lower total cholesterol, LDL, and HDL, both males and females are at risk for medication-specific lipid dysregulation [134,135,136,137].
Schizophrenic patients have a reduced life expectancy that can be assigned to higher rates of cardiovascular disease [124]. For example, women following a treatment with clozapine or olanzapine not only have a remarkably higher risk for metabolic dysfunction but also are more prone to future adverse cardiovascular outcomes [134]. Furthermore, women taking antipsychotics are 3.6 times more likely to have a prolonged QTc interval than men, with gender predicting QTc length during treatment more than class or type of antipsychotic and age. Women are also at a higher risk of becoming hyperprolactinemic than men while being treated with second generation antipsychotics that block dopamine (risperidone, amisulpride) [138]. That means that, despite the fact that fewer females report sexual dysfunction whilst taking antipsychotics, women are more at risk of developing sexual side effects than men. For what is more, reduced bone density can be associated with low estrogen levels due to chronic elevation of prolactin levels [139]. Thus, ADRs such as amenorrhea, galactorrhea, and sexual dysfunctions might reduce females’ therapeutic adherence to atypical antipsychotics that interfere with the dopamine pathway [140]. On the other hand, antipsychotics that have α1-antiadrenergic and anticholinergic properties are associated with sexual dysfunctions in male patients [141,142,143]. Moreover, acute dystonic reactions, tardive dystonia, and akathisia are more likely to occur in young men than in women [122].
Taking all of the above into account, women appear to have a superior response to antipsychotic therapy, although there are also studies claiming the opposite. However, it seems that reported sex-related differences regarding ADRs are steadier: women are more at risk of developing metabolic dysfunctions, cardiovascular disorders, and hyperprolactinemia. Sexual dysfunctions are common in both sexes, but the underlying mechanism varies in each pharmacological subclass and generates different symptoms.
7. Conclusions
So far, many animal and human studies have neglected female subjects in order to avoid a hypothesized complexity of feminine hormonal status. The general assumption was that women possess more variability due to changes during the estrus cycle, thus adding a supplementary layer of complexity to the analyses. As a consequence, studies would require a much larger number of subjects in order to reach adequate power. Nowadays, women may be increasingly represented in clinical trials, but, despite that, most studies fail to analyze sex-related differences in results.
Healthcare professionals need to understand the pharmacokinetics and pharmacodynamics of drugs in various populations thoroughly in order to minimize the ADRs and maximize the therapeutic effectiveness. For this to happen, both sexes should be included in medication trials, clinical, preclinical, and experimental studies in sufficient numbers to detect statistically significant differences. Another step forward would imply that medical trials would include a mandatory report of outcomes differentiated by sex.
Author Contributions
Conceptualization, V.B., M.R. and L.D.; methodology, M.R., V.B., A.L. and I.L.; software, C.D. and I.L.; validation, V.B., M.S., M.A. and L.D.; writing—original draft preparation, M.R., M.S. and A.L.; writing—review and editing, V.B., C.A.D. and L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitley, H.P.; Lindsey, W. Sex-Based Differences in Drug Activity. Am. Fam. Physician 2009, 80, 1254–1258. [Google Scholar] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, A.; Fadiran, E.O.; Uhl, K.; Throckmorton, D.C. Adverse Effects in Women: Implications for Drug Development and Regulatory Policies. Expert Rev. Clin. Pharmacol. 2011, 4, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Fadiran, E.; Zhang, L. Effects of Sex Differences in the Pharmacokinetics of Drugs and Their Impact on the Safety of Medicines in Women. Med. Women 2015, 41–68. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, M.; Aweeka, F.; Greenblatt, R.M.; Blaschke, T.F. Sex Differences in Pharmacokinetics and Pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Madla, C.M.; Gavins, F.K.H.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s Talk about Sex: Differences in Drug Therapy in Males and Females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef]
- Krecic-Shepard, M.E.; Barnas, C.R.; Slimko, J.; Jones, M.P.; Schwartz, J.B. Gender-Specific Effects on Verapamil Pharmacokinetics and Pharmacodynamics in Humans. J. Clin. Pharmacol. 2000, 40, 219–230. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex Differences in Pharmacokinetics Predict Adverse Drug Reactions in Women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- De Vries, S.T.; Denig, P.; Ekhart, C.; Burgers, J.S.; Kleefstra, N.; Mol, P.G.M.; van Puijenbroek, E.P. Sex Differences in Adverse Drug Reactions Reported to the National Pharmacovigilance Centre in the Netherlands: An Explorative Observational Study. Br. J. Clin. Pharmacol. 2019, 85, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, L.C.; van der Linden, P.D.; Lagro-Janssen, A.L.M.; van den Bemt, P.M.L.A.; Siiskonen, S.J.; Teichert, M.; Kuiper, J.G.; Herings, R.M.C.; Stricker, B.H.; Visser, L.E. Sex Differences Associated with Adverse Drug Reactions Resulting in Hospital Admissions. Biol. Sex Differ. 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Poovathai, R.; Pondiyadanar, S. Influences of Gender on Postoperative Morphine Consumption. J. Clin. Diagn. Res. JCDR 2014, 8, GC04–GC07. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Sex Differences in Pain and Pain Inhibition: Multiple Explanations of a Controversial Phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-dasilva, M.C.; Rahim-williams, B.; Riley, J.L.; Dentistry, C. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.L.; Robinson, M.E.; Wise, E.A.; Myers, C.D.; Fillingim, R.B. Sex Differences in the Perception of Noxious Experimental Stimuli: A Meta-Analysis. Pain 1998, 74, 181–187. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racine, M.; Tousignant-Laflamme, Y.; Kloda, L.A.; Dion, D.; Dupuis, G.; Choinire, M. A Systematic Literature Review of 10 Years of Research on Sex/Gender and Experimental Pain Perception—Part 1: Are There Really Differences between Women and Men? Pain 2012, 153, 602–618. [Google Scholar] [CrossRef]
- Unruh, A.M. Gender Variations in Clinical Pain Experience. Pain 1996, 65, 123–167. [Google Scholar] [CrossRef]
- Berkley, K. Sex Differences in Pain. J. Am. Dent. Assoc. 1997, 143, 764–765. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Maixner, W. Gender Differences in the Responses to Noxious Stimuli. Pain Forum 1995, 4, 209–221. [Google Scholar] [CrossRef]
- Packiasabapathy, S.; Sadhasivam, S. Gender, Genetics, and Analgesia: Understanding the Differences in Response to Pain Relief. J. Pain Res. 2018, 11, 2729–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Back, S.E.; Payne, R.L.; Wahlquist, A.H.; Carter, R.E.; Stroud, Z.; Haynes, L.; Hillhouse, M.; Brady, K.T.; Ling, W. Comparative Profiles of Men and Women with Opioid Dependence: Results from a National Multisite Effectiveness Trial. Am. J. Drug Alcohol Abuse 2011, 37, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying Sex and Gender Differences in Pain and Analgesia: A Consensus Report. Pain 2007, 132, 26–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kest, B.; Sarton, E.; Dahan, A. Gender differences in opioid-mediated analgesia: Animal and human studies. Anesthesiology 2000, 93, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, T.J.; Ennis, T.; Ogden, J.; Meyer, E.R. Gender Differences in the Reinforcing Properties of Morphine. Pharmacol. Biochem. Behav. 2000, 65, 91–96. [Google Scholar] [CrossRef]
- Craft, R.M. Sex Differences in Opioid Analgesia: “From Mouse to Man”. Clin. J. Pain 2003, 19, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.Y.; Chow, L.H.; Hung, C.C.; Liu, K.; Ger, L.P.; Wang, P.N. Gender and Pain upon Movement Are Associated with the Requirements for Postoperative Patient-Controlled Iv Analgesia: A Prospective Survey of 2298 Chinese Patients. Can. J. Anesth. 2002, 49, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.L.; Tong, W.N.; Irwin, M.; Ng, K.F.J.; Lo, J.R.; Chan, W.S.; Yang, J. The Efficacy, Applicability and Side-Effects of Postoperative Intravenous Patient-Controlled Morphine Analgesia: An Audit of 1233 Chinese Patients. Anaesth. Intensive Care 1996, 24, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.W.; Hodsman, N.B.A.; McLintock, T.T.C.; Gillies, G.W.A.; Kenny, G.N.C.; McArdle, C.S. The Influence of Patient Characteristics on the Requirements for Postoperative Analgesia. Anaesthesia 1989, 44, 2–6. [Google Scholar] [CrossRef]
- Sarton, E.; Olofsen, E.; Romberg, R.; Den Hartigh, J.; Kest, B.; Nieuwenhuijs, D.; Burm, A.; Teppema, L.; Dahan, A. Sex Differences in Morphine Analgesia: An Experimental Study in Healthy Volunteers. Anesthesiology 2000, 93, 1245–1254. [Google Scholar] [CrossRef]
- Aubrun, F.; Salvi, N.; Coriat, P.; Riou, B. Sex- and Age-Related Differences in Morphine Requirements for Postoperative Pain Relief. Anesthesiology 2005, 103, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Carr, D.B. Women Experience More Pain and Require More Morphine Than Men to Achieve a Similar Degree of Analgesia. Anesth. Analg. 2003, 97, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Comer, S.D.; Cooper, Z.D.; Kowalczyk, W.J.; Sullivan, M.A.; Evans, S.M.; Bisaga, A.M.; Vosburg, S.K. Evaluation of Potential Sex Differences in the Subjective and Analgesic Effects of Morphine in Normal, Healthy Volunteers. Psychopharmacology 2010, 208, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Fillingim, R.B.; Ness, T.J.; Glover, T.L.; Campbell, C.M.; Hastie, B.A.; Price, D.D.; Staud, R. Morphine Responses and Experimental Pain: Sex Differences in Side Effects and Cardiovascular Responses but Not Analgesia. J. Pain 2005, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, P.E.; Jarvis, D.A. Age Is the Best Predictor of Postoperative Morphine Requirements. Pain 1996, 64, 357–364. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Farrar, J.T.; Baumgarten, M.; Boston, R.; Carr, D.B.; Strom, B.L. Side Effects of Opioids during Short-Term Administration: Effect of Age, Gender, and Race. Clin. Pharmacol. Ther. 2003, 74, 102–112. [Google Scholar] [CrossRef]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A. Difference in Risk Factors for Postoperative Nausea and Vomiting. J. Am. Soc. Anesthesiol. 2003, 98, 46–52. [Google Scholar] [CrossRef]
- Myles, P.S.; McLeod, A.D.M.; Hunt, J.O.; Fletcher, H. Sex Differences in Speed of Emergence and Quality of Recovery after Anaesthesia: Cohort Study. Br. Med. J. 2001, 322, 710–711. [Google Scholar] [CrossRef] [Green Version]
- Zun, L.S.; Downey, L.V.A.; Gossman, W.; Rosenbaum, J.; Sussman, G. Gender Differences in Narcotic-Induced Emesis in the ED. Am. J. Emerg. Med. 2002, 20, 151–154. [Google Scholar] [CrossRef]
- Sarton, E.; Teppema, L.; Dahan, A. Sex Differences in Morphine-Induced Ventilatory Depression Reside within the Peripheral Chemoreflex Loop. J. Am. Soc. Anesthesiol. 1999, 90, 1329–1338. [Google Scholar] [CrossRef]
- Dahan, A.; Sarton, E.; Teppema, L.; Olievier, G. Sex-Related Differences in the Influence of Morphine on Ventilatory Control in Humans. Anesthesiology 1998, 88, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P. Morphine Responses in Humans: A Retrospective Analysis of Sex Differences. Drug Alcohol Depend. 2001, 63, 23–28. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Gear, R.W. Sex Differences in Opioid Analgesia: Clinical and Experimental Findings. Eur. J. Pain 2004, 8, 413–425. [Google Scholar] [CrossRef]
- Niesters, M.; Dahan, A.; Kest, B.; Zacny, J.; Stijnen, T.; Aarts, L.; Sarton, E. Do Sex Differences Exist in Opioid Analgesia ? A Systematic Review and Meta-Analysis of Human Experimental and Clinical Studies. Pain 2010, 151, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Averitt, D.L.; Eidson, L.N.; Doyle, H.H.; Murphy, A.Z. Neuronal and Glial Factors Contributing to Sex Differences in Opioid Modulation of Pain. Neuropsychopharmacology 2019, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Ho, I. Sex Differences in Opioid Analgesia and Addiction: Interactions among Opioid Receptors and Estrogen Receptors. Mol. Pain 2013, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gear, R.; Miaskowski, C.; Gordon, N. Kappa-Opioids Produce Significantly Greater Analgesia in Women than in Men. Nat. Med. 1996, 2, 1248–1250. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Ness, T.J.; Glover, T.L.; Campbell, C.M.; Price, D.D.; Staud, R. Experimental Pain Models Reveal No Sex Differences in Pentazocine Analgesia in Humans. Anesthesiology 2004, 100, 1263–1270. [Google Scholar] [CrossRef]
- Mogil, J.S.; Wilson, S.G.; Chesler, E.J.; Rankin, A.L.; Nemmani, K.V.S.; Lariviere, W.R.; Groce, M.K.; Wallace, M.R.; Kaplan, L.; Staud, R.; et al. The Melanocortin-1 Receptor Gene Mediates Female-Specific Mechanisms of Analgesia in Mice and Humans. Proc. Natl. Acad. Sci. USA 2003, 100, 4867–4872. [Google Scholar] [CrossRef] [Green Version]
- Olofsen, E.; Romberg, R.; Bijl, H.; Mooren, R.; Engbers, F.; Kest, B.; Dahan, A. Alfentanil and Placebo Analgesia. Anesthesiology 2005, 103, 130–139. [Google Scholar] [CrossRef]
- Romberg, R.; Olofsen, E.; Sarton, E.; Den Hartigh, J.; Taschner, P.E.M.; Dahan, A. Pharmacokinetic-Pharmacodynamic Modeling of Morphine-6-Glucuronide-Induced Analgesia in Healthy Volunteers: Absence of Sex Differences. Anesthesiology 2004, 100, 120–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, A.; Kest, B.; Waxman, A.R.; Sarton, E. Sex-Specific Responses to Opiates: Animal and Human Studies. Anesth. Analg. 2008, 107, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Koons, A.L.; Greenberg, M.R.; Cannon, R.D.; Beauchamp, G.A. Women and the Experience of Pain and Opioid Use Disorder: A Literature-Based Commentary. Clin. Ther. 2018, 40, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, S.M.; Young, E.A.; Kerber, K.B.; Kornstein, S.; Farabaugh, A.H.; Mitchell, J.; Wisniewski, S.R.; Balasubramani, G.K.; Trivedi, M.H.; Rush, A.J. Gender Differences in Depression: Findings from the STAR*D Study. J. Affect. Disord. 2005, 87, 141–150. [Google Scholar] [CrossRef]
- LeGates, T.A.; Kvarta, M.D.; Thompson, S.M. Sex Differences in Antidepressant Efficacy. Neuropsychopharmacology 2019, 44, 140–154. [Google Scholar] [CrossRef] [Green Version]
- Keers, R.; Aitchison, K.J. Gender Differences in Antidepressant Drug Response. Int. Rev. Psychiatry 2010, 22, 485–500. [Google Scholar] [CrossRef]
- Marazziti, D.; Baroni, S.; Picchetti, M.; Piccinni, A.; Carlini, M.; Vatteroni, E.; Falaschi, V.; Lombardi, A.; Dell’osso, L. Pharmacokinetics and Pharmacodinamics of Psychotropic Drugs: Effect of Sex. CNS Spectr. 2013, 18, 118–127. [Google Scholar] [CrossRef]
- Kloiber, S.; Ising, M.; Reppermund, S.; Horstmann, S.; Dose, T.; Majer, M.; Zihl, J.; Pfister, H.; Unschuld, P.G.; Holsboer, F.; et al. Overweight and Obesity Affect Treatment Response in Major Depression. Biol. Psychiatry 2007, 62, 321–326. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Petersen, T.; Iosifescu, D.V.; Burns, A.M.; Nierenberg, A.A.; Alpert, J.E.; Rosenbaum, J.F.; Fava, M. Obesity among Outpatients with Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2005, 8, 59–63. [Google Scholar] [CrossRef]
- Khan, A.; Schwartz, K.A.; Kolts, R.L.; Brown, W.A. BMI, Sex, and Antidepressant Response. J. Affect. Disord. 2007, 99, 101–106. [Google Scholar] [CrossRef]
- Bigos, K.L.; Pollock, B.G.; Stankevich, B.A.; Bies, R.R. Sex Differences in the Pharmacokinetics and Pharmacodynamics of Antidepressants: An Updated Review. Gend. Med. 2009, 6, 522–543. [Google Scholar] [CrossRef]
- Smith, K.A.; Fairburn, C.G.; Cowen, P.J. Relapse of Depression after Vapid Depletion of Tryptophan. Lancet 1997, 349, 915–919. [Google Scholar] [CrossRef]
- Maes, M.; Jacobs, M.P.; Suy, E.; Minner, B.; Leclercq, C.; Christiaens, F.; Raus, J. Suppressant Effects of Dexamethasone on the Availability of Plasma L-tryptophan and Tyrosine in Healthy Controls and in Depressed Patients. Acta Psychiatr. Scand. 1990, 81, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Akhter, S.; Afridi, M.I. Gender Based Response to Fluoxetine Hydrochloride Medication in Endogenous Depression. J. Coll. Physicians Surg. Pak. 2004, 14, 161–165. [Google Scholar] [PubMed]
- Ejsing, T.B.; Linnet, K. Influence of P-Glycoprotein Inhibition on the Distribution of the Tricyclic Antidepressant Nortriptyline over the Blood-Brain Barrier. Hum. Psychopharmacol. 2005, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Uhr, M.; Grauer, M.T.; Holsboer, F. Differential Enhancement of Antidepressant Penetration into the Brain in Mice with Abcb1ab (Mdr1ab) P-Glycoprotein Gene Disruption. Biol. Psychiatry 2003, 54, 840–846. [Google Scholar] [CrossRef]
- Damoiseaux, V.A.; Proost, J.H.; Jiawan, V.C.R.; Melgert, B.N. Sex Differences in the Pharmacokinetics of Antidepressants: Influence of Female Sex Hormones and Oral Contraceptives. Clin. Pharmacokinet. 2014, 53, 509–519. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Davis, P.G. Combination Treatment with Benzodiazepines and SSRIs for Comorbid Anxiety and Depression: A Review. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 222–228. [Google Scholar] [CrossRef]
- Smith, W.T.; Londborg, P.D.; Glaudin, V.; Painter, J.R. Short Term Treatment of Fluoxetine with Clonazepam in the Treatment of Depression: A Double-Blind Study. Am. J. Psychiatry 1998, 155, 1339–1351. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Preskorn, S.H.; Cotreau, M.M.; Horst, W.D.; Harmatz, J.S. Fluoxetine Impairs Clearance of Alprazolam but Not of Clonazepam. Clin. Pharmacol. Ther. 1992, 52, 479–486. [Google Scholar] [CrossRef]
- Lemberger, L.; Rowe, H.; Bosomworth, J.C.; Tenbarge, J.B.; Bergstrom, R.F. The Effect of Fluoxetine on the Pharmacokinetics and Psychomotor Responses of Diazepam. Clin. Pharmacol. Ther. 1988, 43, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; Scordo, M.G.; D’Arrigo, C. Metabolic Drug Interactions with New Psychotropic Agents. Fundam. Clin. Pharmacol. 2003, 17, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Carpenter, L. Sex Differences in Recurent Depression: Are There Any That Are Significant? Am. J. Psychiatry 1988, 145, 41–45. [Google Scholar] [PubMed]
- Kim, J.; Kim, S.; Stewart, R.; Kim, S.; Yoon, Y.; Jung, S. Predictors of 12-Week Remission in a Nationwide Cohort of People with Depressive Disorders: The CRESCEND Study. J. Clin. Psychiatry 2011, 55, 391–393. [Google Scholar] [CrossRef]
- Berlanga, C.; Flores-Ramos, M. Different Gender Response to Serotonergic and Noradrenergic Antidepressants. A Comparative Study of the Efficacy of Citalopram and Reboxetine. J. Affect. Disord. 2006, 95, 119–123. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of Outcomes with Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Martényi, F.; Dossenbach, M.; Mraz, K.; Metcalfe, S. Gender Differences in the Efficacy of Fluoxetine and Maprotiline in Depressed Patients: A Double-Blind Trial of Antidepressants with Serotonergic or Norepinephrinergic Reuptake Inhibition Profile. Eur. Neuropsychopharmacol. 2001, 11, 227–232. [Google Scholar] [CrossRef]
- Kornstein, S.G.; Schatzberg, A.F.; Thase, M.E.; Yonkers, K.A.; McCullough, J.P.; Keitner, G.I.; Gelenberg, A.J.; Davis, S.M.; Harrison, W.M.; Keller, M.B. Gender Differences in Treatment Response to Sertraline versus Imipramine in Chronic Depression. Am. J. Psychiatry 2000, 157, 1445–1452. [Google Scholar] [CrossRef]
- Baca, E.; Garcia-Garcia, M.; Porras-Chavarino, A. Gender Differences in Treatment Response to Sertraline versus Imipramine in Patients with Nonmelancholic Depressive Disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 57–65. [Google Scholar] [CrossRef]
- Khan, A.; Brodhead, A.E.; Schwartz, K.A.; Kolts, R.L.; Brown, W.A. Sex Differences in Antidepressant Response in Recent Antidepressant Clinical Trials. J. Clin. Psychopharmacol. 2005, 25, 318–324. [Google Scholar] [CrossRef]
- Pande, A.C.; Birkett, M.; Fechner-Bates, S.; Haskett, R.F.; Greden, J.F. Fluoxetine versus Phenelzine in Atypical Depression. Biol. Psychiatry 1996, 40, 1017–1020. [Google Scholar] [CrossRef]
- Thase, M.E.; Entsuah, R.; Cantillon, M.; Kornstein, S.G. Relative Antidepressant Efficacy of Venlafaxine and SSRIs: Sex-Age Interactions. J. Women’s Health 2005, 14, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, T.; Storosum, J.G.; Elferink, A.J.A.; Van Zwieten, B.J.; Fouwels, A.; Van Den Brink, W. Response to Tricyclic Antidepressants: Independent of Gender? Am. J. Psychiatry 2004, 161, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Quitkin, F.M.; Stewart, J.W.; McGrath, P.J. Are There Differences between Women’s and Men’s Antidepressant Responses? Prim. Care Companion J. Clin. Psychiatry 2002, 4, 205. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.G.; Steyerberg, E.W.; Stage, K.B.; Passchier, J.; Kragh-soerensen, P. Are Gender Differences Important for the Clinical Effects of Antidepressants? Am. J. Psychiatry 2003, 160, 1643–1650. [Google Scholar] [CrossRef] [Green Version]
- Parker, G.; Parker, K.; Austin, M.P.; Mitchell, P.; Brotchie, H. Gender Differences in Response to Differing Antidepressant Drug Classes: Two Negative Studies. Psychol. Med. 2003, 33, 1473–1477. [Google Scholar] [CrossRef]
- Marsh, W.; Deligiannidis, K. Sex-Related Differences in Antidepressant Response: When to Adjust Treatment. Curr. Psychiatr. 2010, 9, 25–30. [Google Scholar]
- Coppen, A.; Bailey, J. Enhancement of the Antidepressant Action of FLuoxetine by Folic Acid: A Randomised, Placebo Controlled Trial. J. Affect. Disord. 2000, 60, 121–130. [Google Scholar] [CrossRef]
- Altshuler, L.L.; Bauer, M.; Frye, M.A.; Gitlin, M.J.; Mintz, J.; Szuba, M.P.; Leight, K.L.; Whybrow, P.C. Does Thyroid Supplementation Accelerate Tricyclic Antidepressant Response? A Review and Meta-Analysis of the Literature. Am. J. Psychiatry 2001, 158, 1617–1622. [Google Scholar] [CrossRef]
- Zemishlany, Z.; Weizman, A. The Impact of Mental Illness on Sexual Dysfunction. Adv. Psychosom. Med. 2008, 29, 89–106. [Google Scholar] [CrossRef]
- Clayton, A.; Keller, A.; McGarvey, E.L. Burden of Phase-Specific Sexual Dysfunction with SSRIs. J. Affect. Disord. 2006, 91, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.; Nutt, D. Antidepressants. Psychiatry 2007, 6, 289–294. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Judge, R. Treatment of Depression with Associated Anxiety: Comparison of Tricyclic Antidepressant and Selective Serotonin Reuptake Inhibitors. Acta Psychiatr. Scand. Suppl. 2000, 101, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Peretti, S.; Judge, R.; Hindmarch, I. Safety and Tolerability Considerations: Tricyclic Antidepressants vs. Selective Serotonin Reuptake Inhibitors. Acta Psychiatr. Scand. 2000, 101, 17–25. [Google Scholar] [CrossRef]
- Samba Reddy, D. Sex Differences in the Anticonvulsant Activity of Neurosteroids. J. Neurosci. Res. 2017, 95, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Verrotti, A.; Greco, R.; Giannuzzi, R.; Chiarelli, F.; Latini, G. Old and New Antiepileptic Drugs for the Treatment of Idiopathic Generalized Epilepsies. Curr. Clin. Pharmacol. 2008, 2, 249–259. [Google Scholar] [CrossRef]
- Herzog, A.G. Hormonal Therapies: Progesterone. Neurotherapeutics 2009, 6, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Younus, I.; Reddy, D.S. Seizure Facilitating Activity of the Oral Contraceptive Ethinyl Estradiol. Epilepsy Res. 2016, 121, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Herzog, A.G. Differential Impact of Antiepileptic Drugs on the Effects of Contraceptive Methods on Seizures: Interim Findings of the Epilepsy Birth Control Registry. Seizure 2015, 28, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Perucca, E.; Battino, D.; Tomson, T. Gender Issues in Antiepileptic Drug Treatment. Neurobiol. Dis. 2014, 72, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Johannessen Landmark, C.; Johannessen, S.I.; Tomson, T. Host Factors Affecting Antiepileptic Drug Delivery-Pharmacokinetic Variability. Adv. Drug Deliv. Rev. 2012, 64, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Blum, A.S.; Farina, E.L.; Maestri, X.E.; Newman, J.; Garcia, E.; Krishnamurthy, K.B.; Hoch, D.B.; Replansky, S.; Fowler, K.M.; et al. Valproate and Lamotrigine Level Variation with Menstrual Cycle Phase and Oral Contraceptive Use. Neurology 2009, 72, 911–914. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Guillebaud, J. Contraception for Women Taking Antiepileptic Drugs. J. Fam. Plan. Reprod. Health Care 2011, 37, 60. [Google Scholar] [CrossRef] [Green Version]
- Patsalos, P.N. Drug Interactions with the Newer Antiepileptic Drugs (AEDs)—Part 2: Pharmacokinetic and Pharmacodynamic Interactions between AEDs and Drugs Used to Treat Non-Epilepsy Disorders. Clin. Pharmacokinet. 2013, 52, 1045–1061. [Google Scholar] [CrossRef]
- Boruban, M.C. Tamoxifen Inhibits Cytochrome P450 2C9. J. Chemother. 2006, 18, 421–424. [Google Scholar] [CrossRef]
- Cialis 2.5 mg, 5 mg, 10 mg & 20 mg Film-Coated Tablets—Summary of Product Characteristics (SmPC)—(Emc). Available online: https://www.medicines.org.uk/emc/medicine/11363#INTERACTIONS (accessed on 20 January 2021).
- Tomson, T.; Landmark, C.J.; Battino, D. Antiepileptic Drug Treatment in Pregnancy: Changes in Drug Disposition and Their Clinical Implications. Epilepsia 2013, 54, 405–414. [Google Scholar] [CrossRef]
- Reimers, A.; Helde, G.; Bråthen, G.; Brodtkorb, E. Lamotrigine and Its N2-Glucuronide during Pregnancy: The Significance of Renal Clearance and Estradiol. Epilepsy Res. 2011, 94, 198–205. [Google Scholar] [CrossRef]
- Petrenaite, V.; Sabers, A.; Hansen-Schwartz, J. Seizure Deterioration in Women Treated with Oxcarbazepine during Pregnancy. Epilepsy Res. 2009, 84, 245–249. [Google Scholar] [CrossRef]
- Tomson, T.; Palm, R.; Källén, K.; Ben-Menachem, E.; Söderfeldt, B.; Danielsson, B.; Johansson, R.; Luef, G.; Öhman, I. Pharmacokinetics of Levetiracetam during Pregnancy, Delivery, in the Neonatal Period, and Lactation. Epilepsia 2007, 48, 1111–1116. [Google Scholar] [CrossRef]
- Verrotti, A.; D’Egidio, C.; Mohn, A.; Coppola, G.; Parisi, P.; Chiarelli, F. Antiepileptic Drugs, Sex Hormones, and PCOS. Epilepsia 2011, 52, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Mintzer, S.; Pack, A.M.; Gidal, B.E.; Vecht, C.J.; Schmidt, D. Enzyme Induction with Antiepileptic Drugs: Cause for Concern? Epilepsia 2013, 54, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Isojärvi, J.I.; Taubøll, E.; Herzog, A.G. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drugs 2005, 19, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Drislane, F.W.; Schomer, D.L.; Pennell, P.B.; Bromfield, E.B.; Dworetzky, B.A.; Farina, E.L.; Frye, C.A. Differential Effects of Antiepileptic Drugs on Neuroactive Steroids in Men with Epilepsy. Epilepsia 2006, 47, 1945–1948. [Google Scholar] [CrossRef]
- Bauer, J.; Isojärvi, J.I.T.; Herzog, A.G.; Reuber, M.; Polson, D.; Taubøll, E.; Genton, P.; Van der Ven, H.; Roesing, B.; Luef, G.J.; et al. Reproductive Dysfunction in Women with Epilepsy: Recommendations for Evaluation and Management. J. Neurol. Neurosurg. Psychiatry 2002, 73, 121–125. [Google Scholar] [CrossRef]
- Rubin, L.H.; Haas, G.L.; Keshavan, M.S.; Sweeney, J.A.; Maki, P.M. Sex Difference in Cognitive Response to Antipsychotic Treatment in First Episode Schizophrenia. Neuropsychopharmacology 2008, 33, 290–297. [Google Scholar] [CrossRef]
- Usall, J.; Araya, S.; Ochoa, S.; Busquets, E.; Gost, A.; Márquez, M. Gender Differences in a Sample of Schizophrenic Outpatients. Compr. Psychiatry 2001, 42, 301–305. [Google Scholar] [CrossRef]
- Häfner, H. Gender Differences in Schizophrenia. Psychoneuroendocrinology 2003, 28, 17–54. [Google Scholar] [CrossRef]
- Seeman, M.V. Gender Differences in the Prescribing of Antipsychotic Drugs. Am. J. Psychiatry 2004, 161, 1324–1333. [Google Scholar] [CrossRef]
- Crawford, M.B.; DeLisi, L.E. Issues Related to Sex Differences in Antipsychotic Treatment. Curr. Opin. Psychiatry 2016, 29, 211–217. [Google Scholar] [CrossRef]
- Smith, S. Gender Differences in Antipsychotic Prescribing. Int. Rev. Psychiatry 2010, 22, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Zyprexa—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/zyprexa-epar-product-information_en.pdf (accessed on 1 March 2021).
- Sainz, J.; Prieto, C.; Crespo-facorro, B. Sex Differences in Gene Expression Related to Antipsychotic Induced Weight Gain. PLoS ONE 2019, 14, e0215477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, B.; Mueller, J.K.; Leweke, F.M.; Bumb, J.M. How Gender Affects the Pharmacotherapeutic Approach to Treating Psychosis—A Systematic Review. Expert Opin. Pharmacother. 2017, 18, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Men and Women Respond Differently to Antipsychotic Drugs. Neuropharmacology 2020, 163, 107631. [Google Scholar] [CrossRef]
- Usall, J.; Suarez, D.; Haro, J.M. Gender Differences in Response to Antipsychotic Treatment in Outpatients with Schizophrenia. Psychiatry Res. 2007, 153, 225–231. [Google Scholar] [CrossRef]
- Salokangas, R.K.R. Gender and the Use of Neuroleptics in Schizophrenia. Schizophr. Res. 2004, 66, 41–49. [Google Scholar] [CrossRef]
- Melkersson, K.I.; Hulting, A.L.; Rane, A.J. Dose Requirement and Prolactin Elevation of Antipsychotics in Male and Female Patients with Schizophrenia or Related Psychoses. Br. J. Clin. Pharmacol. 2001, 51, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Walther, S.; Moggi, F.; Horn, H.; Moskvitin, K.; Abderhalden, C.; Maier, N.; Strik, W.; Müller, T.J. Rapid Tranquilization of Severely Agitated Patients with Schizophrenia Spectrum Disorders: A Naturalistic, Rater-Blinded, Randomized, Controlled Study with Oral Haloperidol, Risperidone, and Olanzapine. J. Clin. Psychopharmacol. 2014, 34, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Haslemo, T.; Eikeseth, P.H.; Tanum, L.; Molden, E.; Refsum, H. The Effect of Variable Cigarette Consumption on the Interaction with Clozapine and Olanzapine. Eur. J. Clin. Pharmacol. 2006, 62, 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Zullino, D.F.; Delessert, D.; Eap, C.B.; Preisig, M.; Baumann, P. Tobacco and Cannabis Smoking Cessation Can Lead to Intoxication with Clozapine or Olanzapine. Int. Clin. Psychopharmacol. 2002, 17, 141–143. [Google Scholar] [CrossRef]
- Lowe, E.J.; Ackman, M.L. Impact of Tobacco Smoking Cessation on Stable Clozapine or Olanzapine Treatment. Ann. Pharmacother. 2010, 44, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Kraal, A.Z.; Ward, K.M.; Ellingrod, V.L. Sex Differences in Antipsychotic Related Metabolic Functioning in Schizophrenia Spectrum Disorders. Psychopharmacol. Bull. 2017, 47, 8–21. [Google Scholar] [PubMed]
- Russell, J.M.; Mackell, J.A. Bodyweight Gain Associated with Atypical Antipsychotics: Epidemiology and Therapeutic Implications. CNS Drugs 2001, 15, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Homel, P.; Casey, D.; Allison, D.B. Changes in Body Mass Index for Individuals with and without Schizophrenia, 1987-1996. Schizophr. Res. 2002, 55, 277–284. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Aichhorn, W.; Whitworth, A.B.; Weiss, E.M.; Marksteiner, J. Second-Generation Antipsychotics: Is There Evidence for Sex Differences in Pharmacokinetic and Adverse Effect Profiles? Drug Saf. 2006, 29, 587–598. [Google Scholar] [CrossRef]
- Klibanski, A.; Neer, R.; Beitins, I.; Ridgway, C. Decreased Bone Density in Hyperprolactinemic Women. N. Engl. J. Med. 1980, 303, 1511–1514. [Google Scholar] [CrossRef]
- Hamner, M.B.; Arana, G.W. Hyperprolactinaemia in Antipsychotic-Treated Patients: Guidelines for Avoidance and Management. CNS Drugs 1998, 10, 209–222. [Google Scholar] [CrossRef]
- Debusk, R.; Drory, Y.; Goldstein, I.; Jackson, G.; Kaul, S.; Kimmel, S.E.; Kostis, J.B.; Kloner, R.A.; Lakin, M.; Meston, C.M.; et al. Management of Sexual Dysfunction in Patients with Cardiovascular Disease: Recommendations of the Princeton Consensus Panel. Am. J. Cardiol. 2000, 86, 62–68. [Google Scholar] [CrossRef]
- Knegtering, H.; Van Der Moolen, A.E.G.M.; Castelein, S.; Kluiter, H.; Van Den Bosch, R.J. What Are the Effects of Antipsychotics on Sexual Dysfunctions and Endocrine Functioning? Psychoneuroendocrinology 2003, 28, 109–123. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Popkin, M.K. Antipsychotic Drug Therapy and Sexual Dysfunction in Men. Am. J. Psychiatry 1982, 139, 633–637. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).