Functional Capacity in Patients Who Recovered from Mild COVID-19 with Exertional Dyspnea
Abstract
:1. Introduction
2. Methods
2.1. Research Design
- 1.
- Mild disease–symptomatic patients without evidence of viral pneumonia or hypoxia.
- 2.
- Moderate disease–patients with clinical signs of pneumonia but no signs of severe pneumonia, including oxygen saturation (SpO2) ≥ 90% on room air.
- 3.
- Severe disease–severe pneumonia: clinical signs of pneumonia plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air.
- 4.
- Critical disease–COVID-19 related ARDS.
2.2. Patients
2.3. Study Protocol
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. PFTs, 6MWT and Echocardiography
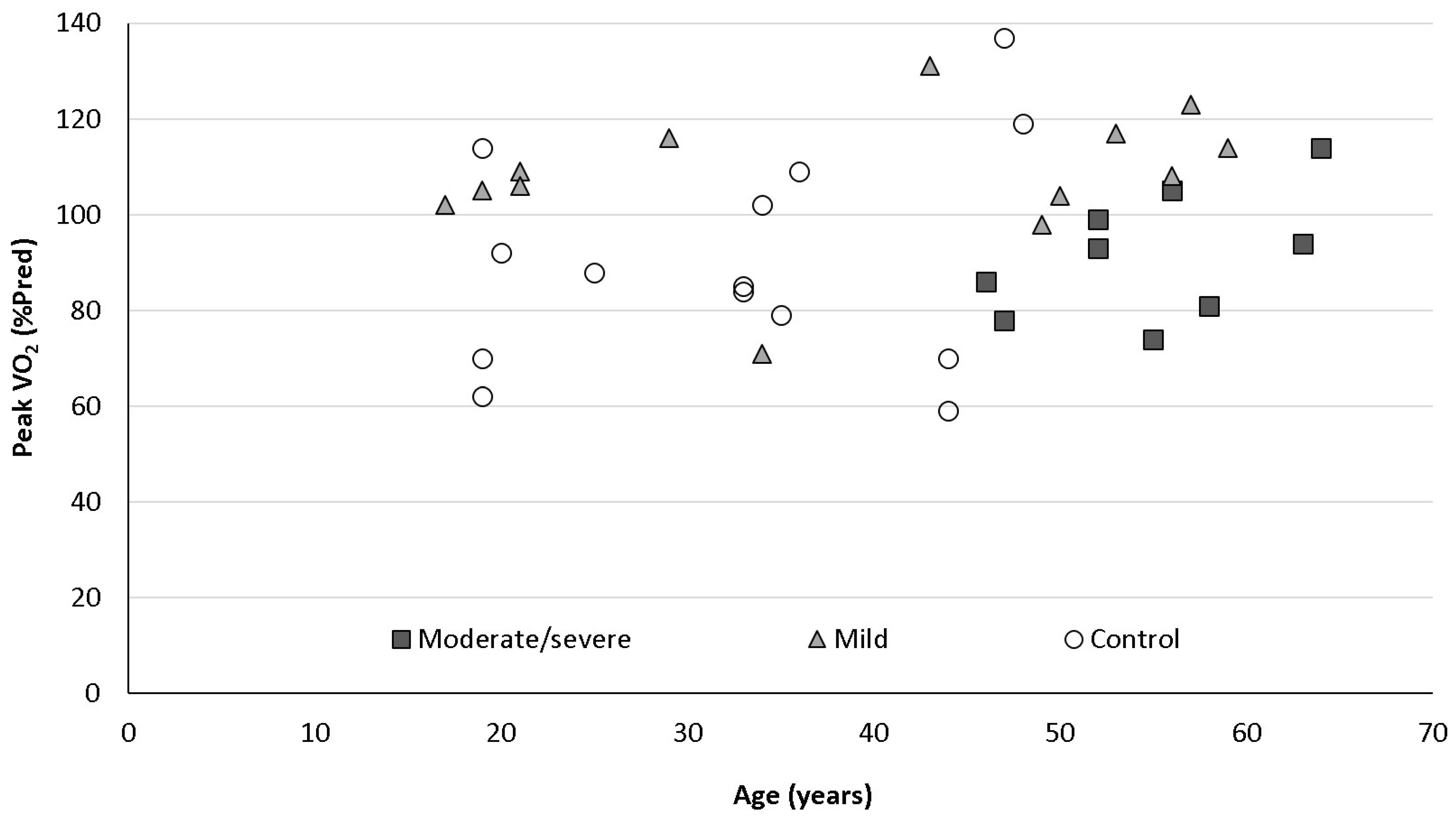
3.3. CPET Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Yek, C.; Warner, S.; Wiltz, J.L.; Sun, J.; Adjei, S.; Mancera, A.; Silk, B.J.; Gundlapalli, A.V.; Harris, A.M.; Boehmer, T.K.; et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series—465 Health Care Facilities, United States, December 2020–October 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients With COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.S.; Wong, K.T.; Ko, F.W.; Tam, L.S.; Chan, D.P.; Woo, J.; Sung, J.J. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005, 128, 2247–2261. [Google Scholar] [CrossRef] [Green Version]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.B.; Jun, K.I.; Kim, G.; Choi, J.P.; Rhee, J.Y.; Cheon, S.; Lee, C.H.; Park, J.S.; Kim, Y.; Joh, J.S.; et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J. Korean Med. Sci. 2018, 33, e169. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.; Yu, M.W.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental Morbidities and Chronic Fatigue in Severe Acute Respiratory Syndrome Survivors: Long-term Follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems during the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, R.; Geng, Q.; Mo, X.; Zhan, C.; Jian, W.; Li, S.; Zheng, J. Cardiopulmonary Exercise Test might be Helpful for Insight Interpretation of Impaired Pulmonary Function on Recovered COVID-19 Patients. Eur. Respir. J. 2021, 57, 2004265. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Mohr, A.; Dannerbeck, L.; Lange, T.J.; Pfeifer, M.; Blaas, S.; Salzberger, B.; Hitzenbichler, F.; Koch, M. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip. Respir. Med. 2021, 16, 732. [Google Scholar] [CrossRef] [PubMed]
- Clavario, P.; De Marzo, V.; Lotti, R.; Barbara, C.; Porcile, A.; Russo, C.; Beccaria, F.; Bonavia, M.; Bottaro, L.C.; Caltabellotta, M.; et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int. J. Cardiol. 2021, 340, 113–118. [Google Scholar] [CrossRef]
- Alba, G.A.; Ziehr, D.R.; Rouvina, J.N.; Hariri, L.P.; Knipe, R.S.; Medoff, B.D.; Hibbert, K.A.; Kowal, A.; Hoenstine, C.; Ginns, L.C.; et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. EClinicalMedicine 2021, 39, 101066. [Google Scholar] [CrossRef]
- Barbagelata, L.; Masson, W.; Iglesias, D.; Lillo, E.; Migone, J.F.; Orazi, M.L.; Maritano Furcada, J. Cardiopulmonary Exercise Testing in Patients with Post-COVID-19 Syndrome. Med. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cassar, M.P.; Tunnicliffe, E.M.; Petousi, N.; Lewandowski, A.J.; Xie, C.; Mahmod, M.; Samat, A.H.A.; Evans, R.A.; Brightling, C.E.; Ho, L.P.; et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health—Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine 2021, 41, 101159. [Google Scholar] [CrossRef]
- Clinical Management of COVID-19: Interim Guidance, 27 May 2020. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 31 October 2021).
- Culver, B.H.; Graham, B.L.; Coates, A.L.; Wanger, J.; Berry, C.E.; Clarke, P.K.; Hallstrand, T.S.; Hankinson, J.L.; Kaminsky, D.A.; MacIntyre, N.R.; et al. ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am. J. Respir. Crit. Care Med. 2017, 196, 1463–1472. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95 yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Ross, R.M. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 1451. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.E.; Sun, X.G.; Whipp, B.J. Principles of Exercise Testing and Interpretation, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Huang, Y.; Tan, C.; Wu, J.; Chen, M.; Wang, Z.; Luo, L.; Zhou, X.; Liu, X.; Huang, X.; Yuan, S.; et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Cares-Marambio, K.; Montenegro-Jiménez, Y.; Torres-Castro, R.; Vera-Uribe, R.; Torralba, Y.; Alsina-Restoy, X.; Vasconcello-Castillo, L.; Vilaró, J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chronic Respir. Dis. 2021, 18, 14799731211002240. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Abdallah, S.J.; Voduc, N.; Corrales-Medina, V.F.; McGuinty, M.; Pratt, A.; Chopra, A.; Law, A.; Garuba, H.A.; Thavorn, K.; Reid, R.E.R.; et al. Symptoms, Pulmonary Function, and Functional Capacity Four Months after COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 1912–1917. [Google Scholar] [CrossRef]
- Porfidia, A.; Valeriani, E.; Pola, R.; Porreca, E.; Rutjes, A.W.S.; Di Nisio, M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb. Res. 2020, 196, 67–74. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Lagou, S.; Kontopantelis, E.; Stergiou, G.S.; Syrigos, K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc. Med. 2021, 26, 415–425. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Kravchenko, D.; Isaak, A.; Zimmer, S.; Mesropyan, N.; Reinert, M.; Faron, A.; Pieper, C.C.; Heine, A.; Velten, M.; Nattermann, J.; et al. Cardiac MRI in Patients with Prolonged Cardiorespiratory Symptoms after Mild to Moderate COVID-19. Radiology 2021, 301, E419–E425. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Khamsi, R. Rogue antibodies could be driving severe COVID-19. Nature 2021, 590, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rubin, F.; Ludwig, B.; Rietzsch, H.; Schwarz, P.E.H.; Rodionov, R.N.; Khunti, K.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; et al. Consequences of the COVID-19 pandemic on patients with metabolic diseases. Nat. Metab. 2021, 3, 289–292. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Voit-Bak, K.; Donate, T.; Rodionov, R.N.; Gainetdinov, R.R.; Tselmin, S.; Kanczkowski, W.; Müller, G.M.; Achleitner, M.; Wang, J.; et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol. Psychiatry 2021, 27, 34–37. [Google Scholar] [CrossRef]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent Exertional Intolerance After COVID-19: Insights from Invasive Cardiopulmonary Exercise Testing. Chest 2022, 161, 54–63. [Google Scholar] [CrossRef]

| Mild (n = 13) | Moderate/Severe (n = 9) | Control Group (n = 14) | |
|---|---|---|---|
| Age (years) | 37 ± 16 * | 53 ± 4 | 33 ± 11 |
| Gender (%F) | 62% | 45% | 29% |
| BMI (kg/m2) | 29 ± 7 | 31 ± 3 | 27 ± 6 |
| Hospitalization (days) | 0 | 7 ± 5 | NR |
| Supplemental oxygen (% needed) | 0 | 78% | NR |
| Lung disease (%) | 0 | 0 | 0 |
| Heart disease (%) | 0 | 0 | 0 |
| Hypertension (%) | 8% | 33% | 0 |
| Diabetes mellitus (%) | 0 | 22% | 0 |
| History of cancer (%) | 0 | 11% | 0 |
| Active smoker (%) | 8% | 22% | 21% |
| Mild (n = 13) | Moderate/Severe (n = 9) | Control Group (n = 14) | |
|---|---|---|---|
| FEV1 (% of predicted) | 98 ± 13% | 85 ± 17% | 95 ± 11% |
| FVC (% of predicted) | 99 ± 18 | 81 ± 16 | 97 ± 10 |
| FEV1/FVC | 0.82 ± 0.08 | 0.83 ± 0.03 | 0.82 ± 0.06 |
| TLC (% of predicted) | 88 ± 11 | 88 ± 9 | NA |
| RV (% of predicted) | 100 ± 28 | 114 ± 13 | NA |
| DLCO (% of predicted) | 85 ± 9 * | 64 ± 8 | NA |
| 6MWD (meter) | 594 ± 128 | 593 ± 89 | NA |
| Mild (n = 13) | Moderate/Severe (n = 9) | Control Group (n = 14) | |
|---|---|---|---|
| PeakVO2 (ml/kg/min) | 33 ± 9.9 * | 23 ± 2.7 | 35 ± 10 |
| PeakVO2 (%pred) | 108 ± 14 ᶲ | 92 ± 13 | 91 ± 23 |
| RER | 1.15 ± 0.17 | 1.18 ± 0.18 | 1.24 ± 0.1 |
| PeakHR (bpm) | 177 ± 19 | 164 ± 18 | 183 ± 13 |
| PeakHR (%pred) | 100 ± 6 | 96 ± 11 | 99 ± 6.2 |
| VE/VCO2 slope | 31 ± 7.7 | 34 ± 6.6 | 29 ± 6.4 |
| PeakO2 pulse (%pred) | 101 ± 29 ^ | 95 ± 12.3 | 93 ± 26 |
| SpO2 pretest (%) | 98 ± 1.5 | 99 ± 1 | 99 ± 1 |
| Peak SpO2 (%) | 98 ± 1.3 | 96 ± 4 | 98 ± 1.6 |
| VE (L/min) | 108 ± 31 | 87 ± 18 | 115 ± 33 |
| MVV (L/min) | 133 ± 31 | 113 ± 28 | 124 ± 42 |
| BR (L) | 27 ± 12.6 | 26 ± 13 | 42 ± 30 |
| BR (%) | 20 ± 9.5 | 21 ± 11.3 | 27 ± 18 |
| Normal BR (n = 29) | Low/Borderline BR (n = 7) | |
|---|---|---|
| Age (years) | 37 ± 15 | 46 ± 11 |
| Gender (F/M) | 15/14 | 1/6 |
| BMI (kg/m2) | 28 ± 6 | 30 ± 7 |
| FEV1/FVC | 0.83 + 0.06 | 0.8 ± 0.06 |
| FEV1 (% of predicted) | 97 ± 11 * | 78 ± 13 |
| FVC (% of predicted) | 97 ± 14 * | 80 ± 16 |
| PeakVO2 (ml/kg/min) | 30.6 ± 9.5 | 33.8 ± 12 |
| PeakVO2 (%pred) | 95 ± 19 | 105 ± 22 |
| RER | 1.19 ± 0.1 | 1.18 ± 0.16 |
| Peak HR (bpm) | 178 ± 17 | 169 ± 20 |
| Peak HR (%pred) | 99 ± 6.5 | 96 ± 10.6 |
| VE/VCO2 slope | 31 ± 7.5 | 30 ± 4.5 |
| Peak O2 pulse (%pred) | 93 ± 25 * | 108 ± 13 |
| SpO2 pretest (%) | 97 ± 1.2 | 97 ± 5 |
| Peak SpO2 (%) | 98 ± 1.4 | 97 ± 5 |
| VE (L/min) | 101 ± 27 | 124 ± 38 |
| MVV (L/min) | 128 ± 35 | 110 ± 31 |
| BR (L) | 39 ± 18 * | 4 ± 12 |
| BR (%) | 28 ± 9 * | 3.7 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotan, Y.; Weiner, E.; Zucker-Toledano, M.; Solomonov, A.; Fuchs, E.; Dawood, H.; Mor, E.; Hanna, M.; Naser-Aldeen, R.; Bentur, L.; et al. Functional Capacity in Patients Who Recovered from Mild COVID-19 with Exertional Dyspnea. J. Pers. Med. 2022, 12, 874. https://doi.org/10.3390/jpm12060874
Dotan Y, Weiner E, Zucker-Toledano M, Solomonov A, Fuchs E, Dawood H, Mor E, Hanna M, Naser-Aldeen R, Bentur L, et al. Functional Capacity in Patients Who Recovered from Mild COVID-19 with Exertional Dyspnea. Journal of Personalized Medicine. 2022; 12(6):874. https://doi.org/10.3390/jpm12060874
Chicago/Turabian StyleDotan, Yaniv, Elite Weiner, Merav Zucker-Toledano, Anna Solomonov, Eyal Fuchs, Hanna Dawood, Elad Mor, Moneera Hanna, Rihan Naser-Aldeen, Lea Bentur, and et al. 2022. "Functional Capacity in Patients Who Recovered from Mild COVID-19 with Exertional Dyspnea" Journal of Personalized Medicine 12, no. 6: 874. https://doi.org/10.3390/jpm12060874
APA StyleDotan, Y., Weiner, E., Zucker-Toledano, M., Solomonov, A., Fuchs, E., Dawood, H., Mor, E., Hanna, M., Naser-Aldeen, R., Bentur, L., & Bar-Yoseph, R. (2022). Functional Capacity in Patients Who Recovered from Mild COVID-19 with Exertional Dyspnea. Journal of Personalized Medicine, 12(6), 874. https://doi.org/10.3390/jpm12060874






