Endocrine and Metabolic Illnesses in Young Adults with Prader–Willi Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Measurements
2.3. Definitions of Endocrine and Metabolic Illnesses
2.4. Growth Hormone Treatment
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics of Young Adults with PWS
3.2. Comparison of Health Problems between PWS Cohort and Age-, Sex-, and BMI-Matched Healthy Controls
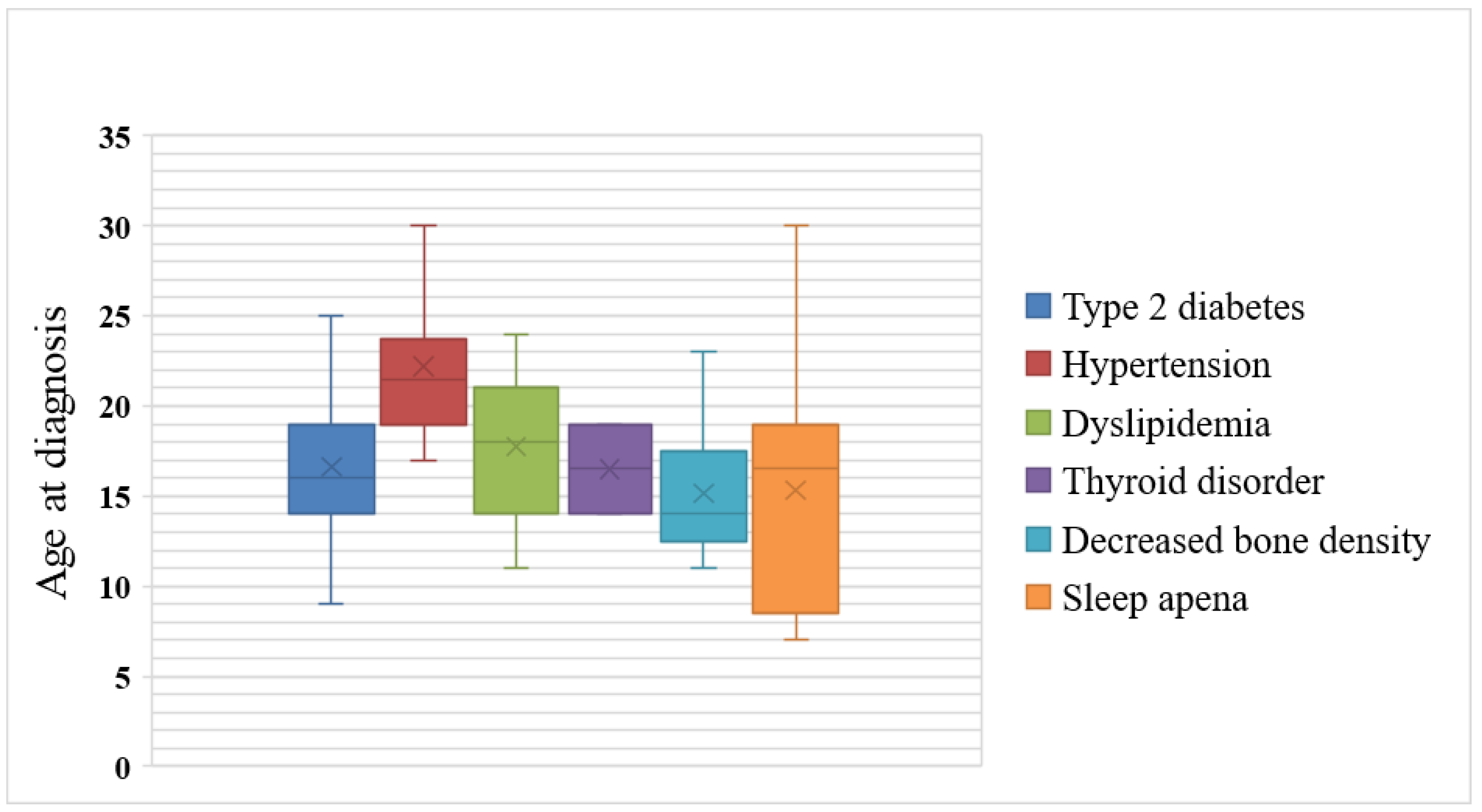
3.3. Age at Diagnosis of Endocrine and Metabolic Illnesses
3.4. Type 2 Diabetes Mellitus
3.5. Hypertension
3.6. Dyslipidemia
3.7. Thyroid Disorders
3.8. Decreased Bone Density
3.9. Sleep Apnea
3.10. Adrenal Insufficiency
3.11. Growth Hormone Treatment in Young Adults with PWS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscogiuri, G.; Formoso, G.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Colao, A. Prader-Willi syndrome: An uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 2019, 20, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.S.; Emerick, J.E. Growth Hormone Therapy in Adults with Prader-Willi Syndrome. Diseases 2015, 3, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Ortiga, R.; Klibanski, A.; Tritos, N.A. Effects of recombinant human growth hormone therapy in adults with Prader-Willi syndrome: A meta-analysis. Clin. Endocrinol. 2012, 77, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.L.; Tony, M.; Hoybye, C.; Allen, D.B.; Tauber, M.; Christiansen, J.S. 2011 Growth Hormone in Prader-Willi Syndrome Clinical Care Guidelines Workshop Participants. GrowthHormone Research Society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1072-87. [Google Scholar]
- Proffitt, J.; Osann, K.; McManus, B.; Kimonis, V.E.; Heinemann, J.; Butler, M.G.; Stevenson, D.A.; Gold, J.-A. Contributing factors of mortality in Prader–Willi syndrome. Am. J. Med. Genet. Part A 2018, 179, 196–205. [Google Scholar] [CrossRef]
- Pellikaan, K.; Rosenberg, A.G.; Kattentidt-Mouravieva, A.A.; Kersseboom, R.; Bos-Roubos, A.G.; Veen-Roelofs, J.M.C.; van Wieringen, N.; Hoekstra, F.M.E.; van der Berg, S.A.A.; van der Lely, A.J.; et al. Missed Diagnoses and Health Problems in Adults With Prader-Willi Syndrome: Recommendations for Screening and Treatment. J. Clin. Endocrinol. Metab. 2020, 105, e4671–e4687. [Google Scholar] [CrossRef]
- Zheng, Y.; Manson, J.E.; Yuan, C.; Liang, M.H.; Grodstein, F.; Stampfer, M.J.; Hu, F.B. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA 2017, 318, 255–269. [Google Scholar] [CrossRef]
- Rhee, E.-J.; Kim, H.C.; Kim, J.H.; Lee, E.Y.; Kim, B.J.; Kim, E.M.; Song, Y.; Lim, J.H.; Kim, H.J.; Choi, S.; et al. 2018 Guidelines for the management of dyslipidemia in Korea. Korean J. Intern. Med. 2019, 34, 1171. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.S.; Hwang, J.S.; Lee, J.A.; Kim, D.H.; Park, K.D.; Cheon, G.J.; Shin, C.H.; Yang, S.W. Bone Mineral Density According to Age, Bone Age, and Pubertal Stages in Korean Children and Adolescents. J. Clin. Densitom. 2010, 13, 68–76. [Google Scholar] [CrossRef]
- Gasco, V.; Caputo, M.; Lanfranco, F.; Ghigo, E.; Grottoli, S. Management of GH treatment in adult GH deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 13–24. [Google Scholar] [CrossRef]
- Butler, M.G.; Manzardo, A.M.; Janice, L.F. Prader-Willi Syndrome: Clinical Genetics and Diagnostic Aspects with Treatment Approaches. Curr. Pediatr. Rev. 2016, 12, 136–166. [Google Scholar] [CrossRef] [PubMed]
- Coupaye, M.; Lorenzini, F.; Lloret-Linares, C.; Molinas, C.; Pinto, G.; Diene, G.; Poitou, C. Growth hormone therapy for children and adolescents with Prader-Willi syndrome is associated with improved body composition and metabolic status in adulthood. J. Clin. Endocrinol. Metab. 2013, 98, E328–E335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fintini, D.; Grugni, G.; Bocchini, S.; Brufani, C.; Di Candia, S.; Corrias, A.; Delvecchio, M.; Salvatoni, A.; Ragusa, L.; Greggio, N.; et al. Disorders of glucose metabolism in Prader–Willi syndrome: Results of a multicenter Italian cohort study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Kim, J.; Cho, S.Y.; Jin, D.-K. Prevalence and risk factors for type 2 diabetes mellitus with Prader–Willi syndrome: A single center experience. Orphanet J. Rare Dis. 2017, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Ng, N.B.H.; Low, Y.W.; Rajgor, D.D.; Low, J.M.; Lim, Y.Y.; Loke, K.Y.; Lee, Y.S. The effects of glucagon-like peptide (GLP)-1 receptor agonists on weight and glycaemic control in Prader–Willi syndrome: A systematic review. Clin. Endocrinol. 2021, 96, 144–154. [Google Scholar] [CrossRef]
- Coupaye, M.; Tauber, M.; Cuisset, L.; Laurier, V.; Bieth, E.; Lacorte, J.-M.; Oppert, J.-M.; Clément, K.; Poitou, C. Effect of Genotype and Previous GH Treatment on Adiposity in Adults With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 4895–4903. [Google Scholar] [CrossRef]
- Laurier, V.; Lapeyrade, A.; Copet, P.; Demeer, G.; Silvie, M.; Bieth, E.; Coupaye, M.; Poitou, C.; Lorenzini, F.; Labrousse, F.; et al. Medical, psychological and social features in a large cohort of adults with Prader-Willi syndrome: Experience from a dedicated centre in France. J. Intellect. Disabil. Res. 2014, 59, 411–421. [Google Scholar] [CrossRef]
- Vaiani, E.; Herzovich, V.; Chaler, E.; Chertkoff, L.; Rivarola, M.A.; Torrado, M.; Belgorosky, A. Thyroid Axis Dysfunction in Patients with Prader-Willi Syndrome during the First 2 Years of Life. Clin. Endocrinol. 2010, 73, 546–550. [Google Scholar] [CrossRef]
- Vestergaard, P.; Kristensen, K.; Bruun, J.M.; Ostergaard, J.R.; Heickendorff, L.; Mosekilde, L. Reduced bone mineral density and increased bone turnover in Prader-Willi syndrome compared with controls matched for sex and body mass index--a cross-sectional study. J. Pediatr. 2004, 144, 614–619. [Google Scholar] [CrossRef]
- Donze, S.H.; Kuppens, R.J.; Bakker, N.E.; Velden, J.A.V.A.-V.D.; Hokken-Koelega, A.C. Bone mineral density in young adults with Prader-Willi syndrome: A randomized, placebo-controlled, crossover GH trial. Clin. Endocrinol. 2018, 88, 806–812. [Google Scholar] [CrossRef]
- Cataldi, M.; Arnaldi, D.; Tucci, V.; De Carli, F.; Patti, G.; Napoli, F.; Pace, M.; Maghnie, M.; Nobili, L. Sleep disorders in Prader-Willi syndrome, evidence from animal models and humans. Sleep Med. Rev. 2021, 57, 101432. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Ambroziak, A.; Wysocka-Mincewicz, M.; Świercz, A.; Jędrzejczak, M.; Szalecki, M. Comparison of Frequency and Severity of Sleep-Related Breathing Disorders in Children with Simple Obesity and Paediatric Patients with Prader–Willi Syndrome. J. Pers. Med. 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; on behalf of the Italian Society of Cardiology (SIC) Working Group on Heart Failure members; Lombardi, C.; Castagna, F.; Mattaliano, P.; Filardi, P.P.; Agostoni, P. Heart failure and sleep disorders. Nat. Rev. Cardiol. 2016, 13, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Bs, A.C.C.; Dai, X.; Walsh, J.M.; Sterni, L.M.; Prichett, L.; Boss, E.F.; Seal, S.M.; Ryan, M.A. Outcomes of Adenotonsillectomy for Obstructive Sleep Apnea in Prader-Willi Syndrome: Systematic Review and Meta-analysis. Laryngoscope 2020, 131, 898–906. [Google Scholar]
- Nixon, G.M.; Rodda, C.P.; Davey, M.J. Longitudinal Association between Growth Hormone Therapy and Obstructive Sleep Apnea in a Child with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Grugni, G.; Beccaria, L.; Corrias, A.; Crinò, A.; Cappa, M.; De Medici, C.; Di Candia, S.; Gargantini, L.; Ragusa, L.; Salvatoni, A.; et al. Central adrenal insufficiency in young adults with Prader-Willi Syndrome. Clin. Endocrinol. 2013, 79, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.G.W.; Pellikaan, K.; Poitou, C.; Goldstone, A.; Høybye, C.; Markovic, T.; Grugni, G.; Crinò, A.; Caixàs, A.; Coupaye, M.; et al. Central Adrenal Insufficiency Is Rare in Adults With Prader–Willi Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, e2563–e2571. [Google Scholar] [CrossRef]
- Angulo, M.A.; Butler, M.G.; Hossain, W.A.; Castro-Magana, M.; Corletto, J. Central adrenal insufficiency screening with morning plasma cortisol and ACTH levels in Prader-Willi syndrome. J. Pediatr. Endocrinol. Metab. 2022. [Google Scholar] [CrossRef]
- Passone, C.G.B.; Franco, R.R.; Ito, S.S.; Trindade, E.; Polak, M.; Damiani, D.; Bernardo, W.M. Growth hormone treatment in Prader-Willi syndrome patients: Systematic review and meta-analysis. BMJ Paediatr. Open 2020, 4, e000630. [Google Scholar] [CrossRef]
- Rosenberg, A.G.W.; Passone, C.D.G.B.; Pellikaan, K.; Damiani, D.; van der Lely, A.J.; Polak, M.; Bernardo, W.M.; de Graaff, L.C.G. Growth Hormone Treatment for Adults With Prader-Willi Syndrome: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2021, 106, 3068–3091. [Google Scholar] [CrossRef]
- Butler, M.G.; Manzardo, A.M.; Heinemann, J.; Loker, C.; Loker, J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet. Med. 2016, 19, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-S.; Kim, J.; Cho, J.; Cho, S.Y.; Jin, D.-K. Tailored management of life-threatening complications related to severe obesity in a young adult with Prader-Willi syndrome. Ann. Pediatr. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
| Young Adult PWS, n = 68 | |
|---|---|
| Age (years) (range) | 24.5 ± 4.2 (19.0~34.0) |
| Age at diagnosis with PWS (range) | 6.9 ± 5.7 (0.1~28.5) |
| Sex(male), n (%) | 39 (57.3) |
| Genotype (deletion), n (%) | 44 (64.7) |
| BMI in kg/m2 | 34.6 ± 11.7 (17.5~78.9) |
| <22.9, n (%) | 5 (7.3) |
| 23–24.9, n (%) | 6 (8.8) |
| 25–29.9, n (%) | 15 (22.0) |
| 30–34.9, n (%) | 20 (29.4) |
| 35<, n (%) | 22 (32.3) |
| Obesity, n (%) | 58 (85.2) |
| History of growth hormone treatment, n (%) | 58 (85.2) |
| Death, n (%) | 2 (2.9) |
| Metabolic syndrome, n (%) | 24 (35.3) |
| Type 2 diabetes, n (%) | 34 (50.0) |
| Hypertension, n (%) | 21 (30.8) |
| Dyslipidemia, n (%) | 26 (38.2) |
| Thyroid disorder, n (%) | 2 (2.9) |
| Decreased bone density, n (%) | 18 (26.4) |
| Sleep apnea, n (%) | 22 (32.3) |
| Adrenal insufficiency, n (%) | 0 (0) |
| Scoliosis, n (%) | 41 (60.2) |
| Behavior disorder, n (%) | 24 (45.2) |
| Soft tissue infection, n (%) | 4 (5.8) |
| PWS (n = 68) | Control (n = 204) | p-Value | |
|---|---|---|---|
| Metabolic syndrome, n (%) | 24 (35.3) | 9 (4.4) | <0.001 |
| Type 2 diabetes, n (%) | 34 (50.0) | 11 (5.4) | <0.001 |
| Hypertension, n (%) | 21 (30.8) | 33 (16.1) | 0.0085 |
| Dyslipidemia, n (%) | 26 (38.2) | 29 (14.2) | <0.001 |
| Thyroid disorder, n (%) | 2 (2.9) | 5 (2.4) | 0.5574 |
| Decreased bone density, n (%) | 18 (26.4) | 2 (0.9) | <0.001 |
| Sleep apnea, n (%) | 22 (32.3) | 9 (4.4) | <0.001 |
| Number (%) | |
|---|---|
| Comorbidities | |
| Diabetic nephropathy | 16 (47.0) |
| Diabetic neuropathy | 6 (17.6) |
| Diabetic retinopathy | 1 (2.9) |
| Hypertension | 21 (61.7) |
| Diabetic foot | 2 (5.8) |
| Heart failure | 2 (5.8) |
| Antidiabetics | |
| OHA alone | 11 (32.3) |
| Combination therapy | 6 (17.6) |
| Monotherapy | 5 (14.7) |
| Insulin alone | 0 (0) |
| OHA and GLP-1 receptor agonist | 2 (5.8) |
| OHA and insulin | 12 (35.2) |
| OHA and insulin and GLP-1 receptor agonist | 9 (26.4) |
| (a) | |||||||
| Non-rhGHTreatment (n = 10) | History of rhGH Treatment (n = 48) | Currently rhGH Treatment (n = 10) | p-Value | ||||
| Age (Years) (Range) | 28.5 ± 4.8 (20–33) | 23.5 ± 3.4 (19–31) | 23.6 ± 4.3 (19–32) | 0.757 | |||
| Age of starting rhGH Tx. (years) | 8.2 ± 3.6 (2–16) | 9.6 ± 6.0 (3–22.4) | 0.350 | ||||
| Age at diagnosis with PWS (years) | 11.6 ± 9.4 (0.1–28.5) | 5.5 ± 4.2 (0.1–13.7) | 6.7 ± 5.4 (0.1–22.4) | 0.869 | |||
| Sex (male), n (%) | 8 (80) | 26 (54.1) | 5 (50) | 0.443 | |||
| Genotype (deletion), n (%) | 6 (60) | 30 (62.5) | 8 (80) | 0.340 | |||
| BMI, kg/m2 (±SD) (range) | 41.65 ± 14.78 (26–78) | 32.58 ± 11.1 (18–58) | 37.8 ± 8.7 (25–53) | 0.048 | |||
| p-Value * | p-Value * | p-Value * | |||||
| Metabolic syndrome, n (%) | 5 (50) | <0.001 | 16 (33.3) | <0.001 | 3 (30) | 0.013 | |
| Type 2 diabetes, n (%) | 5 (50) | <0.001 | 25 (52.1) | <0.001 | 4 (40) | 0.003 | |
| Hypertension, n (%) | 6 (60) | 0.003 | 12 (25) | 0.112 | 2 (20) | 0.509 | |
| Dyslipidemia, n (%) | 4 (40) | 0.05 | 17 (35.4) | 0.001 | 5 (50) | 0.011 | |
| Thyroid disorder, n (%) | 0 (0) | 0.785 | 2 (4.2) | 0.398 | 0 (0) | 0.785 | |
| Decreased bone density, n (%) | 2 (20) | 0.011 | 14 (29.2) | <0.001 | 2 (20) | 0.011 | |
| Sleep apnea, n (%) | 3 (30) | 0.013 | 16 (33.3) | <0.001 | 3 (30) | 0.013 | |
| (b) | |||||||
| Outcome | Variable | Crude OR | Adjusted ORa | ||||
| Coef (95% CI) | p-Value | Coef (95% CI) | p-Value | ||||
| Metabolic syndrome | Non-rhGH treatment | Ref | 0.571 | Ref | 0.937 | ||
| History of rhGH treatment | 2.333 (0.373–14.613) | 0.365 | 1.461 (0.180–11.856) | 0.723 | |||
| Currently rhGH treatment | 1.167 (0.266–5.123) | 0.838 | 1.131 (0.245–5.221) | 0.875 | |||
| Type 2 diabetes | Non-rhGH treatment | Ref | 0.787 | Ref | 0.692 | ||
| History of rhGH treatment | 1.500 (0.255–8.817) | 0.654 | 0.929 (0.121–7.131) | 0.943 | |||
| Currently rhGH treatment | 1.630 (0.408–6.521) | 0.489 | 1.623 (0.383–6.875) | 0.511 | |||
| Hypertension | Non-rhGH treatment | Ref | 0.091 | Ref | 0.508 | ||
| History of rhGH treatment | 6.000 (0.812–44.351) | 0.079 | 3.317 (0.363–30.347) | 0.288 | |||
| Currently rhGH treatment | 1.333 (0.248–7.165) | 0.737 | 1.323 (0.237–7.391) | 0.749 | |||
| Dyslipidemia | Non-rhGH treatment | Ref | 0.647 | Ref | 0.447 | ||
| History of rhGH treatment | 0.429 (0.068–2.684) | 0.365 | 0.258 (0.032–2.094) | 0.205 | |||
| Currently rhGH treatment | 0.600 (0.152–2.362) | 0.465 | 0.599 (0.150–2.396) | 0.468 | |||
| Decreased bone density | Non-rhGH treatment | Ref | 0.740 | Ref | 0.854 | ||
| History of rhGH treatment | 1.000 (0.112–8.947) | 1.000 | 1.374 (0.115–16.384) | 0.801 | |||
| Currently rhGH treatment | 1.647 (0.310–8.748) | 0.558 | 1.622 (0.292–9.018) | 0.581 | |||
| Sleep apnea | Non-rhGH treatment | Ref | 0.965 | Ref | 0.690 | ||
| History of rhGH treatment | 1.000 (0.148–6.772) | 1.000 | 0.555 (0.064–4.818) | 0.594 | |||
| Currently rhGH treatment | 1.167 (0.266–5.123) | 0.838 | 1.209 (0.266–5.493) | 0.806 | |||
| BMI≥30 | Non-rhGH treatment | Ref | 0.060 | Ref | 0.127 | ||
| History of rhGH treatment | 0.444 (0.034–5.880) | 0.538 | 0.152 (0.009–2.643) | 0.196 | |||
| Currently rhGH treatment | 0.121 (0.014–1.029) | 0.053 | 0.106 (0.012–0.948) | 0.045 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, E.-S.; Kim, M.-S.; Kim, C.; Jeon, K.; Kim, S.; Cho, S.Y.; Jin, D.-K. Endocrine and Metabolic Illnesses in Young Adults with Prader–Willi Syndrome. J. Pers. Med. 2022, 12, 858. https://doi.org/10.3390/jpm12060858
Noh E-S, Kim M-S, Kim C, Jeon K, Kim S, Cho SY, Jin D-K. Endocrine and Metabolic Illnesses in Young Adults with Prader–Willi Syndrome. Journal of Personalized Medicine. 2022; 12(6):858. https://doi.org/10.3390/jpm12060858
Chicago/Turabian StyleNoh, Eu-Seon, Min-Sun Kim, Chiwoo Kim, Kyeongman Jeon, Seonwoo Kim, Sung Yoon Cho, and Dong-Kyu Jin. 2022. "Endocrine and Metabolic Illnesses in Young Adults with Prader–Willi Syndrome" Journal of Personalized Medicine 12, no. 6: 858. https://doi.org/10.3390/jpm12060858
APA StyleNoh, E.-S., Kim, M.-S., Kim, C., Jeon, K., Kim, S., Cho, S. Y., & Jin, D.-K. (2022). Endocrine and Metabolic Illnesses in Young Adults with Prader–Willi Syndrome. Journal of Personalized Medicine, 12(6), 858. https://doi.org/10.3390/jpm12060858







