A Double-Blind Randomized Trial to Investigate Mechanisms of Antidepressant-Related Dysfunctional Arousal in Depressed or Anxious Youth at Familial Risk for Bipolar Disorder
Abstract
1. Introduction
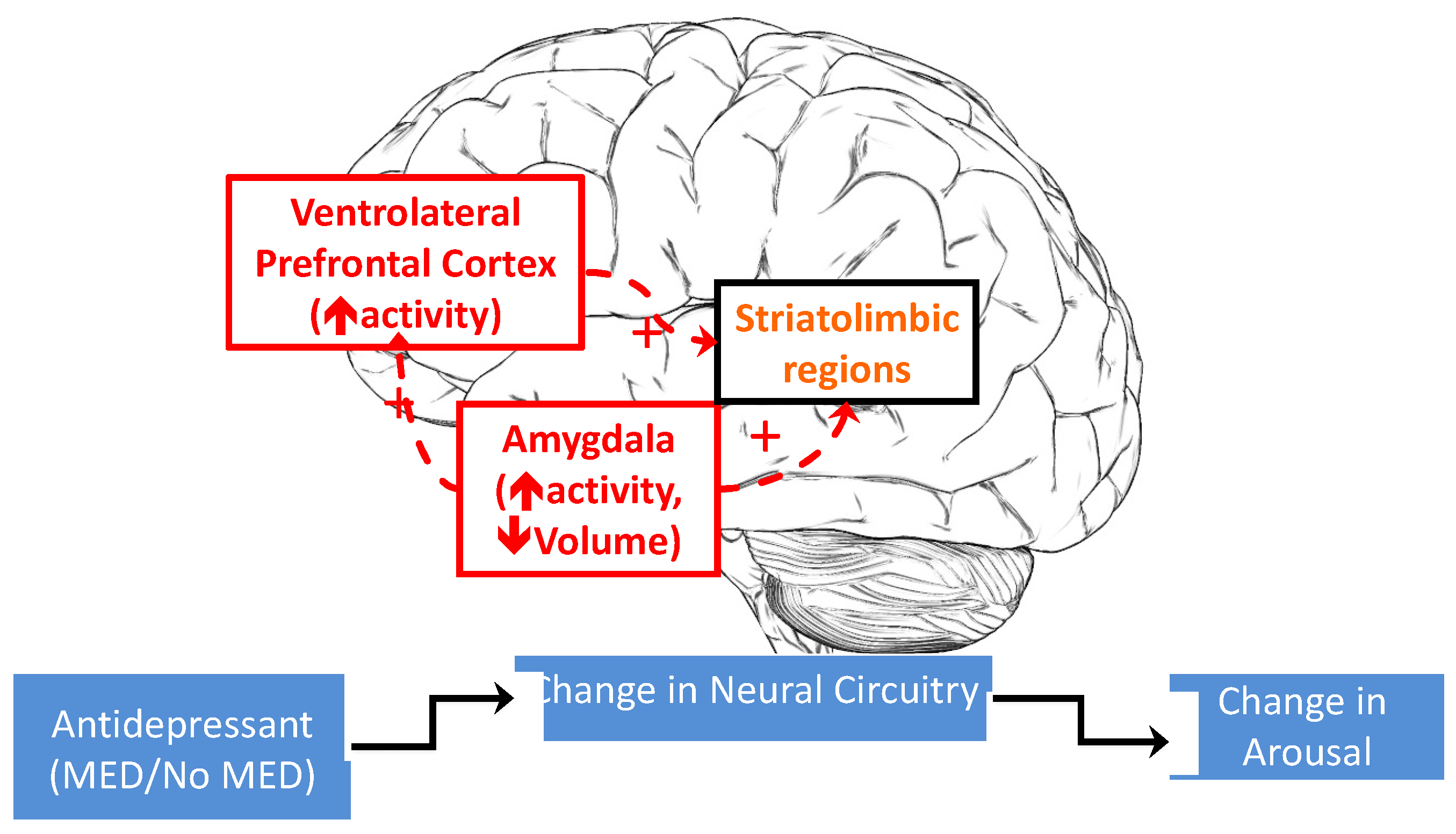
1.1. Risk Factors and Putative Mechanisms Underlying Emotional and Physiological Arousal during Antidepressant Treatment
1.2. Dynamic Functional Networks Subserving Hyperarousal
1.3. Escitalopram Pharmacogenetics in High-Risk Youth
2. Study Protocol
2.1. Setting and Study Population
2.2. Randomization
2.3. Medication Dosing and Accountability
2.4. Primary Clinical Outcome and Neural and Pharmacogenetic Mediators of Outcome
2.4.1. Neural Mediators
2.4.2. Pharmacogenetic Mediators
2.5. Hypotheses and Analytic Plan
2.6. Limitations
3. Impact of the Study
4. Ethics Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsey, L.B.; Ong, H.H.; Schildcrout, J.S.; Shi, Y.; Tang, L.A.; Hicks, J.K.; El Rouby, N.; Cavallari, L.H.; Tuteja, S.; Aquilante, C.L.; et al. Prescribing Prevalence of Medications with Potential Genotype-Guided Dosing in Pediatric Patients. JAMA Netw. Open 2020, 3, e2029411. [Google Scholar] [CrossRef]
- Merikangas, K.R.; He, J.P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Koechlin, H.; Zion, S.R.; Werner, C.; Pine, D.S.; Kirsch, I.; Kessler, R.C.; Kossowsky, J. Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents. JAMA Psychiatry 2017, 74, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Welge, J.A.; Wehry, A.M.; Keeshin, B.R.; Rynn, M.A. Efficacy and Tolerability of Antidepressants in Pediatric Anxiety Disorders: A Systematic Review and Meta-Analysis. Depress. Anxiety 2015, 32, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Mills, J.A.; Suresh, V.; Peris, T.S.; Walkup, J.T.; Croarkin, P.E. Combining selective serotonin reuptake inhibitors and cognitive behavioral therapy in youth with depression and anxiety. J. Affect. Disord. 2022, 298, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.; Singh, M.; Chang, K. Antidepressants and psychostimulants in pediatric populations: Is there an association with mania? Paediatr. Drugs 2011, 13, 225–243. [Google Scholar] [CrossRef]
- Joseph, M.F.; Youngstrom, E.A.; Soares, J.C. Antidepressant-coincident mania in children and adolescents treated with selective serotonin reuptake inhibitors. Future Neurol. 2009, 4, 87–102. [Google Scholar] [CrossRef]
- Martin, A.; Young, C.; Leckman, J.F.; Mukonoweshuro, C.; Rosenheck, R.; Leslie, D. Age effects on antidepressant-induced manic conversion. Arch. Pediatr. Adolesc. Med. 2004, 158, 773–780. [Google Scholar] [CrossRef]
- Safer, D.J.; Zito, J.M. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: Children versus adolescents. J. Child. Adolesc. Psychopharmacol. 2006, 16, 159–169. [Google Scholar] [CrossRef]
- Zuckerman, M.L.; Vaughan, B.L.; Whitney, J.; Dodds, A.; Yakhkind, A.; MacMillan, C.; Raches, D.; Pravdova, I.; DeMaso, D.R.; Beardslee, W.R.; et al. Tolerability of selective serotonin reuptake inhibitors in thirty-nine children under age seven: A retrospective chart review. J. Child. Adolesc. Psychopharmacol. 2007, 17, 165–174. [Google Scholar] [CrossRef]
- Goodwin, F.K.; Jamison, K.R. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Diler, R.S.; Birmaher, B.; Axelson, D.; Obreja, M.; Monk, K.; Hickey, M.B.; Goldstein, B.; Goldstein, T.; Sakolsky, D.; Iyengar, S.; et al. Dimensional psychopathology in offspring of parents with bipolar disorder. Bipolar Disord. 2011, 13, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Hillegers, M.H.; Reichart, C.G.; Wals, M.; Verhulst, F.C.; Ormel, J.; Nolen, W.A. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005, 7, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Geller, B.; Zimerman, B.; Williams, M.; Bolhofner, K.; Craney, J.L. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am. J. Psychiatry 2001, 158, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Hafeman, D.; Merranko, J.; Zwicker, A.; Goldstein, B.; Goldstein, T.; Axelson, D.; Monk, K.; Hickey, M.B.; Sakolsky, D.; et al. Role of Polygenic Risk Score in the Familial Transmission of Bipolar Disorder in Youth. JAMA Psychiatry 2022, 79, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.S.; Holt-Gosselin, B.; Hagan, K.E.; Fleming, S.L.; Nimarko, A.F.; Gotlib, I.H.; Singh, M.K. Intrinsic connectivity and family dynamics: Striato-limbic markers of risk and resilience in youth at familial risk for mood disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging, 2022; in press. [Google Scholar] [CrossRef]
- Nimarko, A.F.; Fischer, A.S.; Hagan, K.E.; Gorelik, A.J.; Lu, Y.; Young, C.J.; Singh, M.K. Neural Correlates of Positive Emotion Processing That Distinguish Healthy Youths at Familial Risk for Bipolar Versus Major Depressive Disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2021, 60, 887–901. [Google Scholar] [CrossRef]
- Nimarko, A.F.; Gorelik, A.J.; Carta, K.E.; Gorelik, M.G.; Singh, M.K. Neural correlates of reward processing distinguish healthy youth at familial risk for bipolar disorder from youth at familial risk for major depressive disorder. Transl. Psychiatry 2022, 12, 31. [Google Scholar] [CrossRef]
- Tulisiak, A.K.; Klein, J.A.; Harris, E.; Luft, M.J.; Schroeder, H.K.; Mossman, S.A.; Varney, S.T.; Keeshin, B.R.; Cotton, S.; Strawn, J.R. Antidepressant Prescribing by Pediatricians: A Mixed-Methods Analysis. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 15–24. [Google Scholar] [CrossRef]
- Baumer, F.M.; Howe, M.; Gallelli, K.; Simeonova, D.I.; Hallmayer, J.; Chang, K.D. A pilot study of antidepressant-induced mania in pediatric bipolar disorder: Characteristics, risk factors, and the serotonin transporter gene. Biol. Psychiatry 2006, 60, 1005–1012. [Google Scholar] [CrossRef]
- Findling, R.L.; Correll, C.U.; Nyilas, M.; Forbes, R.A.; McQuade, R.D.; Jin, N.; Ivanova, S.; Mankoski, R.; Carson, W.H.; Carlson, G.A. Aripiprazole for the treatment of pediatric bipolar I disorder: A 30-week, randomized, placebo-controlled study. Bipolar Disord. 2013, 15, 138–149. [Google Scholar] [CrossRef]
- Geller, B.; Cooper, T.B.; Zimerman, B.; Frazier, J.; Williams, M.; Heath, J.; Warner, K. Lithium for prepubertal depressed children with family history predictors of future bipolarity: A double-blind, placebo-controlled study. J. Affect. Disord. 1998, 51, 165–175. [Google Scholar] [CrossRef]
- Findling, R.L.; Frazier, T.W.; Youngstrom, E.A.; McNamara, N.K.; Stansbrey, R.J.; Gracious, B.L.; Reed, M.D.; Demeter, C.A.; Calabrese, J.R. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J. Clin. Psychiatry 2007, 68, 781–788. [Google Scholar] [CrossRef] [PubMed]
- DelBello, M.P.; Adler, C.M.; Whitsel, R.M.; Stanford, K.E.; Strakowski, S.M. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J. Clin. Psychiatry 2007, 68, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.; Ribeiro, A.; Matthews, J.; Kow, L.-M. Concepts and mechanisms of generalized central nervous system arousal. Ann. N. Y. Acad. Sci. 2008, 1129, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, L.; Moncrieff, J. The psychoactive effects of antidepressants and their association with suicidality. Curr. Drug Saf. 2011, 6, 115–121. [Google Scholar] [CrossRef]
- Beasley, C.M.; Potvin, J.H. Fluoxetine: Activating and sedating effects. Int. Clin. Psychopharmacol. 1993, 8, 271–275. [Google Scholar] [CrossRef]
- Ricci, L.A.; Melloni, R.H. Repeated fluoxetine administration during adolescence stimulates aggressive behavior and alters serotonin and vasopressin neural development in hamsters. Behav. Neurosci. 2012, 126, 640–653. [Google Scholar] [CrossRef]
- Licht, C.M.M.; de Geus, E.J.C.; van Dyck, R.; Penninx, B.W.J.H. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol. Psychiatry 2010, 68, 861–868. [Google Scholar] [CrossRef]
- Strakowski, S.M.; Eliassen, J.C.; Lamy, M.; Cerullo, M.A.; Allendorfer, J.B.; Madore, M.; Lee, J.H.; Welge, J.A.; DelBello, M.P.; Fleck, D.E.; et al. Functional magnetic resonance imaging brain activation in bipolar mania: Evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol. Psychiatry 2011, 69, 381–388. [Google Scholar] [CrossRef]
- Nielen, M.M.A.; Heslenfeld, D.J.; Heinen, K.; Van Strien, J.W.; Witter, M.P.; Jonker, C.; Veltman, D.J. Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain Cogn. 2009, 71, 387–396. [Google Scholar] [CrossRef]
- Strawn, J.R.; Adler, C.M.; McNamara, R.K.; Welge, J.A.; Bitter, S.M.; Mills, N.P.; Barzman, D.H.; Cerullo, M.A.; Chang, K.D.; Strakowski, S.M.; et al. Antidepressant tolerability in anxious and depressed youth at high risk for bipolar disorder: A prospective naturalistic treatment study. Bipolar Disord. 2014, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Liu, S.C.; Tsou, H.H.; Liu, S.W.; Lin, K.M.; Lu, S.C.; Hsiao, M.C.; Hsiao, C.F.; Liu, C.Y.; Chen, C.H.; et al. CYP1A2 genetic polymorphisms are associated with early antidepressant escitalopram metabolism and adverse reactions. Pharmacogenomics 2013, 14, 1191–1201. [Google Scholar] [CrossRef]
- Chua, E.W.; Foulds, J.; Miller, A.L.; Kennedy, M.A. Novel CYP2D6 and CYP2C19 variants identified in a patient with adverse reactions towards venlafaxine monotherapy and dual therapy with nortriptyline and fluoxetine. Pharmacogenet. Genom. 2013, 23, 494–497. [Google Scholar] [CrossRef]
- Ladouceur, C.D.; Diwadkar, V.A.; White, R.; Bass, J.; Birmaher, B.; Axelson, D.A.; Phillips, M.L. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev. Cogn. Neurosci. 2013, 5, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Williams, S.C.; Cleare, A.J.; Brammer, M.J.; Walsh, N.D.; Kim, J.; Andrew, C.M.; Pich, E.M.; Williams, P.M.; Reed, L.J. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry 2004, 61, 877–889. [Google Scholar] [CrossRef]
- Bigos, K.L.; Pollock, B.G.; Aizenstein, H.J.; Fisher, P.M.; Bies, R.R.; Hariri, A.R. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 2008, 33, 3221–3225. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.E.; Bonnemann, C.; Emrich, H.M. Cortico-limbic mechanisms of affect regulation in the therapy of depression. Psychiatr. Prax. 2007, 34 (Suppl. 3), S287–S291. [Google Scholar] [CrossRef]
- Daray, F.M.; Thommi, S.B.; Ghaemi, S.N. The pharmacogenetics of antidepressant-induced mania: A systematic review and meta-analysis. Bipolar Disord. 2010, 12, 702–706. [Google Scholar] [CrossRef]
- Demjaha, A.; MacCabe, J.H.; Murray, R.M. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr. Bull. 2012, 38, 209–214. [Google Scholar] [CrossRef][Green Version]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020–11025. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Furman, D.J.; Chang, C.; Thomason, M.E.; Dennis, E.; Gotlib, I.H. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol. Psychiatry 2011, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Craddock, R.C.; Holtzheimer, P.E.; Hu, X.P.; Mayberg, H.S. Disease state prediction from resting state functional connectivity. Magn. Reson. Med. 2009, 62, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, A.R.; Mujica-Parodi, L.R. A principal component network analysis of prefrontal-limbic functional magnetic resonance imaging time series in schizophrenia patients and healthy controls. Psychiatry Res. 2009, 174, 184–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Etkin, A.; Schatzberg, A.F. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am. J. Psychiatry 2011, 168, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.H.; Parhi, K.K.; Westlund Schreiner, M.; Lenglet, C.; Mueller, B.A.; Klimes-Dougan, B.; Cullen, K.R. Effect of SSRIs on Resting-State Functional Brain Networks in Adolescents with Major Depressive Disorder. J. Clin. Med. 2021, 10, 4322. [Google Scholar] [CrossRef]
- Cullen, K.R.; Klimes-Dougan, B.; Vu, D.P.; Westlund Schreiner, M.; Mueller, B.A.; Eberly, L.E.; Camchong, J.; Westervelt, A.; Lim, K.O. Neural Correlates of Antidepressant Treatment Response in Adolescents with Major Depressive Disorder. J. Child. Adolesc. Psychopharmacol. 2016, 26, 705–712. [Google Scholar] [CrossRef]
- Connolly, C.G.; Ho, T.C.; Blom, E.H.; LeWinn, K.Z.; Sacchet, M.D.; Tymofiyeva, O.; Simmons, A.N.; Yang, T.T. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J. Affect. Disord. 2017, 207, 86–94. [Google Scholar] [CrossRef]
- Di Simplicio, M.; Norbury, R.; Reinecke, A.; Harmer, C.J. Paradoxical effects of short-term antidepressant treatment in fMRI emotional processing models in volunteers with high neuroticism. Psychol. Med. 2014, 44, 241–252. [Google Scholar] [CrossRef]
- Nery, F.G.; Masifi, S.L.; Strawn, J.R.; Duran, L.R.; Weber, W.A.; Welge, J.A.; Adler, C.M.; Strakowski, S.M.; DelBello, M.P. Association between poor tolerability of antidepressant treatment and brain functional activation in youth at risk for bipolar disorder. Braz. J. Psychiatry 2020, 43, 70–74. [Google Scholar] [CrossRef]
- Lehofer, M.; Moser, M.; Hoehn-Saric, R.; McLeod, D.; Liebmann, P.; Drnovsek, B.; Egner, S.; Hildebrandt, G.; Zapotoczky, H.G. Major depression and cardiac autonomic control. Biol. Psychiatry 1997, 42, 914–919. [Google Scholar] [CrossRef]
- Chang, J.S.; Ha, K.; Yoon, I.Y.; Yoo, C.S.; Yi, S.H.; Her, J.Y.; Ha, T.H.; Park, T. Patterns of cardiorespiratory coordination in young women with recurrent major depressive disorder treated with escitalopram or venlafaxine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.R.; Normandin, L.; Kakuma, T. Suicidal children grow up: Relations between family psychopathology and adolescents’ lifetime suicidal behavior. J. Nerv. Ment. Dis. 1998, 186, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer-Rutz, E.; Rasmussen, F.; Lange, T. A life-course study on effects of parental markers of morbidity and mortality on offspring’s suicide attempt. PLoS ONE 2012, 7, e51585. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, L.J.; Kugelmass, S.; Frenkel, E.; Nathan, M.; Mirsky, A.F. Twenty-five-year followup of the Israeli High-Risk Study: Current and lifetime psychopathology. Schizophr. Bull. 1995, 21, 183–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Button, K.S.; Ioannidis, J.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef]
- Barg, T.; Wolfersdorf, M.; Ruppe, A. The influence of various antidepressants on heart rate and electrodermal activity during psychophysiological examinations of depressive patients. Pharmacopsychiatry 1996, 29, 216–219. [Google Scholar] [CrossRef]
- Morris, S.E.; Cuthbert, B.N. Research Domain Criteria: Cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012, 14, 29–37. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Patkar, A.A. Escitalopram for the treatment of major depressive disorder in youth. Expert Opin. Pharmacother. 2011, 12, 2235–2244. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Sauley, B.A.; Welge, J.A. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child. Adolesc. Psychiatry 2018, 57, 235–244.e2. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Valuck, R.; Saseen, J.; MacFall, H.M. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs 2004, 18, 911–932. [Google Scholar] [CrossRef]
- Emslie, G.J.; Ventura, D.; Korotzer, A.; Tourkodimitris, S. Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Maruf, A.A.; Greenslade, A.; Arnold, P.D.; Bousman, C. Antidepressant pharmacogenetics in children and young adults: A systematic review. J. Affect. Disord. 2019, 254, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Mills, J.A.; Schroeder, H.; Mossman, S.A.; Varney, S.T.; Ramsey, L.B.; Poweleit, E.A.; Desta, Z.; Cecil, K.; DelBello, M.P. Escitalopram in Adolescents With Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Psychiatry 2020, 81, 20m13396. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, S.L.; Poweleit, E.A.; Prows, C.A.; Martin, L.J.; Strawn, J.R.; Ramsey, L.B. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth with Anxiety and Depressive Disorders. Front. Pharmacol. 2019, 10, 99. [Google Scholar] [CrossRef]
- Strawn, J.R.; Poweleit, E.A.; Ramsey, L.B. CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. J. Child. Adolesc. Psychopharmacol. 2019, 29, 340–347. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Veldic, M.; Ahmed, A.T.; Blacker, C.J.; Geske, J.R.; Biernacka, J.M.; Borreggine, K.L.; Moore, K.M.; Prieto, M.L.; Vande Voort, J.L.; Croarkin, P.E.; et al. Cytochrome P450 2C19 Poor Metabolizer Phenotype in Treatment Resistant Depression: Treatment and Diagnostic Implications. Front. Pharmacol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Altar, C.A.; Hornberger, J.; Shewade, A.; Cruz, V.; Garrison, J.; Mrazek, D. Clinical validity of cytochrome P450 metabolism and serotonin gene variants in psychiatric pharmacotherapy. Int. Rev. Psychiatry Abingdon Engl. 2013, 25, 509–533. [Google Scholar] [CrossRef]
- Scherf-Clavel, M.; Deckert, J.; Menke, A.; Unterecker, S. Smoking Is Associated With Lower Dose-Corrected Serum Concentrations of Escitalopram. J. Clin. Psychopharmacol. 2019, 39, 485–488. [Google Scholar] [CrossRef]
- Jin, Y.; Pollock, B.G.; Frank, E.; Cassano, G.B.; Rucci, P.; Müller, D.J.; Kennedy, J.L.; Forgione, R.N.; Kirshner, M.; Kepple, G.; et al. Effect of age, weight, and CYP2C19 genotype on escitalopram exposure. J. Clin. Pharmacol. 2010, 50, 62–72. [Google Scholar] [CrossRef]
- Dong, Z.-Q.; Li, X.-R.; He, L.; He, G.; Yu, T.; Sun, X.-L. 5-HTR1A and 5-HTR2A genetic polymorphisms and SSRI antidepressant response in depressive Chinese patients. Neuropsychiatr. Dis. Treat. 2016, 12, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Amitai, M.; Kronenberg, S.; Carmel, M.; Michaelovsky, E.; Frisch, A.; Brent, D.; Apter, A.; Chen, A.; Weizman, A.; Fennig, S. Pharmacogenetics of citalopram-related side effects in children with depression and/or anxiety disorders. J. Neural. Transm. 2016, 123, 1347–1354. [Google Scholar] [CrossRef]
- Laje, G.; Perlis, R.H.; Rush, A.J.; McMahon, F.J. Pharmacogenetics studies in STAR*D: Strengths, limitations, and results. Psychiatr. Serv. 2009, 60, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.D.; Baskak, B.; Uckun, Z.; Artun, N.Y.; Ozdemir, H.; Ozel, T.K.; Ozguven, H.D.; Suzen, H.S. Association between serotonin 2A receptor (HTR2A), serotonin transporter (SLC6A4) and brain-derived neurotrophic factor (BDNF) gene polymorphisms and citalopram/sertraline induced sexual dysfunction in MDD patients. Pharmacogenom. J. 2020, 20, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Voort, J.L.V.; Orth, S.S.; Shekunov, J.; Romanowicz, M.; Geske, J.R.; Ward, J.A.; Leibman, N.I.; Frye, M.A.; Croarkin, P.E. A Randomized Controlled Trial of Combinatorial Pharmacogenetics Testing in Adolescent Depression. J. Am. Acad. Child. Adolesc. Psychiatry 2022, 61, 46–55. [Google Scholar] [CrossRef]
- Kronenberg, S.; Apter, A.; Brent, D.; Schirman, S.; Melhem, N.; Pick, N.; Gothelf, D.; Carmel, M.; Frisch, A.; Weizman, A. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J. Child. Adolesc. Psychopharmacol. 2007, 17, 741–750. [Google Scholar] [CrossRef]
- Park, M.H.; Sanders, E.; Howe, M.; Singh, M.; Hallmayer, J.; Kim, E.; Chang, K. Association of Anxiety Symptoms in Offspring of Bipolar Parents with Serotonin Transporter-Linked Polymorphic Region (5-HTTLPR) Genotype. J. Child. Adolesc. Psychopharmacol. 2015, 25, 458–466. [Google Scholar] [CrossRef]
- Chang, K.; Garrett, A.; Kelley, R.; Howe, M.; Sanders, E.M.; Acquaye, T.; Bararpour, L.; Li, S.; Singh, M.; Jo, B.; et al. Anomalous prefrontal-limbic activation and connectivity in youth at high-risk for bipolar disorder. J. Affect. Disord. 2017, 222, 7–13. [Google Scholar] [CrossRef]
- Strawn, J.R.; Poweleit, E.A.; Mills, J.A.; Schroeder, H.K.; Neptune, Z.A.; Specht, A.M.; Farrow, J.E.; Zhang, X.; Martin, L.J.; Ramsey, L.B. Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial. J. Pers. Med. 2021, 11, 1188. [Google Scholar] [CrossRef]
- Poznanski, E.O.; Grossman, J.A.; Buchsbaum, Y.; Banegas, M.; Freeman, L.; Gibbons, R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J. Am. Acad. Child. Psychiatry 1984, 23, 191–197. [Google Scholar] [CrossRef]
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J. Am. Acad Child. Adolesc. Psychiatry 2002, 41, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- First, M.B. Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Administration Booklet; American Psychiatric Press: Washington, DC, USA, 1997. [Google Scholar]
- Lu, L.; Mills, J.A.; Li, H.; Schroeder, H.K.; Mossman, S.A.; Varney, S.T.; Cecil, K.M.; Huang, X.; Gong, Q.; Ramsey, L.B.; et al. Acute Neurofunctional Effects of Escitalopram in Pediatric Anxiety: A Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Child. Adolesc. Psychiatry 2021, 60, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.M.A.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C.; Endicott, J.; Spitzer, R.L.; Winokur, G. The family history method using diagnostic criteria. Reliability and validity. Arch. Gen. Psychiatry 1977, 34, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B. Psychotherapy for youth at risk for bipolar disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2013, 52, 116–118. [Google Scholar] [CrossRef]
- Miklowitz, D.J.; Schneck, C.D.; Singh, M.K.; Taylor, D.O.; George, E.L.; Cosgrove, V.E.; Howe, M.E.; Dickinson, L.M.; Garber, J.; Chang, K.D. Early intervention for symptomatic youth at risk for bipolar disorder: A randomized trial of family-focused therapy. J. Am. Acad. Child. Adolesc. Psychiatry 2013, 52, 121–131. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef]
- March, J.; Karayal, O.; Chrisman, A. CAPTN: The pediatric adverse event rating scale. In Proceedings of the Scientific—2007 Annual Meeting of the American Academy of Child and Adolescent Psychiatry, Boston, MA, USA, 23–28 October 2007. [Google Scholar]
- Bussing, R.; Murphy, T.K.; Storch, E.A.; McNamara, J.P.; Reid, A.M.; Garvan, C.W.; Goodman, W.K. Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 2013, 205, 253–261. [Google Scholar] [CrossRef][Green Version]
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A children’s global assessment scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef]
- Strawn, J.R.; Bitter, S.M.; Weber, W.A.; Chu, W.J.; Whitsel, R.M.; Adler, C.; Cerullo, M.A.; Eliassen, J.; Strakowski, S.M.; DelBello, M.P. Neurocircuitry of generalized anxiety disorder in adolescents: A pilot functional neuroimaging and functional connectivity study. Depress. Anxiety 2012, 29, 939–947. [Google Scholar] [CrossRef]
- Yamasaki, H.; LaBar, K.S.; McCarthy, G. Dissociable prefrontal brain systems for attention and emotion. Proc. Natl. Acad. Sci. USA 2002, 99, 11447–11451. [Google Scholar] [CrossRef]
- Cerullo, M.A.; Eliassen, J.C.; Smith, C.T.; Fleck, D.E.; Nelson, E.B.; Strawn, J.R.; Lamy, M.; DelBello, M.P.; Adler, C.M.; Strakowski, S.M. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disord. 2014, 16, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T.; Rubia, K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Siegle, G.J.; Carter, C.S.; Thase, M.E. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am. J. Psychiatry 2006, 163, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Axelson, D.A.; Birmaher, B.; Strober, M.A.; Goldstein, B.I.; Ha, W.; Gill, M.K.; Goldstein, T.R.; Yen, S.; Hower, H.; Hunt, J.I.; et al. Course of subthreshold bipolar disorder in youth: Diagnostic progression from bipolar disorder not otherwise specified. J. Am. Acad. Child. Adolesc. Psychiatry 2011, 50, 1001–1016.e3. [Google Scholar] [CrossRef]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. Off. 2017, 19, 215–223. [Google Scholar] [CrossRef]
- Singer, J.D.; Willett, J.B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Kraemer, H.C.; Wilson, G.T.; Fairburn, C.G.; Agras, W.S. Mediators and moderators of treatment effects in randomized clinical trials. Arch. Gen. Psychiatry 2002, 59, 877–883. [Google Scholar] [CrossRef]
- Kraemer, H.C.; Kiernan, M.; Essex, M.; Kupfer, D.J. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008, 27, S101–S108. [Google Scholar] [CrossRef]

| Inclusion Criteria: High-Risk Youth | Inclusion Criteria: Healthy Controls |
|---|---|
| 1. Aged from 12 years, 0 months to 17 years, 11 months | 1. Aged from 12 years, 0 months to 17 years, 11 months |
| 2. At least one parent, step-parent, or guardian with whom the subject lives is willing to participate in research sessions | 2. At least one parent, step-parent, or guardian with whom the subject lives is willing to participate in research sessions |
| 3. The child and relative(s) are able and willing to give written informed assent/consent to participate, respectively | 3. The child and relative(s) are able and willing to give written informed assent/consent to participate, respectively |
| 4. At least one first-degree relative with bipolar I disorder as assessed by the Structured Clinical Interview for DSM (SCID) [83], the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL) [85], and the FH-RDC [86] | 4. No personal psychopathology (except specific phobias) |
| 5. Evidence of current, significant depressive or anxiety symptoms as determined by a current Childhood Depression Rating Scale-Revised (CDRS-R) [81] score > 35 and/or a current Pediatric Anxiety Rating Scale (PARS) score > 15 [82]. | 5. No family history (first- or second-degree) of any mood or psychotic disorders |
| Exclusion Criteria: All Participants | |
| 1. Any history of syndromal bipolar I or II disorder (i.e., history of mania, mixed episode, or major depression with hypomania) | |
| 2. A history of previous antidepressant exposure | |
| 3. A DSM-5 diagnosis of autism spectrum disorders, obsessive compulsive disorder, post-traumatic stress disorder, Tourette’s disorder, or any psychotic disorder including schizophrenia | |
| 4. Evidence of intellectual disability (IQ < 70) as determined by the Weschler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999) | |
| 5. Comorbid neurologic diseases such as seizure disorder | |
| 6. Drug or alcohol abuse or dependence disorders in the 4 months prior to study recruitment, although a lifetime history of substance or alcohol disorders could be present if the child has been abstinent for at least 6 months | |
| 7. Evidence of an unstable medical or psychiatric disorder that required immediate hospitalization or other emergency medical treatment | |
| 8. A positive pregnancy test | |
| 9. Any contraindication for MRI, including metal in the body related to an injury or surgery (e.g., surgical clips, metal fragments in the eyes), piercings that could not be removed, braces, or permanent retainers | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeycutt, D.C.; DelBello, M.P.; Strawn, J.R.; Ramsey, L.B.; Patino, L.R.; Hinman, K.; Welge, J.; Miklowitz, D.J.; Jo, B.; Blom, T.J.; et al. A Double-Blind Randomized Trial to Investigate Mechanisms of Antidepressant-Related Dysfunctional Arousal in Depressed or Anxious Youth at Familial Risk for Bipolar Disorder. J. Pers. Med. 2022, 12, 1006. https://doi.org/10.3390/jpm12061006
Honeycutt DC, DelBello MP, Strawn JR, Ramsey LB, Patino LR, Hinman K, Welge J, Miklowitz DJ, Jo B, Blom TJ, et al. A Double-Blind Randomized Trial to Investigate Mechanisms of Antidepressant-Related Dysfunctional Arousal in Depressed or Anxious Youth at Familial Risk for Bipolar Disorder. Journal of Personalized Medicine. 2022; 12(6):1006. https://doi.org/10.3390/jpm12061006
Chicago/Turabian StyleHoneycutt, Duncan C., Melissa P. DelBello, Jeffrey R. Strawn, Laura B. Ramsey, Luis R. Patino, Kyle Hinman, Jeffrey Welge, David J. Miklowitz, Booil Jo, Thomas J. Blom, and et al. 2022. "A Double-Blind Randomized Trial to Investigate Mechanisms of Antidepressant-Related Dysfunctional Arousal in Depressed or Anxious Youth at Familial Risk for Bipolar Disorder" Journal of Personalized Medicine 12, no. 6: 1006. https://doi.org/10.3390/jpm12061006
APA StyleHoneycutt, D. C., DelBello, M. P., Strawn, J. R., Ramsey, L. B., Patino, L. R., Hinman, K., Welge, J., Miklowitz, D. J., Jo, B., Blom, T. J., Bruns, K. M., Hamill Skoch, S. K., Starace, N., Tallman, M. J., & Singh, M. K. (2022). A Double-Blind Randomized Trial to Investigate Mechanisms of Antidepressant-Related Dysfunctional Arousal in Depressed or Anxious Youth at Familial Risk for Bipolar Disorder. Journal of Personalized Medicine, 12(6), 1006. https://doi.org/10.3390/jpm12061006









