Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. BRCA1/2 Testing, RRM, and RRSO Intent
2.4. Familial Communication Intent
2.5. Statistical Analyses
3. Results
3.1. Demographic Characteristics
3.2. Intent to Undergo BRCA1/2 Testing, RRSO, and RRM
3.3. Decision-Making Predictors for BRCA1/2 Testing, RRSO, and RRM
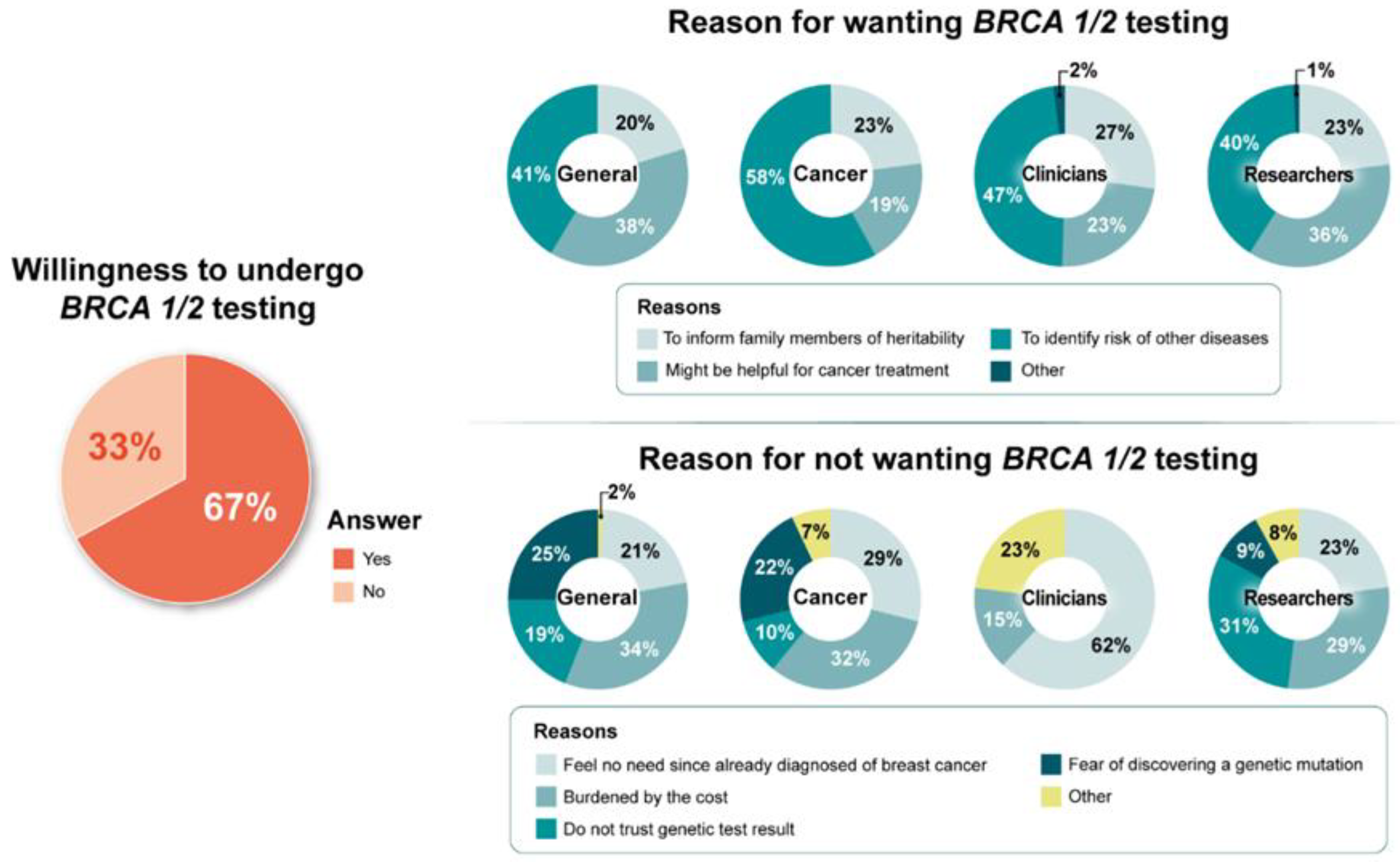
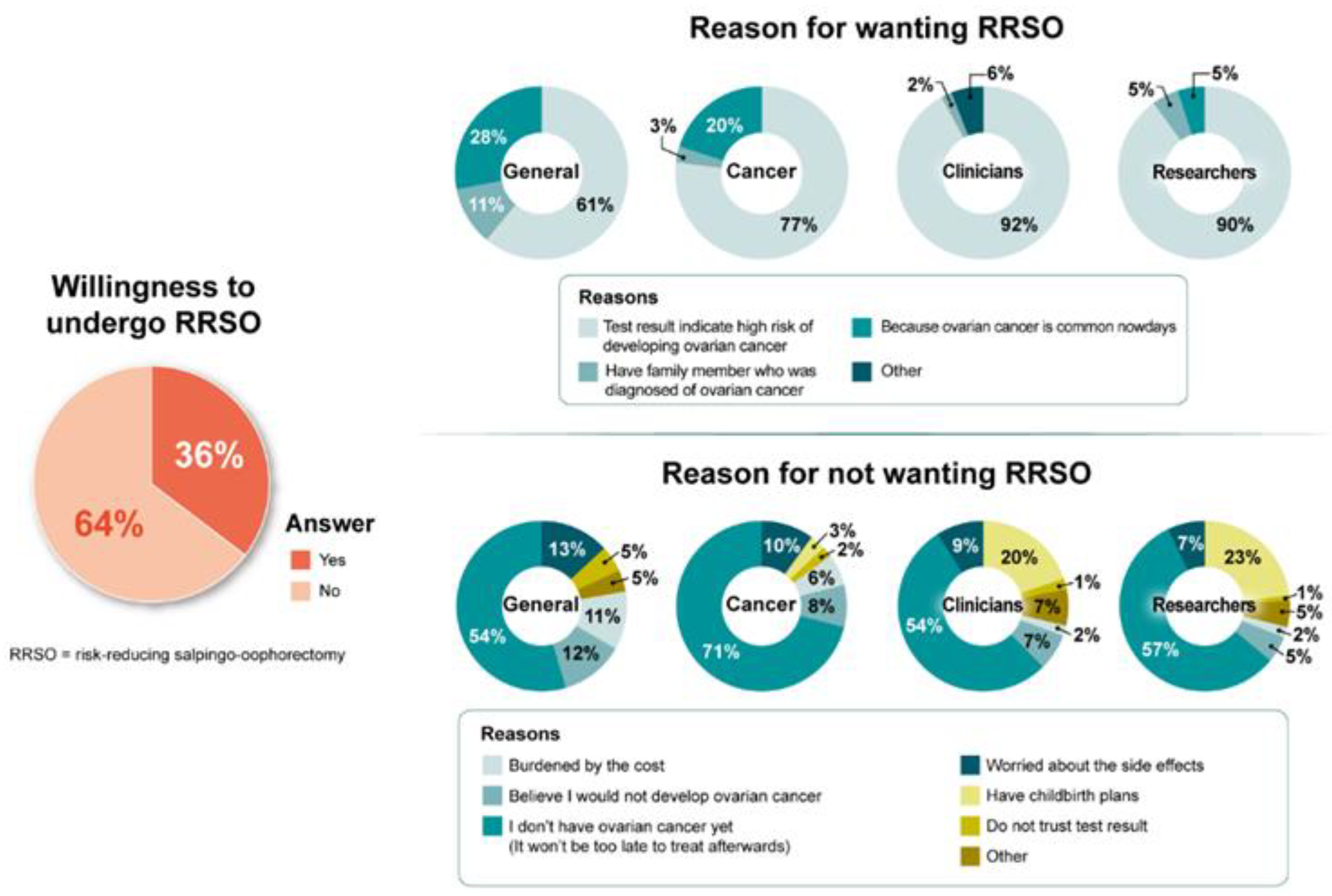
3.4. Barriers and Facilitators of BRCA1/2 Testing, RRSO, and RRM
3.5. Familial Communication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ropka, M.E.; Wenzel, J.; Phillips, E.K.; Siadaty, M.; Philbrick, J.T. Uptake rates for breast cancer genetic testing: A systematic review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 840–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.F.; Balneaves, L.G.; Bottorff, J.L. Women’s decision making about risk-reducing strategies in the context of hereditary breast and ovarian cancer: A systematic review. J. Genet. Couns. 2009, 18, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, A.F.; Dorval, M.; DiGianni, L.S.; Schneider, K.A.; Chittenden, A.; Garber, J.E. Sharing BRCA1/2 test results with first-degree relatives: Factors predicting who women tell. J. Clin. Oncol. 2006, 24, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Seong, M.W.; Park, S.K.; Lee, J.W.; Lee, J.; Kim, L.S.; Lee, J.E.; Kim, S.Y.; Jeong, J.; Han, S.A.; et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: Recent update of the Korean hereditary breast cancer (KOHBRA) study. Breast Cancer Res. Treat. 2015, 151, 157–168. [Google Scholar] [CrossRef]
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Kim, S.W. The Korean hereditary breast cancer study: Review and future perspectives. J. Breast Cancer 2013, 16, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.I.; Lim, M.C.; Lee, D.O.; Kong, S.Y.; Seo, S.S.; Kang, S.; Lee, E.S.; Park, S.Y. Uptake of risk-reducing salpingo-oophorectomy among female BRCA mutation carriers: Experience at the national cancer center of Korea. J. Cancer Res. Clin. Oncol. 2016, 142, 333–340. [Google Scholar] [CrossRef]
- Nakamura, S.; Kwong, A.; Kim, S.W.; Iau, P.; Patmasiriwat, P.; Dofitas, R.; Aryandono, T.; Hu, Z.; Huang, C.S.; Ginsburg, O.; et al. Current status of the management of hereditary breast and ovarian cancer in Asia: First report by the Asian BRCA consortium. Public Health Genom. 2016, 19, 53–60. [Google Scholar] [CrossRef]
- Bottorff, J.L.; Ratner, P.A.; Balneaves, L.G.; Richardson, C.G.; McCullum, M.; Hack, T.; Chalmers, K.; Buxton, J. Women’s interest in genetic testing for breast cancer risk: The influence of sociodemographics and knowledge. Cancer Epidemiol. Biomark. Prev. 2002, 11, 89–95. [Google Scholar]
- Hann, K.E.J.; Freeman, M.; Fraser, L.; Waller, J.; Sanderson, S.C.; Rahman, B.; Side, L.; Gessler, S.; Lanceley, A.; PROMISE Study Team. Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: A systematic review. BMC Public Health 2017, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Claes, E.; Evers-Kiebooms, G.; Boogaerts, A.; Decruyenaere, M.; Denayer, L.; Legius, E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am. J. Med. Genet. A 2003, 116A, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Finlay, E.; Stopfer, J.E.; Burlingame, E.; Evans, K.G.; Nathanson, K.L.; Weber, B.L.; Armstrong, K.; Rebbeck, T.R.; Domchek, S.M. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet. Test. 2008, 12, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, T.S.; Deffenbaugh, A.M.; Reid, J.E.; Hulick, M.; Ward, B.E.; Lingenfelter, B.; Gumpper, K.L.; Scholl, T.; Tavtigian, S.V.; Pruss, D.R.; et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10,000 individuals. J. Clin. Oncol. 2002, 20, 1480–1490. [Google Scholar] [CrossRef]
- Bradbury, A.R.; Dignam, J.J.; Ibe, C.N.; Auh, S.L.; Hlubocky, F.J.; Cummings, S.A.; White, M.; Olopade, O.I.; Daugherty, C.K. How often do BRCA mutation carriers tell their young children of the family’s risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J. Clin. Oncol. 2007, 25, 3705–3711. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Lee, K.A.; Facione, N.C.; Dodd, M.J. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev. Med. 2004, 38, 388–402. [Google Scholar] [CrossRef]
- Nelson, H.D.; Huffman, L.H.; Fu, R.; Harris, E.L.; U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: Systematic evidence review for the U.S. preventive services task force. Ann. Intern. Med. 2005, 143, 362–379. [Google Scholar] [CrossRef] [Green Version]
- Nelson, H.D.; Pappas, M.; Zakher, B.; Mitchell, J.P.; Okinaka-Hu, L.; Fu, R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: Systematic review to update the U.S. preventive services task force recommendation. Ann. Intern. Med. 2014, 160, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.M.; Tracy, M.S.; Meyer, M.E.; Sepucha, K.; Gelber, S.; Hirshfield-Bartek, J.; Troyan, S.; Morrow, M.; Schapira, L.; Come, S.E.; et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: A cross-sectional survey. Ann. Intern. Med. 2013, 159, 373–381. [Google Scholar] [CrossRef]
- Lee, S.H.; Ham, E.M. The relationship between the optimistic bias about cancer and cancer preventive behavior of the Korean, Chinese, American, and Japanese adult residing in Korea. J. Korean Acad. Nurs. 2010, 40, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.L.; Peters, N.; Shea, J.A.; Armstrong, K. Attitudes and misconceptions about predictive genetic testing for cancer risk. Community Genet. 2005, 8, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cragun, D.; Bonner, D.; Kim, J.; Akbari, M.R.; Narod, S.A.; Gomez-Fuego, A.; Garcia, J.D.; Vadaparampil, S.T.; Pal, T. Factors associated with genetic counseling and BRCA testing in a population-based sample of young black women with breast cancer. Breast Cancer Res. Treat. 2015, 151, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaya, W.; Esquivel, P.; Wong, J.H.; Morgan, J.W.; Freeberg, A.; Roy-Chowdhury, S.; Lum, S.S. Disparities in BRCA testing: When insurance coverage is not a barrier. Am. J. Surg. 2009, 198, 562–565. [Google Scholar] [CrossRef]
- Komenaka, I.K.; Nodora, J.N.; Madlensky, L.; Winton, L.M.; Heberer, M.A.; Schwab, R.B.; Weitzel, J.N.; Martinez, M.E. Participation of low-income women in genetic cancer risk assessment and BRCA 1/2 testing: The experience of a safety-net institution. J. Community Genet. 2016, 7, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, S.Y.; Abernethy, A.P. Financial toxicity, part I: A new name for a growing problem. Oncology 2013, 27, 80–81. [Google Scholar]
- Kim, S.Y.; Shin, D.W.; Park, B.; Cho, J.; Oh, J.H.; Kweon, S.S.; Han, H.S.; Yang, H.K.; Park, K.; Park, J.H. Cancer cost communication: Experiences and preferences of patients, caregivers, and oncologists—A nationwide triad study. Support. Care Cancer 2018, 26, 3517–3526. [Google Scholar] [CrossRef]
- Hamblin, A.; Wordsworth, S.; Fermont, J.M.; Page, S.; Kaur, K.; Camps, C.; Kaisaki, P.; Gupta, A.; Talbot, D.; Middleton, M.; et al. Clinical applicability and cost of a 46-gene panel for genomic analysis of solid tumours: Retrospective validation and prospective audit in the UK national health service. PLoS Med. 2017, 14, e1002230. [Google Scholar] [CrossRef]
- Lee, E.G.; Kang, H.J.; Lim, M.C.; Park, B.; Park, S.J.; Jung, S.Y.; Lee, S.; Kang, H.S.; Park, S.Y.; Park, B.; et al. Different patterns of risk reducing decisions in affected or unaffected BRCA pathogenic variant carriers. Cancer Res. Treat. 2019, 51, 280–288. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Yoon, S.Y.; Hassan, T.; Padmanabhan, H.; Yip, C.H.; Keng, W.T.; Thong, M.K.; Annuar, M.A.A.; Taib, N.A.M.; Teo, S.H. Attitudes and training needs of oncologists and surgeons in mainstreaming breast cancer genetic counseling in a low-to-middle income Asian country. J. Genet. Couns. 2022; Online ahead of print. [Google Scholar] [CrossRef]


| Variables | All | General Public | Cancer Patients | Clinicians | Researchers |
|---|---|---|---|---|---|
| (n = 3444) | (n = 1496) | (n = 1500) | (n = 108) | (n = 340) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Gender | |||||
| Female | 1908 (55.4) | 723 (48.3) | 901 (60.1) | 52 (48.1) | 232 (68.2) |
| Male | 1536 (44.6) | 773 (51.7) | 599 (39.9) | 56 (51.9) | 108 (31.8) |
| Age, years | |||||
| Mean (SD) | 47.7 (13.5) | 43.0 (12.0) | 56.0 (11.1) | 40.7 (8.4) | 34.2 (7.9) |
| 20–39 | 1033 (30.0) | 615 (41.1) | 109 (7.3) | 50 (46.3) | 259 (76.2) |
| 40–49 | 796 (23.1) | 381 (25.5) | 313 (20.9) | 40 (37.0) | 62 (18.2) |
| ≥50 | 1615 (46.9) | 500 (33.4) | 1078 (71.9) | 18 (16.7) | 19 (5.6) |
| Education Level | |||||
| ≤High school | 1721 (50.0) | 797 (53.3) | 924 (61.6) | 0 (0) | 0 (0) |
| College graduate | 1363 (39.6) | 687 (45.9) | 522 (34.8) | 15 (13.9) | 139 (40.9) |
| Graduate degree | 360 (10.4) | 12 (0.8) | 54 (3.6) | 93 (86.1) | 201 (59.1) |
| Household monthly income | |||||
| <USD 3000 | 988 (28.7) | 261 (17.4) | 668 (44.5) | 0 (0) | 59 (17.4) |
| USD 3000–4999 | 1961 (56.9) | 1116 (74.6) | 708 (47.2) | 18 (16.7) | 119 (35.0) |
| ≥USD 5000 | 495 (14.4) | 119 (8.0) | 124 (8.3) | 90 (83.3) | 162 (47.6) |
| Living area | |||||
| Metro | 1489 (43.2) | 690 (46.1) | 516 (34.4) | 78 (72.2) | 205 (60.3) |
| Non-metro | 1955 (56.8) | 806 (53.9) | 984 (65.6) | 30 (27.8) | 135 (39.7) |
| Perceived health status | |||||
| Excellent/Good | 1958 (56.9) | 1024 (68.5) | 665 (44.3) | 73 (67.6) | 196 (57.7) |
| Fair/Poor/Very Poor | 1486 (43.1) | 472 (31.5) | 835 (55.7) | 35 (32.4) | 144 (42.3) |
| Variables | All | General Public | Cancer Patients | Clinicians | Researchers |
|---|---|---|---|---|---|
| (n = 3444) | (n = 1496) | (n = 1500) | (n = 108) | (n = 340) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Group | p < 0.001 | ||||
| General public | 1 | ||||
| Cancer patients | 1.71 (1.47–1.99) | ||||
| Clinicians | 5.30 (2.94–9.55) | ||||
| Researchers | 6.53 (4.52–9.44) | ||||
| Gender | p < 0.001 | p = 0.001 | p < 0.001 | p = 0.661 | p = 0.265 |
| Female | 1 | 1 | 1 | 1 | 1 |
| Male | 0.63 (0.55–0.73) | 0.71 (0.58–0.87) | 0.61 (0.49–0.76) | 1.30 (0.41–4.15) | 1.58 (0.69–3.61) |
| Age, years | p < 0.001 | p = 0.706 | p < 0.001 | p = 0.183 | p = 0.557 |
| 20–39 | 1 | 1 | 1 | 1 | 1 |
| 40–49 | 0.91 (0.74–1.11) | 0.90 (0.70–1.17) | 0.63 (0.35–1.12) | 0.30 (0.07–1.25) | 1.76 (0.59–5.21) |
| ≥50 | 0.73 (0.62–0.87) | 0.93 (0.73–1.18) | 0.37 (0.22–0.63) | 0.32 (0.06–1.75) | 1.03 (0.23–4.70) |
| Educational Level | p < 0.001 | p = 0.166 | p < 0.001 | p = 0.461 | p = 0.026 |
| ≤High school | 1 | 1 | 1 | ||
| College graduate | 1.28 (1.10–1.48) | 0.99 (0.81–1.22) | 1.63 (1.28–2.08) | 1 | 1 |
| Graduate degree | 4.18 (3.01–5.79) | 3.64 (0.79–16.74) | 1.08 (0.60–1.95) | 0.48 (0.06–4.01) | 2.24 (1.09–4.61) |
| Household monthly income | p < 0.001 | p = 0.187 | p < 0.001 | p = 0.163 | |
| <USD 3000 | 1 | 1 | 1 | 1 | |
| USD 3000–4999 | 1.15 (0.98–1.34) | 0.94 (0.71–1.23) | 1.82 (1.44–2.30) | 1 | 2.49 (0.95–6.52) |
| ≥USD 5000 | 2.32 (1.80–3.00) | 1.34 (0.86–2.11) | 1.81 (1.17–2.82) | 2.00 (0.84–4.74) | |
| Living area | p = 0.308 | p = 0.060 | p = 0.851 | p = 0.263 | p = 0.089 |
| Metro | 1 | 1 | 1 | 1 | 1 |
| Non-metro | 1.08 (0.93–1.24) | 1.22 (0.99–1.50) | 1.02 (0.81–1.29) | 2.30 (0.48–11.05) | 1.94 (0.88–4.31) |
| Perceived health status | p = 0.458 | p = 0.160 | p = 0.024 | p = 0.024 | p = 0.095 |
| Excellent/Good | 1 | 1 | 1 | 1 | 1 |
| Fair/Poor/Very poor | 1.06 (0.91–1.22) | 1.17 (0.94–1.46) | 0.77 (0.62–0.97) | 6.69 (0.83–53.68) | 0.55 (0.27–1.11) |
| Variables | All | General Public | Cancer Patients | Clinicians | Researchers |
|---|---|---|---|---|---|
| (n = 3444) | (n = 1496) | (n = 1500) | (n = 108) | (n = 340) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Group | p < 0.001 | ||||
| General public | 1 | ||||
| Cancer patients | 1.25 (1.08–1.46) | ||||
| Clinicians | 2.87 (1.93–4.26) | ||||
| Researchers | 0.98 (0.76–1.26) | ||||
| Gender | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.794 | p = 0.003 |
| Female | 1 | 1 | 1 | 1 | 1 |
| Male | 0.68 (0.59–0.79) | 0.56 (0.45–0.69) | 0.64 (0.52–0.80) | 0.90 (0.42–1.94) | 2.07 (1.28–3.33) |
| Age, years | p = 0.009 | p = 0.222 | p = 0.250 | p = 0.671 | p = 0.062 |
| 20–39 | 1 | 1 | 1 | 1 | 1 |
| 40–49 | 1.28 (1.06–1.56) | 1.01 (0.77–1.33) | 1.46 (0.92–2.32) | 1.21 (0.52–2.83) | 1.89 (1.07–3.34) |
| ≥50 | 1.26 (1.07–1.49) | 1.23 (0.96–1.58) | 1.28 (0.84–1.95) | 0.72 (0.25–2.13) | 1.78 (0.69–4.61) |
| Education Level | p = 0.030 | p = 0.028 | p = 0.685 | p = 0.888 | p = 0.819 |
| ≤High school | 1 | 1 | 1 | 1 | |
| College graduate | 1.06 (0.91–1.23) | 1.14 (0.91–1.41) | 1.06 (0.85–1.32) | 0.92 (0.30–2.81) | 1 |
| Graduate degree | 1.37 (1.09–1.73) | 4.40 (1.31–14.75) | 1.25 (0.71–2.17) | 1.06 (0.66–1.68) | |
| Household monthly income | p = 0.026 | p = 0.409 | p = 0.056 | p = 0.793 | p = 0.676 |
| <USD 3000 | 1 | 1 | 1 | 1 | |
| USD 3000–4999 | 1.03 (0.88–1.22) | 1.19 (0.89–1.60) | 1.09 (0.88–1.36) | 1 | 0.76 (0.39–1.46) |
| ≥USD 5000 | 1.34 (1.07–1.67) | 1.30 (0.82–2.07) | 1.61 (1.09–2.37) | 0.87 (0.31–2.45) | 0.77 (0.41–1.44) |
| Living area | p = 0.971 | p = 0.346 | p = 0.114 | p = 0.828 | p = 0.382 |
| Metro | 1 | 1 | 1 | 1 | 1 |
| Non-metro | 1.00 (0.87–1.15) | 0.90 (0.73–1.12) | 1.19 (0.96–1.49) | 0.91 (0.39–2.13) | 0.81 (0.51–1.30) |
| Perceived health status | p = 0.161 | p = 0.075 | p = 0.94 | p = 0.066 | p = 0.741 |
| Excellent/Good | 1 | 1 | 1 | 1 | 1 |
| Fair/Poor/Very Poor | 1.11 (0.96–1.27) | 1.23 (0.98–1.55) | 1.01 (0.82–1.24) | 0.47 (0.21–1.06) | 1.08 (0.68–1.71) |
| Variables | All | General Public | Cancer Patients | Clinicians | Researchers |
|---|---|---|---|---|---|
| (n = 3444) | (n = 1496) | (n = 1500) | (n = 108) | (n = 340) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Group | |||||
| General public | 1 | ||||
| Cancer patients | 1.09 (0.92–1.30) | ||||
| Clinicians | 2.32 (1.40–3.84) | ||||
| Researchers | 0.83 (0.59–1.17) | ||||
| Gender | |||||
| Female | 1 | 1 | 1 | 1 | |
| Male | 0.66 (0.57–0.76) | 0.54 (0.43–0.67) | 0.66 (0.53–0.82) | 1.85 (1.12–3.04) | |
| Age, years | |||||
| 20–39 | 1 | 1 | |||
| 40–49 | 1.24 (1.01–1.53) | 1.55 (0.85–2.81) | |||
| ≥50 | 1.39 (1.13–1.71) | 1.56 (0.59–4.10) | |||
| Educational Level | |||||
| ≤High school | 1 | 1 | |||
| College graduate | 1.19 (1.01–1.41) | 1.24 (0.99–1.54) | |||
| Graduate degree | 1.32 (0.92–1.88) | 5.19 (1.53–17.63) | |||
| Household monthly income | |||||
| <USD 3000 | 1 | 1 | |||
| USD 3000–4999 | 1.05 (0.88–1.25) | 1.05 (0.84–1.30) | |||
| ≥USD 5000 | 1.16 (0.89–1.51) | 1.49 (1.01–2.20) | |||
| Living area | |||||
| Metro | |||||
| Non-metro | |||||
| Perceived health status | |||||
| Excellent/Good | |||||
| Fair/Poor/Very Poor |
| Variables | All | General Public | Cancer Patients | Clinicians | Researchers |
|---|---|---|---|---|---|
| (n = 3444) | (n = 1496) | (n = 1500) | (n = 108) | (n = 340) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Group | p = 0.224 | ||||
| General public | 1 | ||||
| Cancer patients | 0.94 (0.80–1.11) | ||||
| Clinicians | 1.43 (0.95–2.17) | ||||
| Researchers | 1.07 (0.92–1.39) | ||||
| Gender | p < 0.001 | p < 0.001 | p = 0.059 | p = 0.940 | p = 0.006 |
| Female | 1 | 1 | 1 | 1 | 1 |
| Male | 0.76 (0.65–0.89) | 0.57 (0.45–0.72) | 0.79 (0.62–1.01) | 0.97 (0.44–2.15) | 2.02 (1.23–3.30) |
| Age, years | p = 0.660 | p = 0.353 | p = 0.397 | p = 0.126 | p = 0.315 |
| 20–39 | 1 | 1 | 1 | 1 | 1 |
| 40–49 | 1.09 (0.88–1.34) | 1.02 (0.76–1.37) | 1.27 (0.77–2.10) | 0.66 (0.28–1.58) | 1.47 (0.81–2.66) |
| ≥50 | 1.00 (0.84–1.19) | 1.20 (0.92–1.57) | 1.05 (0.66–1.67) | 0.28 (0.07–1.08) | 1.67 (0.63–4.42) |
| Educational Level | p = 0.019 | p = 0.097 | p = 0.177 | p = 0.284 | p = 0.596 |
| ≤High school | 1 | 1 | 1 | 1 | |
| College graduate | 1.23 (1.05–1.45) | 1.17 (0.93–1.47) | 1.25 (0.98–1.60) | 0.54 (0.18–1.64) | 1 |
| Graduate degree | 1.28 (0.99–1.64) | 2.99 (0.95–9.36) | 1.23 (0.67–2.28) | 0.88 (0.54–1.42) | |
| Household monthly income | p = 0.040 | p = 0.177 | p = 0.013 | p = 0.042 | p = 0.366 |
| ≤USD 3000 | 1 | 1 | 1 | 1 | |
| USD 3000–4999 | 1.19 (1.00–1.42) | 1.35 (0.98–1.85) | 1.13 (0.88–1.44) | 1 | 0.64 (0.33–1.25) |
| ≥USD 5000 | 1.34 (1.05–1.71) | 1.23 (0.75–2.04) | 1.87 (1.24–2.81) | 0.34 (0.12–0.96) | 0.65 (0.35–1.24) |
| Living area | p = 0.234 | p = 0.129 | p = 0.354 | p = 0.296 | p = 0.5 |
| Metro | 1 | 1 | 1 | 1 | 1 |
| Non-metro | 0.91 (0.78–1.06) | 0.84 (0.67–1.05) | 1.12 (0.88–1.44) | 0.61 (0.24–1.56) | 0.85 (0.52–1.38) |
| Perceived health status | p = 0.530 | ||||
| Excellent/Good | 1 | ||||
| Fair/Poor/Very Poor | 1.05 (0.90–1.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.J.; Cho, S.; Joo, J.; Ryu, K.H.; Lee, S.; Cho, J.; Lim, M.C.; Jung, S.-Y.; Han, J.H.; Lee, E.S.; et al. Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey. J. Pers. Med. 2022, 12, 818. https://doi.org/10.3390/jpm12050818
Chang YJ, Cho S, Joo J, Ryu KH, Lee S, Cho J, Lim MC, Jung S-Y, Han JH, Lee ES, et al. Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey. Journal of Personalized Medicine. 2022; 12(5):818. https://doi.org/10.3390/jpm12050818
Chicago/Turabian StyleChang, Yoon Jung, Seungyeon Cho, Jungnam Joo, Kum Hei Ryu, Sangwon Lee, Juhee Cho, Myong Cheol Lim, So-Youn Jung, Jai Hong Han, Eun Sook Lee, and et al. 2022. "Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey" Journal of Personalized Medicine 12, no. 5: 818. https://doi.org/10.3390/jpm12050818
APA StyleChang, Y. J., Cho, S., Joo, J., Ryu, K. H., Lee, S., Cho, J., Lim, M. C., Jung, S.-Y., Han, J. H., Lee, E. S., & Kong, S.-Y. (2022). Differences in Willingness to Undergo BRCA1/2 Testing and Risk Reducing Surgery among the General Public, Cancer Patients, and Healthcare Professionals: A Large Population-Based Survey. Journal of Personalized Medicine, 12(5), 818. https://doi.org/10.3390/jpm12050818






