Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and CBC Analyses
2.2. Statistical Analyses
3. Results
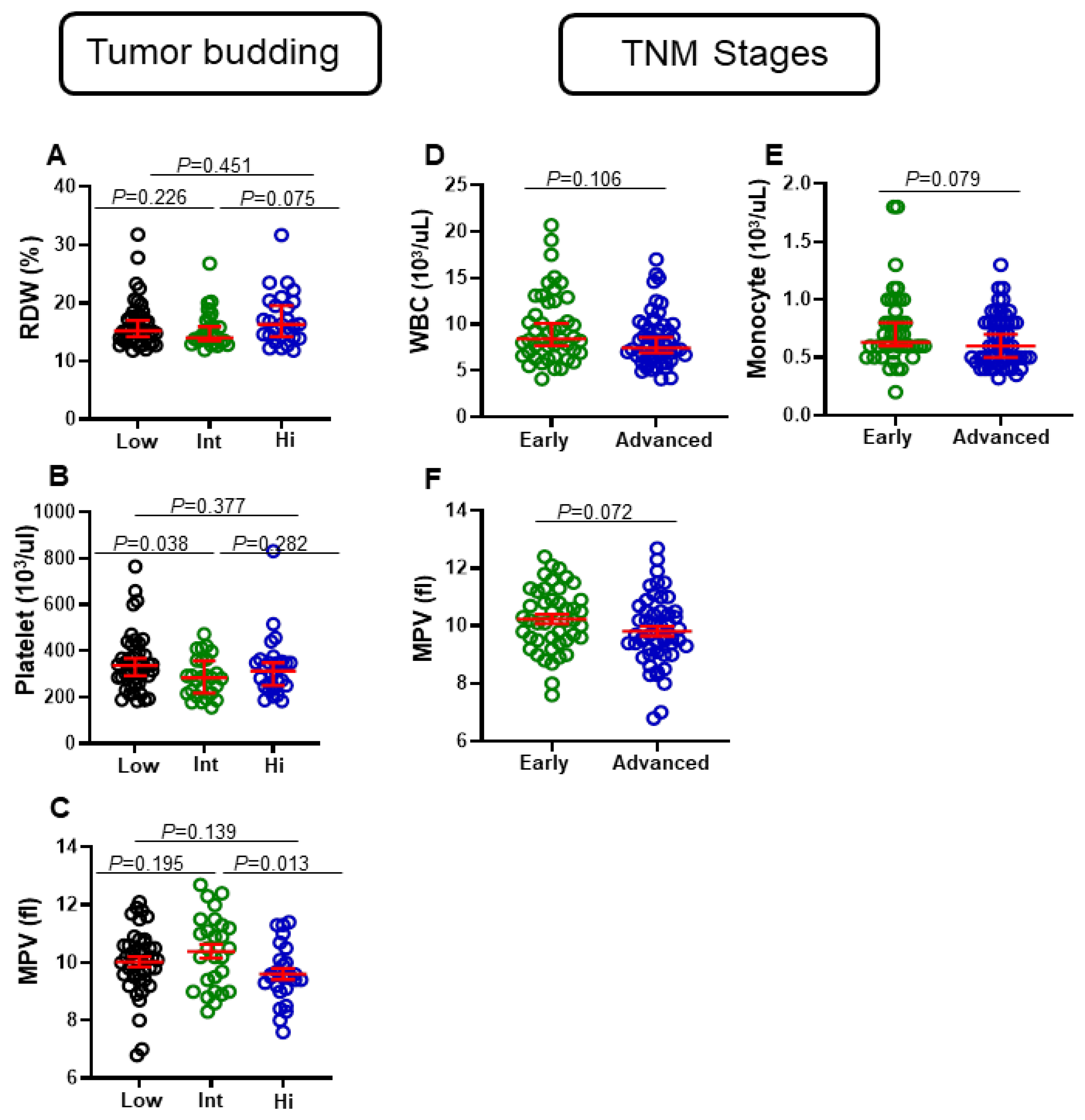
3.1. Differential Levels of Red Cell Distribution Width (RDW), Platelet, and Mean Platelet Volume (MPV) in CRC Patients with Varying Tumor Budding Status
3.2. Differential Levels of Eosinophils, WBC, Monocytes, and MPV in CRC Patients with Different Tumor Stages
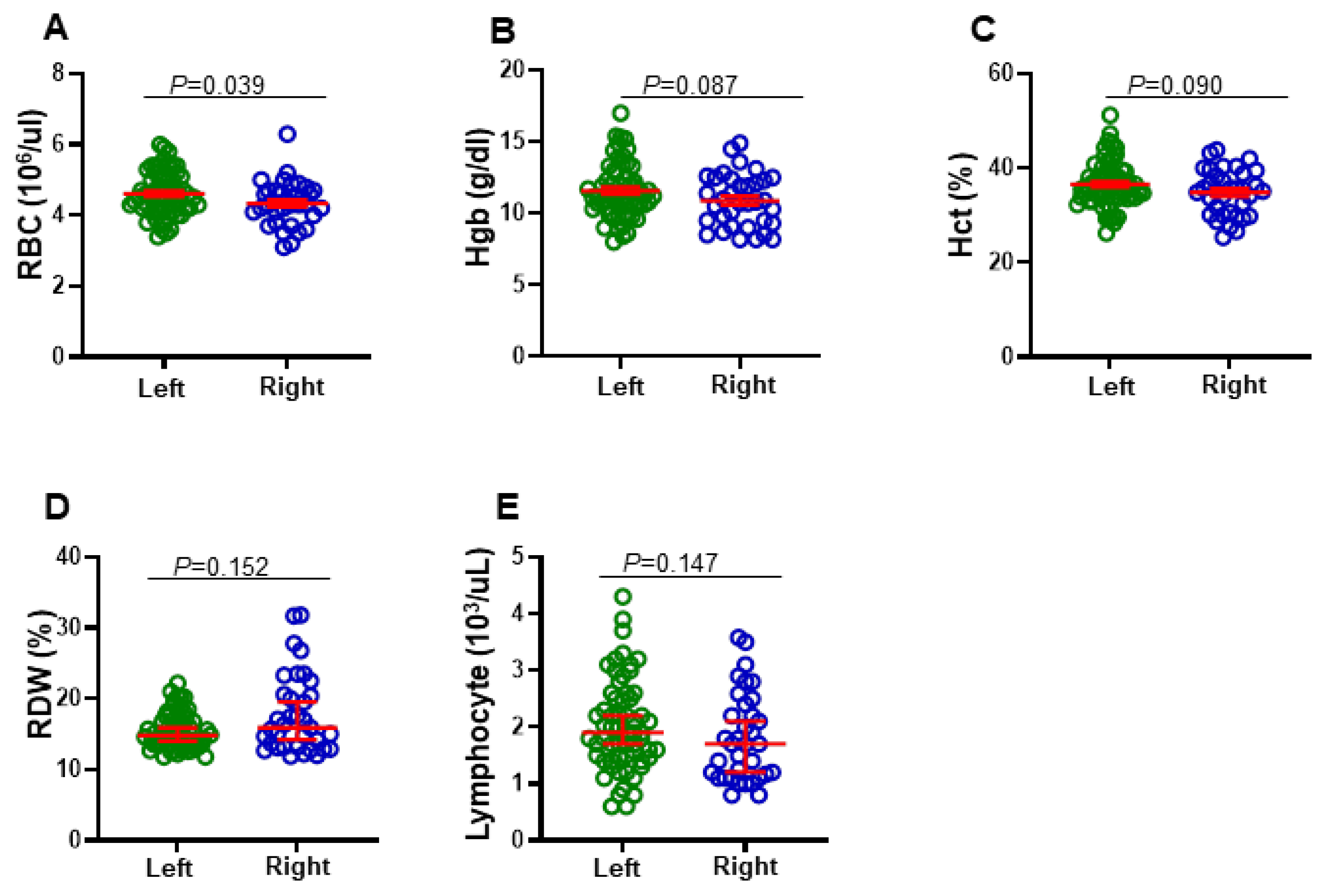
3.3. Differential Levels of RBC, Hgb, Hct, RDW, and Lymphocytes in Right-Sided and Left-Sided CRC Patients
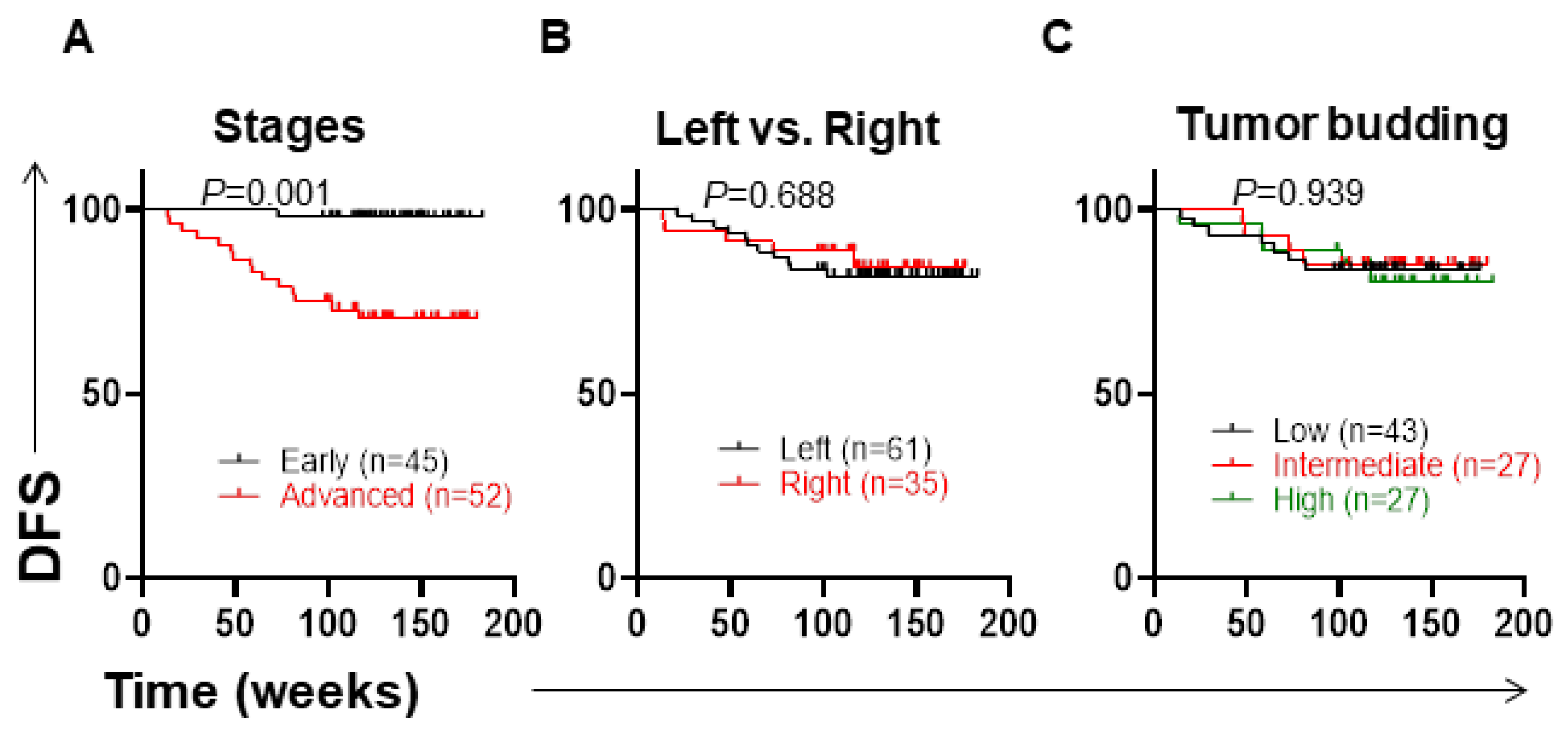
3.4. Clinicopathological Parameters and Disease-Free Survival
3.5. Low Levels of MPV and High Numbers of Eosinophils Are Associated with Shorter DFS
3.6. Associations of CBC Parameters with DFS in Left-Sided and Right-Sided CRC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | complete blood count |
| CRC | colorectal cancer |
| DFS | disease-free survival |
| Hgb | hemoglobin |
| Hct | hematocrit |
| MPV | mean platelet volume |
| MCH | mean corpuscular hemoglobin |
| MDSs | myelodysplastic syndromes |
| OS | overall survival |
| RDW | red cell distribution width |
| RBC | red blood cells |
| TNM | tumor, nodes, and metastases |
| WBC | white blood cells. |
References
- Ferlay, J. Cancer incidence, mortality and prevalence worldwide. In GLOBOCAN 2002; IARC Press: Lyon, France, 2004. [Google Scholar]
- Venook, A. Critical Evaluation of Current Treatments in Metastatic Colorectal Cancer. Oncologist 2005, 10, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Thélin, C.; Sikka, S. Epidemiology of colorectal cancer—Incidence, lifetime risk factors statis-tics and temporal trends. In Screening for Colorectal Cancer with Colonoscopy; IntechOpen Limited: London, UK, 2015; pp. 61–77. [Google Scholar]
- Baran, B.; Ozupek, N.M.; Tetik, N.Y.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Solak Mekić, M.; Pedišić, I.; Šobat, H.; Vučićević Boras, V.; Kirac, I.; Štefančić, L.; Šekerija, M.; Vrdoljak, B.; Vrdoljak, D.V. The role of complete blood count parameters in patients with colorectal cancer. Acta Clinica. Croatica. 2018, 57, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Sylman, J.L.; Mitrugno, A.; Atallah, M.; Tormoen, G.W.; Shatzel, J.J.; Tassi Yunga, S.; Wagner, T.H.; Leppert, J.T.; Mallick, P.; McCarty, O.J.T. The Predictive Value of Inflammation-Related Peripheral Blood Measurements in Cancer Staging and Prognosis. Front. Oncol. 2018, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Glebov, O.K.; Rodriguez, L.M.; Nakahara, K.; Jenkins, J.; Cliatt, J.; Humbyrd, C.J.; DeNobile, J.; Soballe, P.; Simon, R.; Wright, G.; et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol. Prev. Biomark. 2003, 12, 755–762. [Google Scholar]
- Kocarnik, J.M.; Shiovitz, S.; Phipps, A.I. Molecular phenotypes of colorectal cancer and potential clinical applications. Gastroenterol. Rep. 2015, 3, 269–276. [Google Scholar] [CrossRef]
- Luvián-Morales, J.; González-Trejo, S.; Carrillo, J.F.; Herrera-Goepfert, R.; Aiello-Crocifoglio, V.; Gallardo-Rincón, D.; Ochoa-Carrillo, F.J.; Oñate-Ocaña, L.F. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: A strobe compliant retrospective cohort study. Cancer Med. 2019, 8, 3379–3388. [Google Scholar] [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C.; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Future Oncol. 2005, 1, 37–50. [Google Scholar] [CrossRef]
- Ray, S.; Reddy, P.J.; Jain, R.; Gollapalli, K.; Moiyadi, A.; Srivastava, S. Proteomic technologies for the identification of disease biomarkers in serum: Advances and challenges ahead. Proteomics 2011, 11, 2139–2161. [Google Scholar] [CrossRef]
- Reimers, M.S.; Zeestraten, E.C.M.; Kuppen, P.J.K.; Liefers, G.J.; Van De Velde, C.J.H. Biomarkers in precision therapy in colorectal cancer. Gastroenterol. Rep. 2013, 1, 166–183. [Google Scholar] [CrossRef] [PubMed]
- George-Gay, B.; Parker, K. Understanding the complete blood count with differential. J. PeriAnesthesia Nurs. 2003, 18, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.P.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Laird, P.W.; Weisenberger, D.J.; Lynch, C.F.; French, A.J.; Slager, S.L.; Raissian, Y.; et al. Tumor budding in colorectal carcinoma: Confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am. J. Surg. Pathol. 2015, 39, 1340–1346. [Google Scholar] [CrossRef]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Mikhailidis, D.P.; Kitas, G.D. Mean platelet volume: A link be-tween thrombosis and inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Loktionov, A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J. Gastroenterol. 2019, 25, 3503–3526. [Google Scholar] [CrossRef]
- Verdaguer, H.; Saurí, T.; Macarulla, T. Predictive and prognostic biomarkers in personalized gastrointestinal cancer treatment. J. Gastrointest. Oncol. 2017, 8, 405–417. [Google Scholar] [CrossRef][Green Version]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef]
- Loupakis, F.; Yang, D.; Yau, L.; Feng, S.; Cremolini, C.; Zhang, W.; Maus, M.K.H.; Antoniotti, C.; Langer, C.; Scherer, S.J.; et al. Primary Tumor Location as a Prognostic Factor in Metastatic Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju427. [Google Scholar] [CrossRef]
- Nakagawa-Senda, H.; Hori, M.; Matsuda, T.; Ito, H. Prognostic impact of tumor location in colon cancer: The Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer 2019, 19, 431. [Google Scholar] [CrossRef]
- Meguid, R.A.; Slidell, M.B.; Wolfgang, C.L.; Chang, D.C.; Ahuja, N. Is there a difference in survival between right-versus left-sided colon cancers? Ann. Surg. Oncol. 2008, 15, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Moritani, K.; Hasegawa, H.; Okabayashi, K.; Ishii, Y.; Endo, T.; Kitagawa, Y. Difference in the re-currence rate between right-and left-sided colon cancer: A 17-year experience at a single institution. Surg. Today 2014, 44, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lin, G.; Ye, M.; Tong, D.; Zhao, J.; Zhu, D.; Yu, Q.; Zhang, W.; Li, W. Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: Results from mCRC biomarker study. BMC Cancer 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Li, Y.; Jiang, Z.; Wang, R.-T.; Wang, X.-S. Elevated Mean Platelet Volume is Associated with Presence of Colon Cancer. Asian Pac. J. Cancer Prev. 2015, 15, 10501–10504. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Wang, P.; Hu, X.; Gao, Y.; He, J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumor Biol. 2016, 37, 9323–9331. [Google Scholar] [CrossRef]
- Gu, M.; Zhai, Z.; Huang, L.; Zheng, W.; Zhou, Y.; Zhu, R.; Shen, F.; Yuan, C. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer 2015, 23, 752–760. [Google Scholar] [CrossRef]
- Inagaki, N.; Kibata, K.; Tamaki, T.; Shimizu, T.; Nomura, S. Prognostic impact of the mean plate-let volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer 2014, 83, 97–101. [Google Scholar] [CrossRef]
- Yun, Z.Y.; Zhang, X.; Liu, Y.S.; Liu, T.; Liu, Z.P.; Wang, R.T.; Yu, K.J. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci. Rep. 2017, 7, 6700. [Google Scholar] [CrossRef]
- Wang, X.; Cui, M.-M.; Xu, Y.; Liu, L.; Niu, Y.; Liu, T.; Liu, Z.-P.; Wang, R.-T.; Yu, K.-J. Decreased mean platelet volume predicts poor prognosis in invasive bladder cancer. Oncotarget 2017, 8, 68115–68122. [Google Scholar] [CrossRef]
- Qian, W.; Ge, X.X.; Wu, J.; Gong, F.R.; Wu, M.Y.; Xu, M.D.; Lian, L.; Wang, W.J.; Li, W.; Tao, M. Prognostic evaluation of resectable colorectal cancer using platelet-associated indicators. Oncol. Lett. 2019, 18, 571–580. [Google Scholar] [CrossRef]
- Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Bokemeyer, C.; Langner, C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod. Pathol. 2014, 28, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Yalcin, A.D.; Kargi, A.; Gumuslu, S.; Strauss, L.G. Blood eosinophil and platelet levels, proteomics patterns of trail and CXCL8 correlated with survival in bevacizumab treated metastatic colon cancers. Clin. Lab. 2014, 60, 339–340. [Google Scholar]
- Wei, Y.; Zhang, X.; Wang, G.; Zhou, Y.; Luo, M.; Wang, S.; Hong, C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I–III colorectal cancer. Asia Pac. J. Clin. Oncol. 2018, 14, e243–e251. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, K.; Zhou, H.; Peng, L.; You, W.; Fu, Z. Profiles of immune infiltration in colorectal cancer and their clinical significant: A gene expression-based study. Cancer Med. 2018, 7, 4496–4508. [Google Scholar] [CrossRef]
- Gündüz, S.; Göksu, S.S.; Arslan, D.; Tatli, A.M.; Uysal, M.; Gündüz, U.R.; Sevinç, M.M.; Coşkun, H.S.; Bozcuk, H.; Mutlu, H.; et al. Factors affecting dis-ease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol. Clin. Oncol. 2015, 3, 1109–1112. [Google Scholar] [CrossRef][Green Version]
- Andersen, C.L.; Siersma, V.D.; Hasselbalch, H.; Lindegaard, H.M.; Vestergaard, H.; Felding, P.; Olivarius, N.D.F.; Bjerrum, O.W. Eosinophilia in routine blood samples and the subsequent risk of hematological malignancies and death. Am. J. Hematol. 2013, 88, 843–847. [Google Scholar] [CrossRef]
- Wimazal, F.; Germing, U.; Kundi, M.; Noesslinger, T.; Blum, S.; Geissler, P.; Baumgartner, C.; Pfeilstoecker, M.; Valent, P.; Sperr, W.R. Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes. Cancer 2010, 116, 2372–2381. [Google Scholar] [CrossRef]
- Siddiqui, S.; Jaiswal, R.; Hashmi, G.S. Quantitative analysis of tumor-associated tissue eosinophils and tumor-associated blood eosinophils in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2020, 24, 131–137. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2014, 52, 86–105. [Google Scholar] [CrossRef]
- Wang, P.-F.; Song, S.-Y.; Guo, H.; Wang, T.-J.; Liu, N.; Yan, C.-X. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: A meta-analysis of 49 studies. J. Cancer 2019, 10, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-P.; Huang, Y.; Yang, X.; Feng, J.-F. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediat. Inflamm. 2015, 2015, 854670. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, N.; Matsubara, T.; Kawahara, D.; Mizota, Y.; Ishibashi, S.; Tajima, Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int. J. Clin. Oncol. 2016, 21, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-X.; Chen, P.; Cai, X.-J.; Li, L.-J.; Yu, X.-J.; Pan, D.-F.; Wang, X.-H.; Wang, X.-B.; Cao, F.-J. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin. Chim. Acta 2016, 452, 199–203. [Google Scholar] [CrossRef]
- Warwick, R.; Mediratta, N.; Shackcloth, M.; Shaw, M.; McShane, J.; Poullis, M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2014, 45, 108–113. [Google Scholar] [CrossRef]
- Kos, M.; Hocazade, C.; Kos, F.T.; Uncu, D.; Karakas, E.; Dogan, M.; Uncu, H.G.; Ozdemir, N.; Zengin, N. Evaluation of the effects of red blood cell distribution width on survival in lung cancer patients. Contemp. Oncol. 2016, 20, 153–157. [Google Scholar] [CrossRef]
- Iriyama, N.; Hatta, Y.; Kobayashi, S.; Uchino, Y.; Miura, K.; Kurita, D.; Kodaira, H.; Takahashi, H.; Iizuka, Y.; Inoue, M.; et al. Higher Red Blood Cell Distribution Width Is an Adverse Prognostic Factor in Chronic-phase Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors. Anticancer. Res. 2015, 35, 5473–5478. [Google Scholar]
- Periša, V.; Zibar, L.; Sinčić-Petričević, J.; Knezović, A.; Periša, I.; Barbić, J. Red blood cell distribution width as a simple negative prognostic factor in patients with diffuse large B-cell lymphoma: A retrospective study. Croat. Med. J. 2015, 56, 334–343. [Google Scholar] [CrossRef]
- Han, F.; Shang, X.; Wan, F.; Liu, Z.; Tian, W.; Wang, D.; Liu, Y.; Wang, Y.; Zhang, B.; Ju, Y. Clinical value of the preoperative neutrophil-to-lymphocyte ratio and red blood cell distribution width in patients with colorectal carcinoma. Oncol. Lett. 2017, 15, 3339–3349. [Google Scholar] [CrossRef]
- Ay, S.; Eryilmaz, M.A.; Aksoy, N.; Okus, A.; Unlu, Y.; Sevinc, B. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pac. J. Cancer Prev. 2015, 16, 753–756. [Google Scholar] [CrossRef]
- Cheng, K.C.; Lin, Y.M.; Liu, C.C.; Wu, K.L.; Lee, K.C. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Xing, C.; Hu, X.; Zhang, F.; Peng, Y.; Li, Z.; Lu, T. Prognostic evaluation of colorectal cancer using three new comprehensive indexes related to infection, anemia and coagulation derived from peripheral blood. J. Cancer 2020, 11, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Tampellini, M.; Saini, A.; Alabiso, I.; Bitossi, R.; Brizzi, M.P.; Sculli, C.M.; Berruti, A.; Gorzegno, G.; Magnino, A.; Sperti, E.; et al. The role of haemoglobin level in predicting the response to first-line chemotherapy in advanced colorectal cancer patients. Br. J. Cancer 2006, 95, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Buergy, D.; Wenz, F.; Groden, C.; Brockmann, M.A. Tumor-platelet interaction in solid tumors. Int. J. Cancer 2012, 130, 2747–2760. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Long, Y.; Wang, T.; Gao, Q.; Zhou, C. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: A meta-analysis. Oncotarget 2016, 7, 81849–81861. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, Y.; Fu, Y.; Ye, F.; Xie, X.; Lu, W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2012, 38, 651–657. [Google Scholar] [CrossRef]
- Suzuki, K.; Aiura, K.; Kitagou, M.; Hoshimoto, S.; Takahashi, S.; Ueda, M.; Kitajima, M. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology 2004, 51, 847–853. [Google Scholar]
- Li, N.; Yu, Z.; Zhang, X.; Liu, T.; Sun, Y.-X.; Wang, R.-T.; Yu, K.-J. Elevated mean platelet volume predicts poor prognosis in colorectal cancer. Sci. Rep. 2017, 7, 10261. [Google Scholar] [CrossRef]

| CRC Patients | |
|---|---|
| Number | 97 |
| Median age (range) | 59 (18–96) |
| Gender (Male:Female) | 65:32 |
| TNM stage | |
| I | 10 (0) § |
| II | 35 (1) § |
| III | 39 (8) § |
| IV | 13 (7) § |
| Tumor budding | |
| Low | 43 (7) § |
| Intermediate | 27 (4) § |
| High | 27 (5) § |
| Right/Left-sided | 61 (11) § |
| Left | |
| Right | 35 (5) § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalman, A.; Al-Mterin, M.A.; Abu-Dayeh, A.; Alloush, F.; Murshed, K.; Elkord, E. Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients. J. Pers. Med. 2022, 12, 816. https://doi.org/10.3390/jpm12050816
Alsalman A, Al-Mterin MA, Abu-Dayeh A, Alloush F, Murshed K, Elkord E. Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients. Journal of Personalized Medicine. 2022; 12(5):816. https://doi.org/10.3390/jpm12050816
Chicago/Turabian StyleAlsalman, Alhasan, Mohammad A. Al-Mterin, Ala Abu-Dayeh, Ferial Alloush, Khaled Murshed, and Eyad Elkord. 2022. "Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients" Journal of Personalized Medicine 12, no. 5: 816. https://doi.org/10.3390/jpm12050816
APA StyleAlsalman, A., Al-Mterin, M. A., Abu-Dayeh, A., Alloush, F., Murshed, K., & Elkord, E. (2022). Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients. Journal of Personalized Medicine, 12(5), 816. https://doi.org/10.3390/jpm12050816







