Spasticity Management after Spinal Cord Injury: The Here and Now
Abstract
:1. Introduction
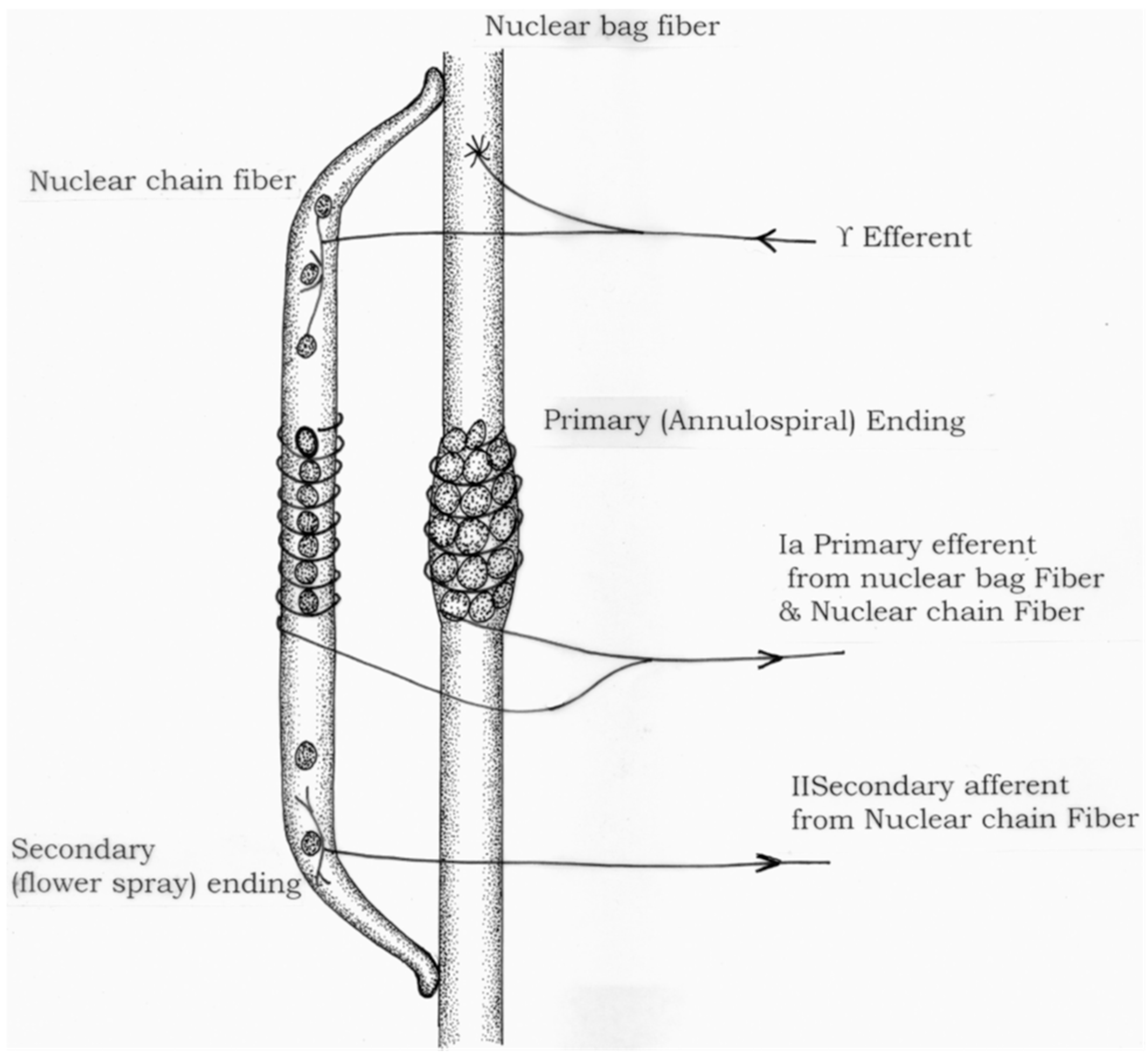
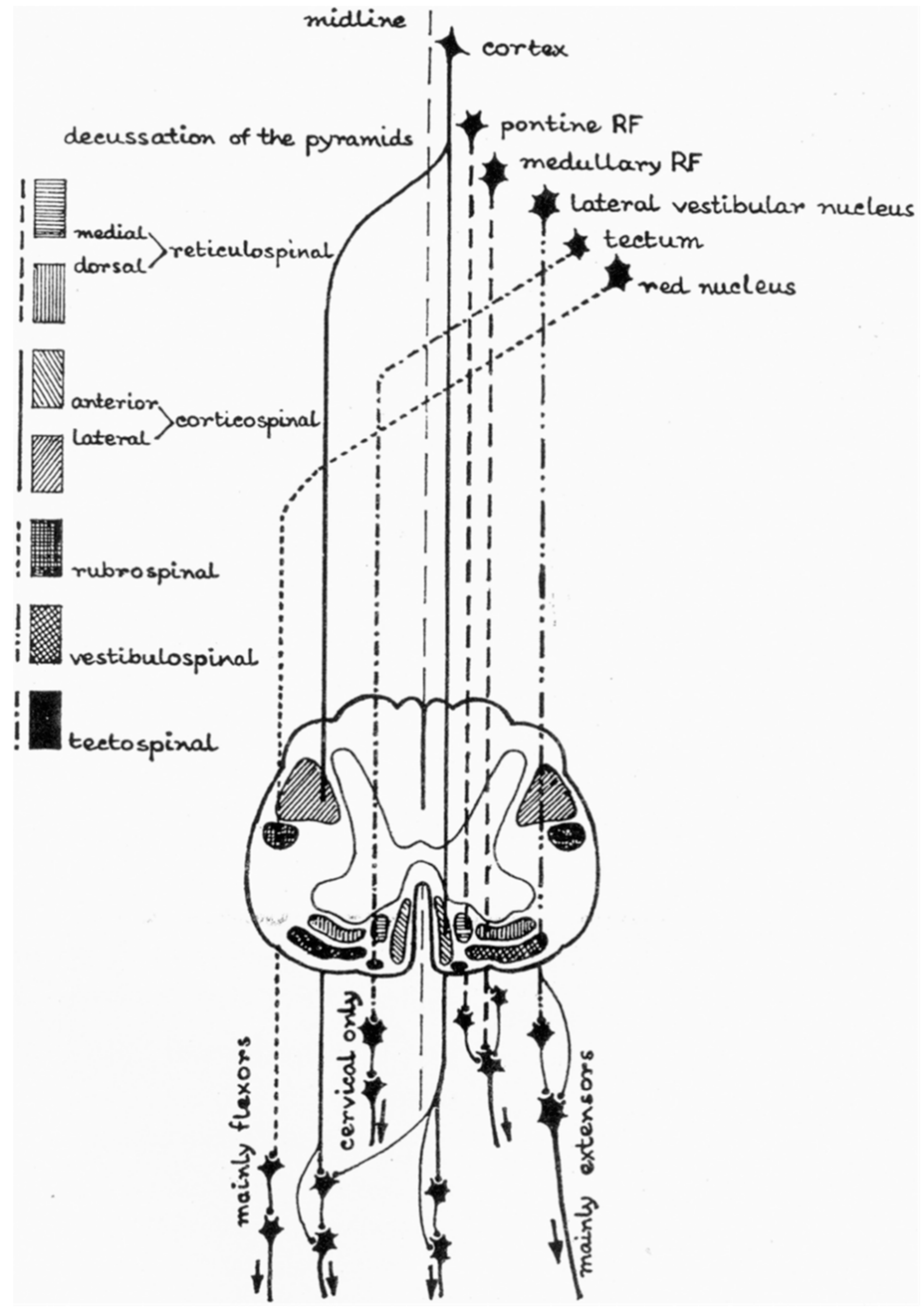
2. Anatomy and Physiology: The Basics
3. Pathophysiology
4. Assessment
5. Management/Treatment
5.1. Conservative Management
5.2. Therapeutic Modalities
5.3. Oral Pharmacological Management
5.3.1. Gaba Agonists
5.3.2. Alpha Agonists
5.3.3. Calcium Antagonists
6. Intrathecal Baclofen
7. Neurolysis
7.1. Botulinim Toxin Injection
7.2. Phenol Neurolysis
8. Non-Pharmacological Neuromodulation
9. Surgical Considerations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenbeg lecture. Neurology 1980, 30, 1303. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.M.; Karunas, R.S.; Waring, W.P., 3rd. Epidemiology of spasticity following traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1990, 71, 566–569. [Google Scholar] [PubMed]
- Holtz, K.A.; Lipson, R.; Noonan, V.K.; Kwon, B.K.; Mills, P.B. Prevalence and Effect of Problematic Spasticity After Traumatic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2017, 98, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Sköld, C.; Levi, R.; Seiger, A. Spasticity after traumatic spinal cord injury: Nature, severity, and location. Arch. Phys. Med. Rehabil. 1999, 80, 1548–1557. [Google Scholar] [CrossRef]
- Skoog, B.; Jakobsson, K.E. Prevalence of Spasticity and Below-Level Neuropathic Pain Related to Spinal Cord Injury Level and Damage to the Lower Spinal Segments. J. Rehabil. Med. Clin. Commun. 2020, 3, 1000039. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakravarty, A. Spasticity mechanisms—For the clinician. Front. Neurol. 2010, 1, 149. [Google Scholar] [CrossRef] [Green Version]
- Boyd, I.A. The response of fast and slow nuclear bag fibres and nuclear chain fibres in isolated cat muscle spindles to fusimotor stimulation, and the effect of intrafusal contraction on the sensory endings. Q. J. Exp. Physiol. Cogn. Med. Sci. 1976, 61, 203–254. [Google Scholar] [CrossRef]
- Sangari, S.; Kirshblum, S.; Guest, J.D.; Oudega, M.; Perez, M.A. Distinct patterns of spasticity and corticospinal connectivity following complete spinal cord injury. J. Physiol.-Lond. 2021, 599, 4441–4454. [Google Scholar] [CrossRef]
- Sangari, S.; Perez, M.A. Imbalanced Corticospinal and Reticulospinal Contributions to Spasticity in Humans with Spinal Cord Injury. J. Neurosci. 2019, 39, 7872–7881. [Google Scholar] [CrossRef] [Green Version]
- Sangari, S.; Lundell, H.; Kirshblum, S.; Perez, M.A. Residual descending motor pathways influence spasticity after spinal cord injury. Ann. Neurol. 2019, 86, 28–41. [Google Scholar] [CrossRef]
- Mc, C.G.; Austin, G.M.; Liu, C.N.; Liu, C.Y. Sprouting as a cause of spasticity. J. Neurophysiol. 1958, 21, 205–216. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Heckmann, C.J.; Gorassini, M.A.; Bennett, D.J. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 2005, 31, 135–156. [Google Scholar] [CrossRef]
- Phadke, C.P.; Balasubramanian, C.K.; Ismail, F.; Boulias, C. Revisiting Physiologic and Psychologic Triggers that Increase Spasticity. Am. J. Phys. Med. Rehabil. 2013, 92, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.A.; Glinsky, J.A.; Katalinic, O.M.; Ben, M. Contracture management for people with spinal cord injuries. NeuroRehabilitation 2011, 28, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Spector, S.A.; Simard, C.P.; Fournier, M.; Sternlicht, E.; Edgerton, V.R. Architectural alterations of rat hind-limb skeletal muscles immobilized at different lengths. Exp. Neurol. 1982, 76, 94–110. [Google Scholar] [CrossRef]
- Diong, J.; Harvey, L.A.; Kwah, L.K.; Eyles, J.; Ling, M.J.; Ben, M.; Herbert, R.D. Incidence and predictors of contracture after spinal cord injury—A prospective cohort study. Spinal Cord 2012, 50, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Hon, A.J.; Kraus, P. Spasticity Management After Spinal Cord Injury. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 159–171. [Google Scholar] [CrossRef]
- Lanig, I.S.; New, P.W.; Burns, A.S.; Bilsky, G.; Benito-Penalva, J.; Bensmail, D.; Yochelson, M. Optimizing the Management of Spasticity in People with Spinal Cord Damage: A Clinical Care Pathway for Assessment and Treatment Decision Making from the Ability Network, an International Initiative. Arch. Phys. Med. Rehabil. 2018, 99, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, P.; Atici, A.; Ozkan, F.U.; Aktas, I.; Kulcu, D.G.; Sarı, A.; Durmus, B. Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord 2017, 55, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.D.; Savoy, S.M.; Corcos, D.; Latash, M.; Gottlieb, G.; Parke, B.; Kroin, J.S. Intrathecal baclofen for severe spinal spasticity. N. Engl. J. Med. 1989, 320, 1517–1521. [Google Scholar] [CrossRef]
- Benz, E.N.; Hornby, T.G.; Bode, R.K.; Scheidt, R.A.; Schmit, B.D. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.V.; Campos, A.R.; Grabljevec, K.; Lopes, A.; Skoog, B.; Burns, A.S. Clinical Assessment of Spasticity in People with Spinal Cord Damage: Recommendations From the Ability Network, an International Initiative. Arch. Phys. Med. Rehabil. 2018, 99, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Cresswell, A.G.; Riek, S. Proprioceptive neuromuscular facilitation stretching: Mechanisms and clinical implications. Sports Med. 2006, 36, 929–939. [Google Scholar] [CrossRef]
- Aydin, G.; Tomruk, S.; Keleş, I.; Demir, S.O.; Orkun, S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: Clinical and electrophysiologic comparison. Am. J. Phys. Med. Rehabil. 2005, 84, 584–592. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Raju, M.; Romagnoli, C.; Annino, G. Transcutaneous spinal cord stimulation effects on spasticity in patients with spinal cord injury: A systematic review. J. Spinal Cord Med. 2021. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Mercuri, N.B. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: A systematic review. J. Spinal Cord Med. 2022, 45, 10–23. [Google Scholar] [CrossRef]
- Holtz, K.A.; Szefer, E.; Noonan, V.K.; Kwon, B.K.; Mills, P.B. Treatment patterns of in-patient spasticity medication use after traumatic spinal cord injury: A prospective cohort study. Spinal Cord 2018, 56, 1176–1183. [Google Scholar] [CrossRef]
- de Sousa, N.; Santos, D.; Monteiro, S.; Silva, N.; Barreiro-Iglesias, A.; Salgado, A.J. Role of Baclofen in Modulating Spasticity and Neuroprotection in Spinal Cord Injury. J. Neurotrauma 2022, 39, 249–258. [Google Scholar] [CrossRef]
- Saulino, M.; Jacobs, B.W. The pharmacological management of spasticity. J. Neurosci. Nurs. 2006, 38, 456–460. [Google Scholar] [PubMed]
- Rabchevsky, A.; Patel, S.; Lyttle, T.; Eldahan, K.; O’Dell, C.; Zhang, Y.; Popovich, P.; Kitzman, P.; Donohue, K. Effects of gabapentin on muscle spasticity and both induced as well as spontaneous autonomic dysreflexia after complete spinal cord injury. Front. Physiol. 2012, 3, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnarsson, S.; Lemming, D.; Alehagen, S.; Berntsson, S.; Ertzgaard, P.; Samuelsson, K. Dosing Patterns in Treatment of Disabling Spasticity with Intrathecal Baclofen. Rehabil. Nurs. 2021, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ivanhoe, C.B.; Tilton, A.H.; Francisco, G.E. Intrathecal Baclofen Therapy for Spastic Hypertonia. Phys. Med. Rehabil. Clin. N. Am. 2001, 12, 923–938. [Google Scholar] [CrossRef]
- Saulino, M.; Anderson, D.J.; Doble, J.; Farid, R.; Gul, F.; Konrad, P.; Boster, A.L. Best Practices for Intrathecal Baclofen Therapy: Troubleshooting. Neuromodulation 2016, 19, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Skoog, B.; Hedman, B. Intrathecal Baclofen Dosage for Long-Term Treatment of Patients with Spasticity Due to Traumatic Spinal Cord Injuries or Multiple Sclerosis. Ann. Rehabil. Med.-ARM 2019, 43, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Lui, J.; Sarai, M.; Mills, P.B. Chemodenervation for treatment of limb spasticity following spinal cord injury: A systematic review. Spinal Cord 2015, 53, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Palazon-Garcia, R.; Alcobendas-Maestro, M.; Esclarin-de Ruz, A.; Benavente-Vaidepenas, A.M. Treatment of spasticity in spinal cord injury with botulinum toxin. J. Spinal Cord Med. 2019, 42, 281–287. [Google Scholar] [CrossRef]
- Benito, J.; Kumru, H.; Murillo, N.; Costa, U.; Medina, J.; Tormos, J.M.; Pascual-Leone, A.; Vidal, J. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top. Spinal Cord Inj. Rehabil. 2012, 18, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Ciampa, A.R.; de Prati, A.C.; Amelio, E.; Cavalieri, E.; Persichini, T.; Colasanti, M.; Musci, G.; Marlinghaus, E.; Suzuki, H.; Mariotto, S. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett. 2005, 579, 6839–6845. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Cavalieri, E.; Amelio, E.; Ciampa, A.R.; de Prati, A.C.; Marlinghaus, E.; Russo, S.; Suzuki, H. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 2005, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, X.; Wang, C.; Tang, T.; Chai, Y. The dose-effect relationship in extracorporeal shock wave therapy: The optimal parameter for extracorporeal shock wave therapy. J. Surg. Res. 2014, 186, 484–492. [Google Scholar] [CrossRef] [PubMed]
| Grade | Spasticity Scale | Deep Tendon Reflexes | |||
|---|---|---|---|---|---|
| Modified Tardieu Scale | Modified Ashworth Scale | Penn Spasm Frequency Scale | |||
| 0 | No resistance throughout the course of passive movement | No increase in tone | No spontaneous spasms | 1+ | Hyporeflexic response |
| 1 | Slight resistance through the course of passive movement, with no clear catch | Slight increase in tone, manifested by a catch and release or minimal resistance at end range of motion | No spontaneous spasms; spasms with rigorous sensory or motor stimulation | 2+ | Normal reflex response |
| 2 | Clear catch interrupting the passive movement, followed by a release | Marked increase in tone, manifested by a catch at mid-range of motion and resistance throughout remainder of motion | Occasional spontaneous spasms and easy induced spasms | 3+ | Brisk reflex response—no clonus elicited |
| 3 | Fatigable clonus (<10 s) while maintaining pressure | Considerable increase in muscle tone, with passive movement difficult | >1 but <10 spontaneous spams in an hour | 4+ | Brisk reflex response with associated clonus |
| 4 | Infatiguable clonus (>10 s) while maintaining pressure | Affect part rigid and fixed in place | >10 spontaneous spasms in an hour | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billington, Z.J.; Henke, A.M.; Gater, D.R., Jr. Spasticity Management after Spinal Cord Injury: The Here and Now. J. Pers. Med. 2022, 12, 808. https://doi.org/10.3390/jpm12050808
Billington ZJ, Henke AM, Gater DR Jr. Spasticity Management after Spinal Cord Injury: The Here and Now. Journal of Personalized Medicine. 2022; 12(5):808. https://doi.org/10.3390/jpm12050808
Chicago/Turabian StyleBillington, Zackery J., Austin M. Henke, and David R. Gater, Jr. 2022. "Spasticity Management after Spinal Cord Injury: The Here and Now" Journal of Personalized Medicine 12, no. 5: 808. https://doi.org/10.3390/jpm12050808
APA StyleBillington, Z. J., Henke, A. M., & Gater, D. R., Jr. (2022). Spasticity Management after Spinal Cord Injury: The Here and Now. Journal of Personalized Medicine, 12(5), 808. https://doi.org/10.3390/jpm12050808






