Diffusion of a Lifelog-Based Digital Healthcare Platform for Future Precision Medicine: Data Provision and Verification Study
Abstract
:1. Introduction
2. Materials and Methods
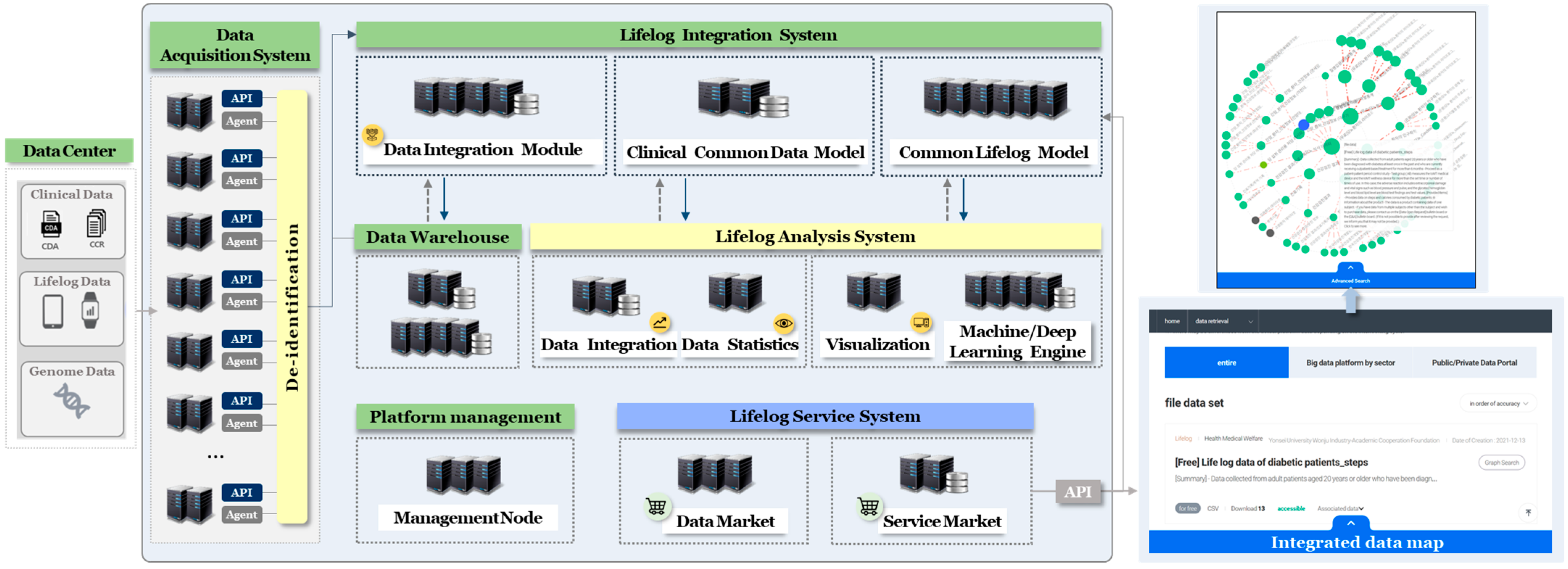
2.1. Data Centers
2.2. Data Acquisition System
2.3. Data Analysis System
2.4. Data Warehouse
2.5. Lifelog Service System
2.5.1. Data Provision
2.5.2. Service Provision
- Submission of research plan for data analysis;
- Check the security pledge and procedures in the control area;
- Approval from information protection manager of the platform;
- Utilization of user’s safety zone;
- Security verification for data export;
- Data export.
2.6. Policies
3. Results
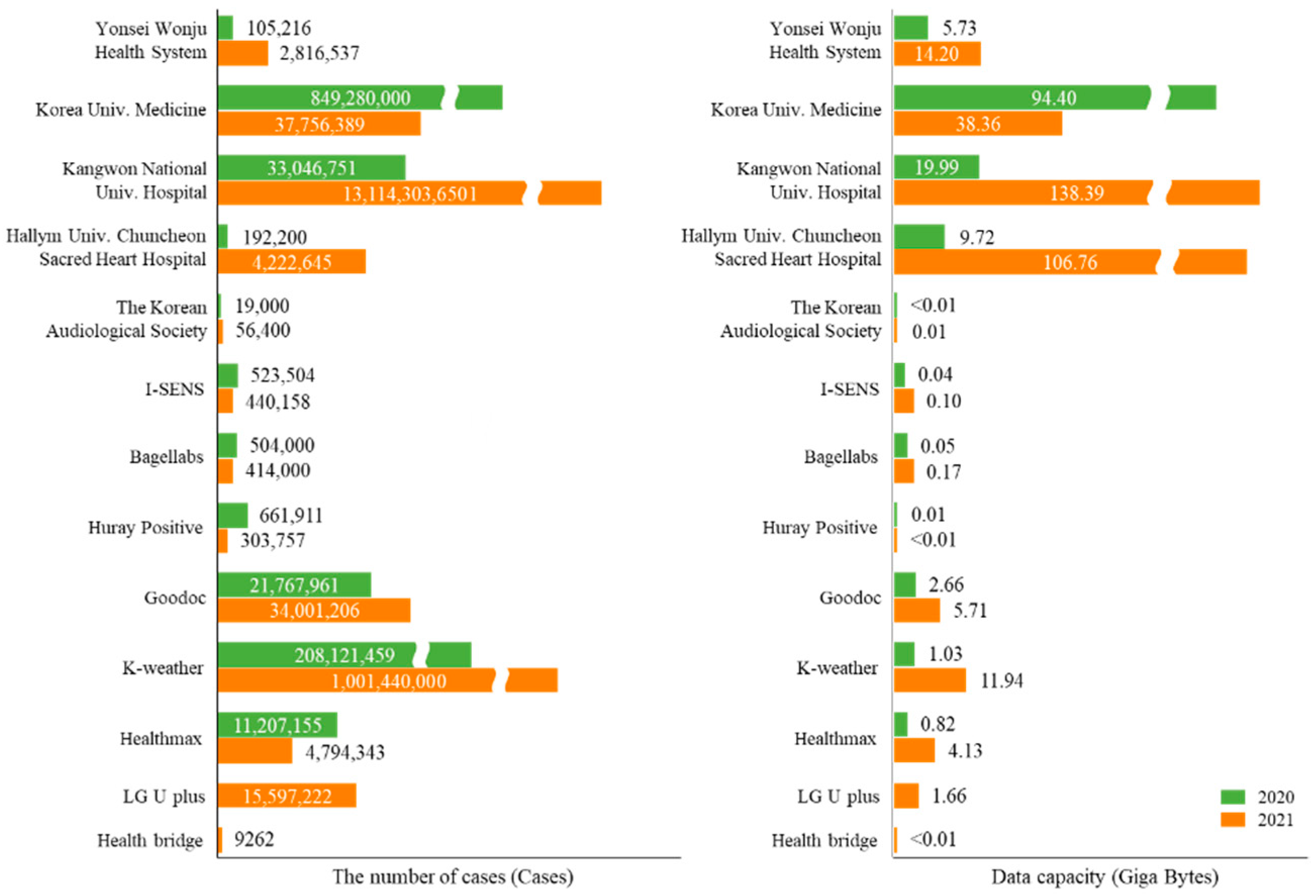
3.1. Data Production
3.2. Data Validation
3.3. Data Provision
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.H.; Urtnasan, E.; Hwang, S.W.; Lee, H.Y.; Lee, J.H.; Koh, S.B.; Youk, H. Concept and Proof of the Lifelog Bigdata Platform for Digital Healthcare and Precision Medicine on the Cloud. Yonsei Med. J. 2022, 63, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Le, N.K.; Nguyen, D.H.; Hoang, T.H.; Nguyen, T.A.; Truong, T.D.; Dinh, D.T.; Luong, Q.-A.; Vo-Ho, V.-K.; Nguyen, V.-T.; Tran, M.-T. Smart lifelog retrieval system with habit-based concepts and moment visualization. In Proceedings of the ACM Workshop on Lifelog Search Challenge, Ottawa, ON, Canada, 10 January 2019. [Google Scholar]
- Kim, J.; Lee, J.; Park, M. Identification of Smartwatch-Collected Lifelog Variables Affecting Body Mass Index in Middle-Aged People Using Regression Machine Learning Algorithms and Shapley Additive Explanations. Appl. Sci. 2022, 12, 3819. [Google Scholar] [CrossRef]
- Offermann, J.; Wilkowska, W.; Poli, A.; Spinsante, S.; Ziefle, M. Acceptance and Preferences of Using Ambient Sensor-Based Lifelogging Technologies in Home Environments. Sensors 2021, 21, 8297. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; Batool, M.; Kim, K. Sustainable Wearable System: Human Behavior Modeling for Life-Logging Activities Using K-Ary Tree Hashing Classifier. Sustainability 2020, 12, 10324. [Google Scholar] [CrossRef]
- Doherty, A.R.; Smeaton, A.F. Automatically Augmenting Lifelog Events Using Pervasively Generated Content from Millions of People. Sensors 2010, 10, 1423–1446. [Google Scholar] [CrossRef] [Green Version]
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]
- Rehman, A.; Haseeb, K.; Saba, T.; Lloret, J.; Tariq, U. Secured Big Data Analytics for Decision-Oriented Medical System Using Internet of Things. Electronics 2021, 10, 1273. [Google Scholar] [CrossRef]
- Hassan, M.; Awan, F.M.; Naz, A.; deAndrés-Galiana, E.J.; Alvarez, O.; Cernea, A.; Fernández-Brillet, L.; Fernández-Martínez, J.L.; Kloczkowski, A. Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: A Review. Int. J. Mol. Sci. 2022, 23, 4645. [Google Scholar] [CrossRef]
- Phan, A.-C.; Phan, T.-C.; Trieu, T.-N. A Systematic Approach to Healthcare Knowledge Management Systems in the Era of Big Data and Artificial Intelligence. Appl. Sci. 2022, 12, 4455. [Google Scholar] [CrossRef]
- Sodhro, A.H.; Zahid, N. AI-Enabled Framework for Fog Computing Driven E-Healthcare Applications. Sensors 2021, 21, 8039. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Wu, D.; Xu, Z.; Wang, H. The Role of Big Data in Aging and Older People’s Health Research: A Systematic Review and Ecological Framework. Sustainability 2021, 13, 11587. [Google Scholar] [CrossRef]
- Stefanicka-Wojtas, D.; Kurpas, D. eHealth and mHealth in Chronic Diseases—Identification of Barriers, Existing Solutions, and Promoters Based on a Survey of EU Stakeholders Involved in Regions4PerMed. J. Pers. Med. 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.L.; Curfman, G. Hypertension—A public health challenge of global proportions. JAMA 2018, 320, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Cho, S.M.J.; Lee, H.; Lee, H.H.; Baek, J.; Heo, J.E. Korea hypertension fact sheet 2020: Analysis of nationwide population-based data. Clin. Hypertens. 2021, 27, 8. [Google Scholar] [CrossRef]
- An, T.J.; Yoon, H.K. Prevalence and socioeconomic burden of chronic obstructive pulmonary disease. J. Korean Med. Assoc. 2018, 61, 533–538. [Google Scholar] [CrossRef]
- Halpin, H.A.; Morales-Suárez-Varela, M.M.; Martin-Moreno, J.M. Chronic disease prevention and the new public health. Public Health Rev. 2010, 32, 120–154. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Lee, S.Y.; Baik, S.Y.; Kim, J.H. MELLO: Medical lifelog ontology for data terms from self-tracking and lifelog devices. Int. J. Med. Inform. 2015, 84, 1099–1110. [Google Scholar] [CrossRef]
- Mezghani, E.; Exposito, E.; Drira, K.; Da Silveira, M.; Pruski, C. A semantic big data platform for integrating heterogeneous wearable data in healthcare. J. Med. Syst. 2015, 39, 185. [Google Scholar] [CrossRef]
- Suciu, G.; Sucium, V.; Martian, A.; Craciunescu, R.; Vulpe, A.; Marcu, I.; Halunga, S.; Fratu, O.L. Big data, internet of things and cloud convergence—An architecture for secure e-health applications. J. Med. Syst. 2015, 39, 141. [Google Scholar] [CrossRef]
- Manogaran, G.; Varatharajan, R.; Lopez, D.; Kumar, P.M.; Sundarasekar, R.; Thota, C. A new architecture of internet of things and big data ecosystem for secured smart healthcare monitoring and alerting system. Future Gener. Comput. Syst. 2018, 82, 375–387. [Google Scholar] [CrossRef]
- Lamb, J.J.; Stone, M.; D’Adamo, C.R.; Volkov, A.; Metti, D.; Aronica, L.; Minich, D.; Leary, M.; Class, M.; Carullo, M.; et al. Personalized Lifestyle Intervention and Functional Evaluation Health Outcomes Survey: Presentation of the LIFEHOUSE Study Using N-of-One Tent–Umbrella–Bucket Design. J. Pers. Med. 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Dragstedt, C.A. Personal health log: Guest editorial. JAMA 1956, 160, 1320. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Li, X.; Koelwyn, G.J.; Milne, S.; Leitao Filho, F.S.; Yang, C.X.; Hernández Cordero, A.I.; Yang, J.; Yang, C.W.T.; Shaipanich, T.; et al. Inhaled Corticosteroids Selectively Alter the Microbiome and Host Transcriptome in the Small Airways of Patients with Chronic Obstructive Pulmonary Disease. Biomedicines 2022, 10, 1110. [Google Scholar] [CrossRef]
- Masuda, Y.; Zimmermann, A.; Viswanathan, M.; Bass, M.; Nakamura, O.; Yamamoto, S. Adaptive enterprise architecture for the digital healthcare industry: A digital platform for drug development. Information 2021, 12, 67. [Google Scholar] [CrossRef]
- Satti, F.A.; Ali, T.; Hussain, J.; Khan, W.A.; Khattak, A.M.; Lee, S. Ubiquitous Health Profile (UHPr): A big data curation platform for supporting health data interoperability. Computing 2020, 102, 2409–2444. [Google Scholar] [CrossRef]
- Rossetto, L.; Baumgartner, M.; Gasser, R.; Heitz, L.; Wang, R.; Bernstein, A. Exploring Graph-querying approaches in LifeGraph. In Proceedings of the 4th Annual on Lifelog Search Challenge, Taipei, Taiwan, 21 August 2021. [Google Scholar]
- Yassein, M.B.; Hmeidi, I.; Al-Harbi, M.; Mrayan, L.; Mardini, W.; Khamayseh, Y. IoT-based healthcare systems: A survey. In Proceedings of the Second International Conference on Data Science, E-Learning and Information Systems, Dubai, United Arab Emirates, 2 December 2019. [Google Scholar]
- Hamm, J.; Stone, B.; Belkin, M.; Dennis, S. Automatic annotation of daily activity from smartphone-based multisensory streams. In Proceedings of the International Conference on Mobile Computing, Applications, and Services, Seattle, DC, USA, 11 October 2012. [Google Scholar]
- Xie, C.; Cai, H.; Yang, Y.; Jiang, L.; Yang, P. User profiling in elderly healthcare services in China: Scalper detection. IEEE J. Biomed. Health Inform. 2018, 22, 1796–1806. [Google Scholar] [CrossRef]
- Herold, R.; Beaver, K. The Practical Guide to HIPAA Privacy and Security Compliance, 2nd ed.; Auerbach Publications: Boca Raton, FL, USA, 2014. [Google Scholar]
- Office for Government Policy Coordination (OPC); Ministry of the Interior Safety (MOIS); Korea Communications Commission (KCC); Financial Services Commission (FSC). Guidelines for De-Identification of Personal Information. 30 June 2016. Available online: https://www.privacy.go.kr/cmm/fms/FileDown.do?atchFileId=FILE_000000000830764&fileSn=0 (accessed on 11 February 2022).
- Integrated Data Map. Available online: https://bigdata–map.kr (accessed on 25 March 2022).
- Data Transaction Based Composition. Available online: https://dataonair.or.kr/data-transaction-based-composition.pdf (accessed on 20 February 2022).
- Moghaddasi, H.; Ghaemi, M.M. A Comparative Study of Three Standards of Data Security in Health Systems. J. Health Biomed. Inform. 2015, 2, 184–194. [Google Scholar]
- Samy, G.N.; Ahmad, R.; Ismail, Z. Threats to health information security. In Proceedings of the 2009 Fifth International Conference on Information Assurance and Security, Xi’an, China, 18 August 2009. [Google Scholar]
- Beckers, K.; Heisel, M.; Côté, I.; Goeke, L.; Güler, S. Structured pattern-based security requirements elicitation for clouds. In Proceedings of the 2013 International Conference on Availability, Reliability and Security, Regensburg, Germany, 2 September 2013. [Google Scholar]
- Kim, D.W.; Kim, H.J.; Myeong, S.H. The Cloud System of Futuristic Vehicles and Security Policies. In Proceedings of the 2020 IEEE International Conference on Big Data and Smart Computing, Busan, Korea, 19 February 2020. [Google Scholar]
- Tep, K.S.; Martini, B.; Hunt, R.; Choo, K.K.R. A taxonomy of cloud attack consequences and mitigation strategies: The role of access control and privileged access management. In Proceedings of the 2015 IEEE International Conference on TrustCom, Helsinki, Finland, 20–22 August 2015. [Google Scholar]
- Verma, A.; Agarwal, G.; Gupta, A.K.; Sain, M. Novel Hybrid Intelligent Secure Cloud Internet of Things Based Disease Prediction and Diagnosis. Electronics 2021, 10, 3013. [Google Scholar] [CrossRef]
- Pulakanam, V. Costs and savings of Six Sigma programs: An empirical study. Qual. Manag. J. 2012, 19, 39–54. [Google Scholar] [CrossRef]
- Antony, J.; Palsuk, P.; Gupta, S.; Mishra, D.; Barach, P. Six Sigma in healthcare: A systematic review of the literature. Int. J. Qual. Reliab. Manag. 2018, 35, 1075–1092. [Google Scholar] [CrossRef]
- Raisinghani, M.S.; Ette, H.; Pierce, R.; Cannon, G.; Daripaly, P. Six Sigma: Concepts, tools, and applications. Ind. Manag. Data Syst. 2005, 105, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Sembiring, N.; Devany, J. Quality control of cutter case at PT. X with six sigma approach. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Prasser, F.; Kohlmayer, F. Putting Statistical Disclosure Control into Practice: The ARX Data Anonymization Tool. In Medical Data Privacy Handbook; Gkoulalas-Divanis, A., Loukides, G., Eds.; Springer: Cham, Switzerland, 2015; pp. 111–148. [Google Scholar]
- Kim, J.Y.; Yadav, D.; Ahn, S.V.; Koh, S.B.; Park, J.T.; Yoon, J.; Yoo, B.S.; Lee, S.H. A prospective study of total sleep duration and incident metabolic syndrome: The ARIRANG study. Sleep Med. 2015, 16, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.S.; Koh, S.B.; Kim, J.Y.; Yoon, J.H.; Sung, J.K.; Youn, Y.J.; Yoo, B.S.; Lee, S.H.; Yoon, J.; Eom, A.; et al. The association between serum adiponectin and carotid intima media thickness in community based cohort in Korea: The ARIRANG study. Mol. Cell. Toxicol. 2011, 7, 33–38. [Google Scholar] [CrossRef]
| Type | Field Name | Type | Description | Input |
|---|---|---|---|---|
| Header | X-CKAN-API-Key | string | Information retrieval with the authentication key | Authentication ID |
| Parameters | q | string | Inquiry by adding conditions for each column | “fields_name”:value |
| fq | list | Applying filters per the column | “fields_name”:value | |
| sort | string | Sorting the result | “sort”:”score desc, metadata_modified desc” | |
| rows | Int *a | The number of rows in the query result | The number of lists to be displayed | |
| start | int | The page in the result | The page number to be displayed | |
| include_private | bool | Whether to retrieve private datasets | “include_private”:true | |
| use_default_schema | bool | Use of the default schema | “use_default_schema”:true | |
| include_drafts | bool | retrieval of draft data | “include_drafts”:true | |
| Result | success | bool | Success or failure of API call | res[“success”] |
| result | Dict *b | Retrieved results | res[“result”] | |
| result.count | int | The number of data in the result | res[“result”][“count”] | |
| result.search_facets | dict | The number of retrieved information by conditions | res[“result”][“search_facets”] | |
| result.result | int | The item list in result | res[“result”][“result”] |
| Data Centers | Data Sources | 2020 | 2021 | ||
|---|---|---|---|---|---|
| Cases | Capacity (GB) | Cases | Capacity (GB) | ||
| Yonsei Wonju Health System | Metabolic syndrome’s lifelog | 53,210 | 4.50 | 67,695 | 7.76 |
| 12-lead ECG | 40,642 | 1.23 | 2,541,855 | 1.90 | |
| Cohort study | 11,364 | <0.01 | 82,635 | 0.01 | |
| Diabetic patient’s lifelog | - | - | 123,194 | 4.52 | |
| COPD patient’s lifelog | - | - | 358 | 0.04 | |
| Integration data | - | - | 800 | <0.01 | |
| Korea University Medicine | CDM data | 849,210,000 | 94.40 | 36,251,389 | 38.33 |
| inPHR data | 70,000 | <0.01 | - | - | |
| CDM extension data | - | - | 1,505,000 | 0.02 | |
| Kangwon National University Hospital | Lifelog data | 625,846 | 0.19 | 56,639,191 | 86.39 |
| Clinical information data | 6,683,156 | 2.00 | 2,619,241,352 | 0.24 | |
| Clinical support data | 9,179,470 | 2.80 | 7,765,402,405 | 17.98 | |
| Health insurance and other data | 16,513,279 | 5.00 | 1,483,360,512 | 27.58 | |
| Clinical and lifelog data of newcomers | 40,000 | <0.01 | 369,460 | <0.01 | |
| Nutritional images | 5000 | 10.00 | 25,000 | 0.07 | |
| Diabetic patient’s lifelog | - | - | 138,529 | <0.01 | |
| Newcomers’ data | - | - | 9,045,808 | 0.07 | |
| Visit and health checkup data | - | - | 511,632 | 0.05 | |
| Cohort’s clinical data | - | - | 1,179,569,762 | 6.00 | |
| Hallym University Chuncheon Sacred Heart Hospital | Smart health data in Kangwon | 45,600 | 2.31 | 1,390,391 | 17.93 |
| Healthy life data in Inje-Yangu | 68,500 | 3.89 | 1,598,230 | 28.73 | |
| Healthy life data in Seoul | 75,600 | 2.35 | 1,202,102 | 13.70 | |
| Chatbot data for dementia | 500 | 1.17 | 9277 | 8.75 | |
| Mild cognitive disorder | - | - | 320 | 0.04 | |
| Telemedicine services | - | - | 22,245 | 37.59 | |
| Dementia data | - | - | 80 | <0.01 | |
| The Korean Audiological Society | Auditory test data | 19,000 | <0.01 | 56,400 | 0.02 |
| Data Centers | Data Sources | 2020 | 2021 | ||
|---|---|---|---|---|---|
| Case | Capacity (GB) | Case | Capacity (GB) | ||
| Bagel labs | Morphotype data | 206,000 | 0.02 | 167,000 | 0.04 |
| Morphotype analysis data | 298,000 | 0.03 | 247,000 | 0.08 | |
| Huray Positive | Self-recorded data | 664,130 | 0.01 | 301,988 | <0.01 |
| Intervention data | 2781 | <0.01 | 1769 | <0.01 | |
| Goodoc | Medical service data | 6,562,939 | 1.37 | 11,642,068 | 2.10 |
| Registry service data | 8,170,880 | 0.64 | 10,722,939 | 1.73 | |
| Medical consulting data | 7,034,037 | 0.64 | 11,632,131 | 1.86 | |
| Insurance service data | 105 | <0.01 | 115 | <0.01 | |
| Vaccination | - | - | 3953 | 0.02 | |
| K-weather | Life-air data for house | 76,039,210 | 0.37 | 222,370,000 | 2.39 |
| Life-air data for school | 110,532,228 | 0.55 | 173,980,000 | 2.58 | |
| Life-air data for crowd facilities | 14,331,629 | 0.07 | 44,400,000 | 0.63 | |
| Health environment index | 432,960 | 0.01 | 1,050,000 | 0.05 | |
| Lifelog data of a vulnerable social group | 6,785,432 | 0.03 | 189,110,000 | 2.16 | |
| Clinical trials in Wonju | - | - | 370,530,000 | 4.11 | |
| I-SENS | Chronic disease analysis data | 523,504 | 0.05 | 440,158 | 0.10 |
| Healthmax | Metabolic syndrome’s data | 11,207,155 | 0.82 | 4,794,343 | 4.14 |
| LG U Plus * | Lifelog on communication | - | - | 15,597,222 | 1.66 |
| Health Bridge * | Lifelog under stress | - | - | 9262 | <0.01 |
| Evaluation Factors | 2020 | 2021 |
|---|---|---|
| The number of opportunities | 906,084,543 | 82,727,257,835 |
| The number of defects | 111,704 | 27,203,636 |
| DPO | 1.23 × 10−4 | 3.28 × 10−4 |
| DPMO | 123 | 329 |
| Defects ratio | 0.01% | 0.03% |
| Data consistency | 99.99% | 99.70 |
| Six-Sigma | 5.17 | 4.91 |
| Parameters | Description | Records at Risk(%) | Highest Risk(%) | Success Risk(%) | De-Identification Method | |||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||
| WNJU_BLOD_ID | Patient ID | - | - | - | - | - | - | Encryption |
| INDVDL_FLNM | Patient name | - | - | - | - | - | - | Remove |
| BRDT | Birthday | 100 | 0 | 100 | 0.51 | 100 | 0.51 | Masking |
| ADDR | Address | 100 | 0 | 100 | 4 | 100 | 4 | Masking |
| MBL_NO | Mobile | 100 | 0 | 100 | 1.58 | 100 | 1.02 | Masking |
| AGE | Age | 15.30 | 0 | 100 | 5.23 | 16.83 | 3.06 | Interval |
| TC | Total cholesterol | 94.89 | 0 | 100 | 5.26 | 53.57 | 1.53 | Interval |
| ALBMN | Albumin | 100 | 0 | 100 | 5.32 | 92.34 | 1.57 | Interval |
| AST | AST | 17.85 | 0 | 100 | <0.1 | 19.89 | <0.1 | Interval |
| ALT | ALT | 40 | 0 | 100 | 0.22 | 27.04 | <0.1 | Interval |
| GGTP | γ-GTP | 100 | 0 | 100 | 0.51 | 97.95 | 0.51 | Interval |
| LDL | LDL | 97.44 | 0 | 100 | 0.51 | 53.06 | 0.51 | Interval |
| HDL | HDL | 30.61 | 0 | 100 | 20 | 23.97 | 2.04 | Interval |
| Cr | Creatin | 100 | 0 | 100 | 8.33 | 97.96 | 2.55 | Interval |
| BUN | Blood urea nitrogen | 86.73 | 0 | 100 | 16.66 | 53.06 | 1.02 | Interval |
| WBC | White blood cell count | 100 | 1.53 | 100 | 33.33 | 98.46 | 4.08 | Interval |
| PLT | Platelet | 100 | 0 | 100 | 20 | 69.38 | 1.53 | Interval |
| API Name | Description | URL | Example of the Output |
|---|---|---|---|
| ckan.logic.action.create.package_create (POST) | Creation of packages | http://platform domain:8080/api/action/package_create | { "help": "http://API url", "success": true, {"author": , … "creator_user_id":"2f53c018-…-8f9d-1875", "isopen": false, "license_id": "version": null, "extras": [ { "key": "paid_gb", "value": "1" } ], … } |
| ckan.logic.action.get.package_search (GET) | Searching for data list and information | http://platform domain:8080/api/action/package_search | { "help": "http://API url", "success": {"author": "yj", … "owner_org": "19f75d75- … -9df8-7231bf67", "period": "yearly", "prodCode": "LI03090002", "species_cd": "LI03200009", "state": "active", … } |
| ckan.logic.action.get.package_show (GET) | Information of the specific package | http://platform domain:8080/api/action/package_show | { "help": "http://API url", "success": {"author": "yj", … "tags": [{ "display_name": "tag_name", "id": "bf71a7ce-a6bf-443d-9d17-3ad2c9d7b3", "name": "tag", "state": "active", "vocabulary_id": null … } |
| ckan.logic.action.create.package_patch (POST) | Updating the information of the specific package | http://platform domain:8080/api/action/package_patch | { "help": "http://API url", "success": {"author": "yj", … "prodCode": "LI032000090002", "species_cd": "LI03200009", "state": "active", "title": "update the title", "type": "dataset", … } |
| ckan.logic.action.delete.package_delete (POST) | Deletion of the package | http://platform domain:8080/api/action/package_delete | { "help": "http://API url", "success": true, “result”:null } |
| ckan.logic.action.create.resource_create (POST) | Registration of package | http://platform domain:8080/api/action/resource_create | {"help":"url": http://API url:8080/dataset/ 88b37228-b57c-46b5-9eaf-e4d256985a4b/ resource/fe49dfba-3f20-43fc-…4762618 /download/iris.csv … } |
| ckan.logic.action.patch.resource_patch (POST) | Updating meta information of attached files in the package | http://platform domain:8080/api/action/resource_patch | { "help": "http://API url", "success": true, {"author": , … "mimetype": "text/csv", "mimetype_inner": null, "name": "resource name", "package_id":"88b37228- ... -e4d256985a4b", … } |
| ckan.logic.action.delete.resource_delete (POST) | Deletion of the file in the package | http://platform domain:8080/api/action/resource_create | { "help": "http://API url", "success": true, “result”:null } |
| ckan.logic.action.get.statistics_list (GET) | Retrieval of the statistic by organizations and resources | http://platform domain:8080/api/action/statistics_list | { "help": "http://API url", "success": true, {"author": , … "results": [{ "title": "YWMC", "resoures_count": 136, "package_count": 58, "name": "yonseuniv", "free": 0, "pay": 58, "format": { "CSV": 99, "ZIP": 37} … } |
| ckan.logic.action.create.schema_create (POST) | Registration of the schema of the package | http://platform domain:8080/api/action/schema_create | { "help": "http://API url?name=schema_create","success": true, "result": { "success": "data insert success" } } |
| ckan.logic.action.get.schema_search (GET) | Retrieval of the schema of the package | http://platform domain:8080/api/action/schema_search | { "help": "http://API url", "success": true, {"author": … "result": { "prodCode": "LI091050111113", "columns": [{"seq": 1,"name": "DTM_AQ ", "data_type": "text", "max_length": 100, … } |
| ckan.logic.action.get.schema_delete (POST) | Deletion of the schema of the package | http://platform domain:8080/api/action/schema_delete | { "help": "http://API url?name=schema_delete","success": true, "result": { "success": "data delete success" } } |
| ckan.logic.action.create.species_create (POST) | Registration of data items | http://platform domain:8080/api/action/species_create | { "help": "http://API url?name=schema_create","success": true, "result": { "success": "LI012000025" } } |
| ckan.logic.action.get.species_list (GET) | Retrieval of data items | http://platform domain:8080/api/action/species_list | { "help": "http://API url", "success": true, {"author": … "result": {"count": 143,"result": [{"species_cd": "LI10200001", "prodcode": "LI10200010011", "metadata_modified":"2021-07-28T06:02:34.015405" … } |
| ckan.logic.action.patch.species_patch (POST) | Updating the information of the data item | http://platform domain:8080/api/action/species_patch | { "help": "http://API url?name=schema_patch", "success": true, "result": { "success": "data patch success" } } |
| ckan.logic.action.delete.species_delete (POST) | Deletion of the data item | http://platform domain:8080/api/action/species_delete | { "help": "http://API url?name=speices_delete","success": true, "result": { "success": "delete success" } } |
| ckan.logic.action.get.organization_list (GET) | Retrieval of the organization list | http://platform domain:8080/api/action/organization_list | { "help": "http://API url", "success": true, {"author":, … "result": ["ywmc", "hallymuniv"…, “koreauniv”] } |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Lee, J.; Hwang, S.; Kim, Y.; Lee, Y.; Urtnasan, E.; Koh, S.B.; Youk, H. Diffusion of a Lifelog-Based Digital Healthcare Platform for Future Precision Medicine: Data Provision and Verification Study. J. Pers. Med. 2022, 12, 803. https://doi.org/10.3390/jpm12050803
Lee K, Lee J, Hwang S, Kim Y, Lee Y, Urtnasan E, Koh SB, Youk H. Diffusion of a Lifelog-Based Digital Healthcare Platform for Future Precision Medicine: Data Provision and Verification Study. Journal of Personalized Medicine. 2022; 12(5):803. https://doi.org/10.3390/jpm12050803
Chicago/Turabian StyleLee, Kyuhee, Jinhyong Lee, Sangwon Hwang, Youngtae Kim, Yeongjae Lee, Erdenebayar Urtnasan, Sang Baek Koh, and Hyun Youk. 2022. "Diffusion of a Lifelog-Based Digital Healthcare Platform for Future Precision Medicine: Data Provision and Verification Study" Journal of Personalized Medicine 12, no. 5: 803. https://doi.org/10.3390/jpm12050803
APA StyleLee, K., Lee, J., Hwang, S., Kim, Y., Lee, Y., Urtnasan, E., Koh, S. B., & Youk, H. (2022). Diffusion of a Lifelog-Based Digital Healthcare Platform for Future Precision Medicine: Data Provision and Verification Study. Journal of Personalized Medicine, 12(5), 803. https://doi.org/10.3390/jpm12050803








