Asthma-COPD Overlap Syndrome: Recent Insights and Unanswered Questions
Abstract
:1. Introduction
2. Definition
3. ACO Pathogenesis
3.1. The Overlapping Mechanisms Hypothesis
3.2. Risk Factors for ACO
3.3. ACO Pathology
3.4. Genetics
3.5. Clinical Manifestations—Diagnosis—Differential Diagnosis
3.6. Medical History
3.7. Laboratory Tests
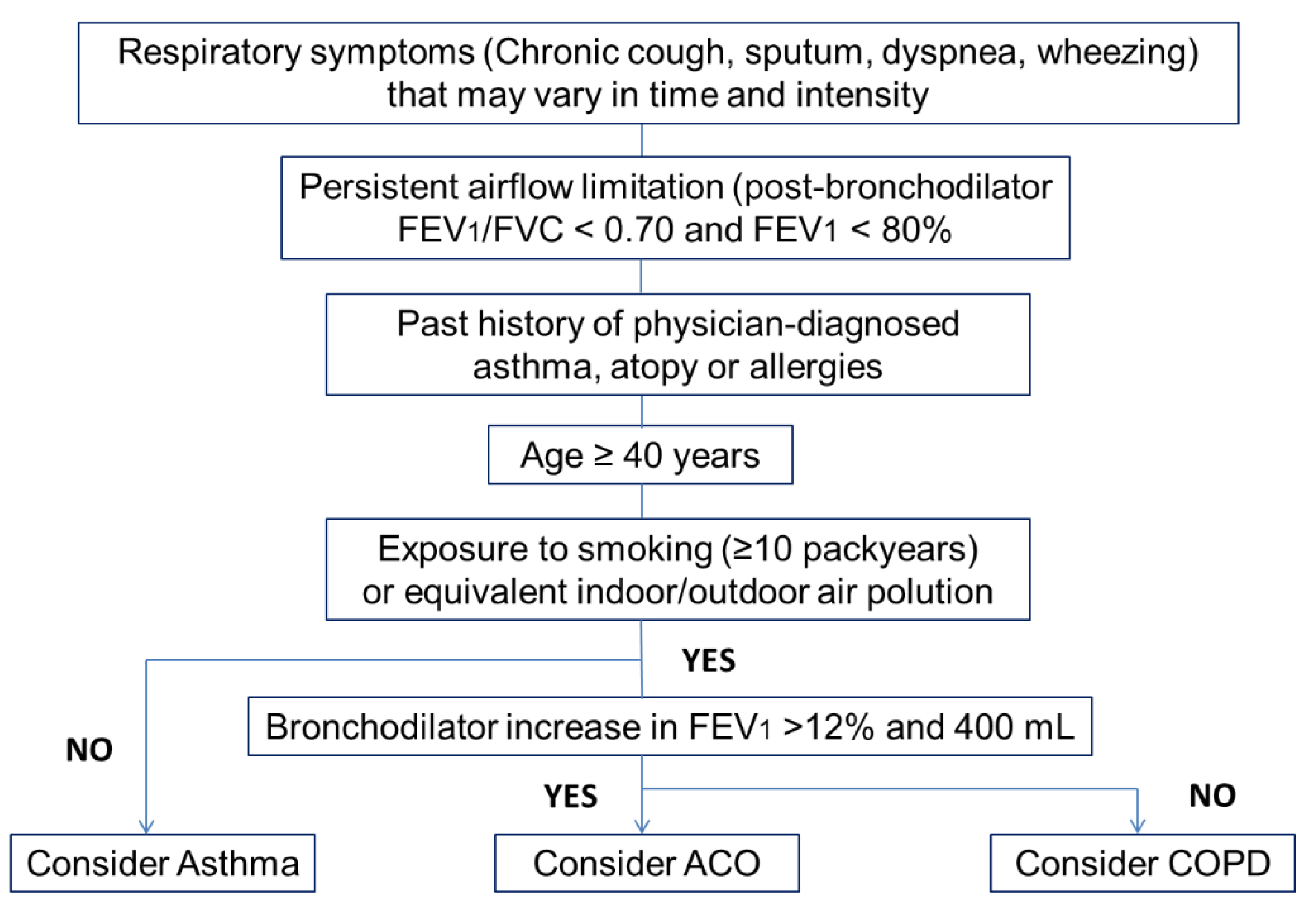
3.8. Diagnosis
- Age ≥ 40 years.
- Persistent respiratory symptoms (chronic cough, sputum, dyspnea, wheezing). These symptoms may vary.
- Airflow limitation not fully reversible, but with historical variability: Postbronchodilator FEV1/FVC < 0.7 or lower limit of normal and bronchodilator increase in FEV1 > 12% and 400 mL.
- Past history of doctor-diagnosed asthma.
- History of atopy or allergies.
- Exposure to a risk factor (e.g., tobacco smoking ≥10 pack-years or equivalent indoor/outdoor air pollution).
Differential Diagnosis
- Bronchiectasis is suspected on the basis of symptoms like chronic productive cough, mucopurulent sputum, recurrent chest infections, and, less frequently, hemoptysis. The diagnosis is usually established based on characteristic findings of bronchial wall thickening and luminal dilatation seen on chest HRCT scans.
- Bronchiolitis obliterans is characterized by concentric fibrotic narrowing of the bronchiolar lumen. It is commonly seen after a viral illness, an inhalation injury, transplantation (eg., bone marrow, lung), or in the context of rheumatic disease. Symptoms include progressive onset of cough and dyspnea, and hypoxemia at rest or with exercise. Findings on chest HRCT scans can include centrilobular bronchial wall thickening, bronchiolar dilation, tree-in-bud nodularity, and a mosaic pattern of attenuation of lung tissue density.
- Central airway obstruction is attributed to a number of benign and malignant processes with slowly progressive dyspnea on exertion, followed by dyspnea under minimal activity. A flow-volume loop, which can be insensitive, and CT with 3-dimensional reconstruction may be helpful, but the gold standard for diagnosis is direct visualization.
- Diffuse panbronchiolitis is characterized by bronchiolitis and chronic sinusitis and it is highly prevalent in Asiatic populations. A prominent clinical feature is cough productive of copious amounts of sputum.
3.9. Challenges in ACO Diagnosis
4. Treatment—Prognosis
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Christenson, S.A.; Steiling, K.; van den Berge, M.; Hijazi, K.; Hiemstra, P.S.; Postma, D.S.; Lenburg, M.E.; Spira, A.; Woodruff, P.G. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 191, 758–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekov, E.; Nuñez, A.; Sin, D.D.; Ichinose, M.; Rhee, C.K.; Maselli, D.J.; Coté, A.; Suppli Ulrik, C.; Maltais, F.; Anzueto, A.; et al. Update on Asthma-COPD Overlap (ACO): A Narrative Review. Int. J. Chron. Obstr. Pulmon. Dis. 2021, 16, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Simpson, J.L. The overlap syndrome of asthma and COPD: What are its features and how important is it? Thorax 2009, 64, 728–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulet, L.P.; Boulay, M.; Dérival, J.L.; Milot, J.; Lepage, J.; Bilodeau, L.; Maltais, F. Asthma-COPD Overlap Phenotypes and Smoking:Comparative features of asthma in smoking or non-smoking patients with an incomplete reversibility of airway obstruction. COPD 2018, 15, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Novella, L.; Soler, C.; Nieto, M.L.; Esteban, V.; Sánchez-Toril, F.; Miravitlles, M. Clinical Characteristics and Risk of Exacerbations Associated With Different Diagnostic Criteria of Asthma-COPD Overlap. Arch. Bronconeumol. (Engl. Ed.) 2020, 56, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Tommola, M.; Ilmarinen, P.; Tuomisto, L.E.; Lehtimäki, L.; Haanpää, J.; Niemelä, O.; Kankaanranta, H. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur. Respir. J. 2017, 49, 1602383. [Google Scholar] [CrossRef] [Green Version]
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2020. Available online: http://www.goldcopd.org (accessed on 8 April 2022).
- Maselli, D.J.; Hanania, N.A. Management of asthma COPD overlap. Ann. Allergy Asthma Immunol. 2019, 123, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, P.G.; van den Berge, M.; Boucher, R.C.; Brightling, C.; Burchard, E.G.; Christenson, S.A.; Han, M.K.; Holtzman, M.J.; Kraft, M.; Lynch, D.A.; et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am. J. Respir. Crit. Care Med. 2017, 196, 375–381. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2014. Available online: http://www.goldcopd.org (accessed on 8 April 2022).
- Soler-Cataluña, J.J.; Cosío, B.; Izquierdo, J.L.; López-Campos, J.L.; Marín, J.M.; Agüero, R.; Baloira, A.; Carrizo, S.; Esteban, C.; Galdiz, J.B.; et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch. Bronconeumol. 2012, 48, 331–337. [Google Scholar] [CrossRef]
- Koblizek, V.; Chlumsky, J.; Zindr, V.; Neumannova, K.; Zatloukal, J.; Zak, J.; Sedlak, V.; Kocianova, J.; Hejduk, K.; Pracharova, S. Chronic Obstructive Pulmonary Disease: Official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2013, 157, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Cosio, B.G.; Soriano, J.B.; López-Campos, J.L.; Calle-Rubio, M.; Soler-Cataluna, J.J.; de-Torres, J.P.; Marín, J.M.; Martínez-Gonzalez, C.; de Lucas, P.; Mir, I.; et al. Defining the Asthma-COPD Overlap Syndrome in a COPD Cohort. Chest 2016, 149, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Miravitlles, M.; Mannino, D.M.; Soriano, J.B.; Price, D.; Celli, B.R.; Leung, J.M.; Nakano, Y.; Park, H.Y.; Wark, P.A.; et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016, 48, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, D.; Corhay, J.L.; Derom, E.; Louis, R.; Marchand, E.; Michils, A.; Ninane, V.; Peché, R.; Pilette, C.; Vincken, W.; et al. A Belgian survey on the diagnosis of asthma-COPD overlap syndrome. Int. J. Chron. Obstr. Pulmon. Dis. 2017, 12, 601–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miravitlles, M.; Alvarez-Gutierrez, F.J.; Calle, M.; Casanova, C.; Cosio, B.G.; López-Viña, A.; Pérez de Llano, L.; Quirce, S.; Roman-Rodríguez, M.; Soler-Cataluña, J.J.; et al. Algorithm for identification of asthma-COPD overlap: Consensus between the Spanish COPD and asthma guidelines. Eur. Respir. J. 2017, 49, 1700068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, C.; Sin, D.D. Asthma-COPD Overlap: What Are the Important Questions? Chest 2021, 161, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Bonten, T.N.; Kasteleyn, M.J.; de Mutsert, R.; Hiemstra, P.S.; Rosendaal, F.R.; Chavannes, N.H.; Slats, A.M.; Taube, C. Defining asthma-COPD overlap syndrome: A population-based study. Eur. Respir. J. 2017, 49, 1602008. [Google Scholar] [CrossRef] [PubMed]
- Barrecheguren, M.; Pinto, L.; Mostafavi-Pour-Manshadi, S.M.; Tan, W.C.; Li, P.Z.; Aaron, S.D.; Benedetti, A.; Chapman, K.R.; Walker, B.; Fitzgerald, J.M.; et al. Identification and definition of asthma-COPD overlap: The CanCOLD study. Respirology 2020, 25, 836–849. [Google Scholar] [CrossRef]
- Diagnosis of Diseases of Chronic Airflow Limitation. Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS). A Joint Project of GINA and GOLD. 2015. Available online: www.ginasthma.org (accessed on 8 April 2022).
- Uchida, A.; Sakaue, K.; Inoue, H. Epidemiology of asthma-chronic obstructive pulmonary disease overlap (ACO). Allergol. Int. 2018, 67, 165–171. [Google Scholar] [CrossRef]
- Jo, Y.S.; Hwang, Y.I.; Yoo, K.H.; Kim, T.H.; Lee, M.G.; Lee, S.H.; Shin, K.C.; In, K.H.; Yoon, H.K.; Rhee, C.K. Effect of Inhaled Corticosteroids on Exacerbation of Asthma-COPD Overlap According to Different Diagnostic Criteria. J. Allergy Clin. Immunol. Pract. 2020, 8, 1625–1633.e6. [Google Scholar] [CrossRef]
- Orie, N.; Sluiter, H.; Charles, I.; Thomas, C. (Eds.) Bronchitis: An International Symposium; Springfield: Assen, The Netherlands, 1961. [Google Scholar]
- Fletcher, C.M. Chronic bronchitis. Its prevalence, nature, and pathogenesis. Am. Rev. Respir. Dis. 1959, 80, 483–494. [Google Scholar] [CrossRef]
- Bateman, E.D.; Reddel, H.K.; van Zyl-Smit, R.N.; Agusti, A. The asthma-COPD overlap syndrome: Towards a revised taxonomy of chronic airways diseases? Lancet Respir. Med. 2015, 3, 719–728. [Google Scholar] [CrossRef]
- Martinez, F.D. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc. Am. Thorac. Soc. 2009, 6, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocks, J.; Sonnappa, S. Early life influences on the development of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2013, 7, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcon, A.; Locatelli, F.; Dharmage, S.C.; Svanes, C.; Heinrich, J.; Leynaert, B.; Burney, P.; Corsico, A.; Caliskan, G.; Calciano, L.; et al. The coexistence of asthma and COPD: Risk factors, clinical history and lung function trajectories. Eur. Respir. J. 2021, 58, 2004656. [Google Scholar] [CrossRef]
- Tonga, K.O.; Chapman, D.G.; Farah, C.S.; Oliver, B.G.; Zimmermann, S.C.; Milne, S.; Sanai, F.; Jetmalani, K.; Berend, N.; Thamrin, C.; et al. Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirology 2020, 25, 613–619. [Google Scholar] [CrossRef]
- Hikichi, M.; Hashimoto, S.; Gon, Y. Asthma and COPD overlap pathophysiology of ACO. Allergol. Int. 2018, 67, 179–186. [Google Scholar] [CrossRef]
- Pascual, R.M.; Peters, S.P. The irreversible component of persistent asthma. J. Allergy Clin. Immunol. 2009, 124, 883–890. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Zhu, J.; Larsen, K.; Simatovic, J.; Feldman, L.; Ryckman, K.; Gershon, A.; Lougheed, M.D.; Licskai, C.; Chen, H.; et al. Progression from Asthma to Chronic Obstructive Pulmonary Disease. Is Air Pollution a Risk Factor? Am. J. Respir. Crit. Care Med. 2016, 194, 429–438. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Backer, V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur. Respir. J. 1999, 14, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Vonk, J.M.; Jongepier, H.; Panhuysen, C.I.; Schouten, J.P.; Bleecker, E.R.; Postma, D.S. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003, 58, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Kurashima, K.; Takaku, Y.; Ohta, C.; Takayanagi, N.; Yanagisawa, T.; Kanauchi, T.; Takahashi, O. Smoking history and emphysema in asthma-COPD overlap. Int. J. Chron. Obstr. Pulmon. Dis. 2017, 12, 3523–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.L.; Palmer, L.J.; Kicic, E.; Maxwell, P.S.; Lagan, S.E.; Ryan, G.F.; Musk, A.W. Decline in lung function in the Busselton Health Study: The effects of asthma and cigarette smoking. Am. J. Respir. Crit. Care Med. 2005, 171, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, S.C.; Chinchilli, V.M.; Rollings, N.J.; Boushey, H.A.; Cherniack, R.; Craig, T.J.; Deykin, A.; DiMango, E.; Fish, J.E.; Ford, J.G.; et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Connett, J.E.; Murray, R.P. Smoking and lung function of Lung Health Study participants after 11 years. Am. J. Respir. Crit. Care Med. 2002, 166, 675–679. [Google Scholar] [CrossRef]
- Thomson, N.C.; Chaudhuri, R.; Livingston, E. Asthma and cigarette smoking. Eur. Respir. J. 2004, 24, 822–833. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Altose, M.D.; Connett, J.E.; Kanner, R.E.; Lee, W.W.; Wise, R.A. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am. J. Respir. Crit. Care Med. 1996, 153, 1802–1811. [Google Scholar] [CrossRef]
- Laucho-Contreras, M.; de Oca, M.M.; Owen, C.A. Asthma COPD Overlap Syndrome: An Approach to A Real -World Endotype in Obstructive Lung Disease? Curr. Pharm. Des. 2016, 22, 6273–6282. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Savic, S.; Siebeneichler, A.; Strobel, W.; Jones, P.W.; Tamm, M.; Stolz, D. A pilot study to test the feasibility of histological characterisation of asthma-COPD overlap. Eur. Respir. J. 2019, 53, 183. [Google Scholar] [CrossRef]
- Pérez-de-Llano, L.; Cosio, B.G. Asthma-COPD overlap is not a homogeneous disorder: Further supporting data. Respir. Res. 2017, 18, 183. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yang, T.; He, R.; Li, A.; Dang, W.; Liu, X.; Chen, M. The Value of Inflammatory Biomarkers in Differentiating Asthma-COPD Overlap from COPD. Int. J. Chron. Obstr. Pulmon. Dis. 2020, 15, 3025–3037. [Google Scholar] [CrossRef]
- Shirai, T.; Hirai, K.; Gon, Y.; Maruoka, S.; Mizumura, K.; Hikichi, M.; Holweg, C.; Itoh, K.; Inoue, H.; Hashimoto, S. Combined Assessment of Serum Periostin and YKL-40 May Identify Asthma-COPD Overlap. J. Allergy Clin. Immunol. Pract. 2019, 7, 134–145.e1. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Vestbo, J.; Agustí, A.; Anderson, G.P.; Bansal, A.T.; Beasley, R.; Bel, E.H.; Janson, C.; Make, B.; Pavord, I.D.; et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur. Respir. J. 2021, 58, 2003927. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.K.; Vasilescu, D.M.; Booth, S.; Hsieh, A.; Katsamenis, O.L.; Fishbane, N.; Elliott, W.M.; Kirby, M.; Lackie, P.; Sinclair, I.; et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: A cross-sectional study. Lancet Respir. Med. 2018, 6, 591–602. [Google Scholar] [CrossRef] [Green Version]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Usmani, O.S.; Han, M.K.; Kaminsky, D.A.; Hogg, J.; Hjoberg, J.; Patel, N.; Hardin, M.; Keen, C.; Rennard, S.; Blé, F.X.; et al. Seven Pillars of Small Airways Disease in Asthma and COPD: Supporting Opportunities for Novel Therapies. Chest 2021, 160, 114–134. [Google Scholar] [CrossRef]
- Hartley, R.A.; Barker, B.L.; Newby, C.; Pakkal, M.; Baldi, S.; Kajekar, R.; Kay, R.; Laurencin, M.; Marshall, R.P.; Sousa, A.R.; et al. Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: A single-center study. J. Allergy Clin. Immunol. 2016, 137, 1413–1422.e12. [Google Scholar] [CrossRef] [Green Version]
- Karayama, M.; Inui, N.; Yasui, H.; Kono, M.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; et al. Physiological and morphological differences of airways between COPD and asthma-COPD overlap. Sci. Rep. 2019, 9, 7818. [Google Scholar] [CrossRef]
- Postma, D.S.; Kerkhof, M.; Boezen, H.M.; Koppelman, G.H. Asthma and chronic obstructive pulmonary disease: Common genes, common environments? Am. J. Respir. Crit. Care Med. 2011, 183, 1588–1594. [Google Scholar] [CrossRef]
- Postma, D.S.; Weiss, S.T.; van den Berge, M.; Kerstjens, H.A.; Koppelman, G.H. Revisiting the Dutch hypothesis. J. Allergy Clin. Immunol. 2015, 136, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.; Swaroop, S.; Kant, S.; Banerjee, M. Signatures for chronic obstructive pulmonary disease (COPD) and asthma: A comparative genetic analysis. Br. J. Biomed. Sci. 2021, 78, 177–183. [Google Scholar] [CrossRef]
- Hardin, M.; Cho, M.; McDonald, M.L.; Beaty, T.; Ramsdell, J.; Bhatt, S.; van Beek, E.J.; Make, B.J.; Crapo, J.D.; Silverman, E.K.; et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur. Respir. J. 2014, 44, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, N.N.; Paré, P.D.; Rafaels, N.; Sin, D.D.; Sandford, A.; Daley, D.; Vergara, C.; Huang, L.; Elliott, W.M.; Pascoe, C.D.; et al. Genome-Wide Association Study Identification of Novel Loci Associated with Airway Responsiveness in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2015, 53, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeidat, M.; Faiz, A.; Li, X.; van den Berge, M.; Hansel, N.N.; Joubert, P.; Hao, K.; Brandsma, C.A.; Rafaels, N.; Mathias, R.; et al. The pharmacogenomics of inhaled corticosteroids and lung function decline in COPD. Eur. Respir. J. 2019, 54, 1900521. [Google Scholar] [CrossRef] [PubMed]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, B.D.; de Jong, K.; Lamontagne, M.; Bossé, Y.; Shrine, N.; Artigas, M.S.; Wain, L.V.; Hall, I.P.; Jackson, V.E.; Wyss, A.B.; et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat. Genet. 2017, 49, 426–432. [Google Scholar] [CrossRef]
- Tho, N.V.; Park, H.Y.; Nakano, Y. Asthma-COPD overlap syndrome (ACOS): A diagnostic challenge. Respirology 2016, 21, 410–418. [Google Scholar] [CrossRef]
- Tashkin, D.P. Is it asthma, COPD, or something in between, and does it matter? Respir. Care 2012, 57, 1354–1356. [Google Scholar] [CrossRef]
- Pascoe, S.J.; Wu, W.; Collison, K.A.; Nelsen, L.M.; Wurst, K.E.; Lee, L.A. Use of clinical characteristics to predict spirometric classification of obstructive lung disease. Int. J. Chron. Obstr. Pulmon. Dis. 2018, 13, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Mirabelli, M.C.; Beavers, S.F.; Chatterjee, A.B. Active asthma and the prevalence of physician-diagnosed COPD. Lung 2014, 192, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Celli, B.; Decramer, M.; Liu, D.; Burkhart, D.; Cassino, C.; Kesten, S. Bronchodilator responsiveness in patients with COPD. Eur. Respir. J. 2008, 31, 742–750. [Google Scholar] [CrossRef]
- Bakakos, A.; Vogli, S.; Dimakou, K.; Hillas, G. Asthma with Fixed Airflow Obstruction: From Fixed to Personalized Approach. J. Pers Med. 2022, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Bujarski, S.; Parulekar, A.D.; Sharafkhaneh, A.; Hanania, N.A. The asthma COPD overlap syndrome (ACOS). Curr. Allergy Asthma Rep. 2015, 15, 509. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.M.; Higgins, I.; Gibson, P.G. Managing older patients with coexistent asthma and chronic obstructive pulmonary disease: Diagnostic and therapeutic challenges. Drugs Aging 2013, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hillas, G.; Papaporfyriou, A.; Dimakou, K.; Papaioannou, A.I. Pharmacological treatment of stable COPD: Need for a simplified approach. Postgrad. Med. 2020, 132, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Hanania, N.A.; Chipps, B.E.; Griffin, N.M.; Yoo, B.; Iqbal, A.; Casale, T.B. Omalizumab effectiveness in asthma-COPD overlap: Post hoc analysis of PROSPERO. J. Allergy Clin. Immunol. 2019, 143, 1629–1633.e2. [Google Scholar] [CrossRef] [Green Version]
- Kupryś-Lipińska, I.; Pałczyński, C.; Molinska, J.; Kuna, P. Omalizumab therapy in a patient with severe asthma and co-existing chronic obstructive pulmonary disease. Postepy Dermatol. Alergol. 2019, 36, 239–241. [Google Scholar] [CrossRef]
- Maltby, S.; Gibson, P.G.; Powell, H.; McDonald, V.M. Omalizumab Treatment Response in a Population With Severe Allergic Asthma and Overlapping COPD. Chest 2017, 151, 78–89. [Google Scholar] [CrossRef]
- Tat, T.S.; Cilli, A. Omalizumab treatment in asthma-COPD overlap syndrome. J. Asthma 2016, 53, 1048–1050. [Google Scholar] [CrossRef]
- Yalcin, A.D.; Celik, B.; Yalcin, A.N. Omalizumab (anti-IgE) therapy in the asthma-COPD overlap syndrome (ACOS) and its effects on circulating cytokine levels. Immunopharmacol. Immunotoxicol. 2016, 38, 253–256. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Chapman, K.R.; Bafadhel, M.; Sciurba, F.C.; Bradford, E.S.; Schweiker Harris, S.; Mayer, B.; Rubin, D.B.; Yancey, S.W.; Paggiaro, P. Mepolizumab for Eosinophil-Associated COPD: Analysis of METREX and METREO. Int. J. Chron. Obstr. Pulmon. Dis. 2021, 16, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Asthma. GINA Workshop Report, Global Strategy for Asthma Management and Prevention. Revised. 2021. Available online: http://www.ginasthma.org (accessed on 8 April 2022).
- Barrecheguren, M.; Esquinas, C.; Miravitlles, M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): Opportunities and challenges. Curr. Opin. Pulm. Med. 2015, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Hardin, M.; Silverman, E.K.; Barr, R.G.; Hansel, N.N.; Schroeder, J.D.; Make, B.J.; Crapo, J.D.; Hersh, C.P. The clinical features of the overlap between COPD and asthma. Respir. Res. 2011, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, P.; Çolak, Y.; Ingebrigtsen, T.S.; Vestbo, J.; Marott, J.L. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: A prospective population-based analysis. Lancet Respir. Med. 2016, 4, 454–462. [Google Scholar] [CrossRef]
- Papaiwannou, A.; Zarogoulidis, P.; Porpodis, K.; Spyratos, D.; Kioumis, I.; Pitsiou, G.; Pataka, A.; Tsakiridis, K.; Arikas, S.; Mpakas, A.; et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): Current literature review. J. Thorac. Dis. 2014, 6 (Suppl. 1), S146–S151. [Google Scholar] [CrossRef]
- Postma, D.S.; Rabe, K.F. The Asthma-COPD Overlap Syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Sorino, C.; Pedone, C.; Scichilone, N. Fifteen-year mortality of patients with asthma-COPD overlap syndrome. Eur. J. Int. Med. 2016, 34, 72–77. [Google Scholar] [CrossRef]
- Wurst, K.E.; Rheault, T.R.; Edwards, L.; Tal-Singer, R.; Agusti, A.; Vestbo, J. A comparison of COPD patients with and without ACOS in the ECLIPSE study. Eur. Respir. J. 2016, 47, 1559–1562. [Google Scholar] [CrossRef]
- Kumbhare, S.; Strange, C. Mortality in Asthma-Chronic Obstructive Pulmonary Disease Overlap in the United States. South Med. J. 2018, 111, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Yasunaga, H.; Matsui, H.; Hasegawa, W.; Jo, T.; Takami, K.; Fushimi, K.; Nagase, T. Comparison of in-hospital mortality in patients with COPD, asthma and asthma-COPD overlap exacerbations. Respirology 2015, 20, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Marott, J.L.; Lange, P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: The Copenhagen General Population Study. Eur. Respir. J. 2018, 52, 1800616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Major Criteria | Minor Criteria | Diagnosis |
|---|---|---|---|
| Gibson, 2009 [3] |
| 3 major criteria | |
| Soler-Cataluna, 2011 [11] | COPD plus:
| COPD plus:
| 2 major criteria OR 1 major criterion AND 2 minor criteria |
| Koblizek, 2013 [12] | COPD plus:
| COPD plus:
| 2 major criteria OR 1 major criterion AND 2 minor criteria |
| GINA/GOLD Criteria, 2014 [10] | More likely COPD if:
| More likely asthma if:
| If ≥3 items are present for either asthma or COPD, the patient is likely to have that disease A similar number of items for asthma and COPD is suggestive for ACO |
| Cosio, 2016 [13] | COPD plus:
| COPD plus:
| 1 major criterion OR 2 minor criteria |
| Sin, 2016 [14] | COPD plus:
| COPD plus:
| 3 major criteria AND 1 minor criterion |
| Cataldo, 2017 [15] | ACO in a COPD patient:
| ACO in a COPD patient:
| 2 major criteria AND 1 minor criterion |
ACO in an asthma patient:
| ACO in an asthma patient:
| ||
| Miravittles, 2017 [16] |
|
| 3 major criteria AND 1 minor criterion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouka, E.; Papaioannou, A.I.; Hillas, G.; Steiropoulos, P. Asthma-COPD Overlap Syndrome: Recent Insights and Unanswered Questions. J. Pers. Med. 2022, 12, 708. https://doi.org/10.3390/jpm12050708
Fouka E, Papaioannou AI, Hillas G, Steiropoulos P. Asthma-COPD Overlap Syndrome: Recent Insights and Unanswered Questions. Journal of Personalized Medicine. 2022; 12(5):708. https://doi.org/10.3390/jpm12050708
Chicago/Turabian StyleFouka, Evangelia, Andriana I. Papaioannou, Georgios Hillas, and Paschalis Steiropoulos. 2022. "Asthma-COPD Overlap Syndrome: Recent Insights and Unanswered Questions" Journal of Personalized Medicine 12, no. 5: 708. https://doi.org/10.3390/jpm12050708
APA StyleFouka, E., Papaioannou, A. I., Hillas, G., & Steiropoulos, P. (2022). Asthma-COPD Overlap Syndrome: Recent Insights and Unanswered Questions. Journal of Personalized Medicine, 12(5), 708. https://doi.org/10.3390/jpm12050708








