Human Papillomavirus Infection and the Risk of Erectile Dysfunction: A Nationwide Population-Based Matched Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Definitions of the Outcomes and Covariates
2.4. Negative Control Exposure
2.5. Statistical Analysis
2.6. Sensitivity Analyses
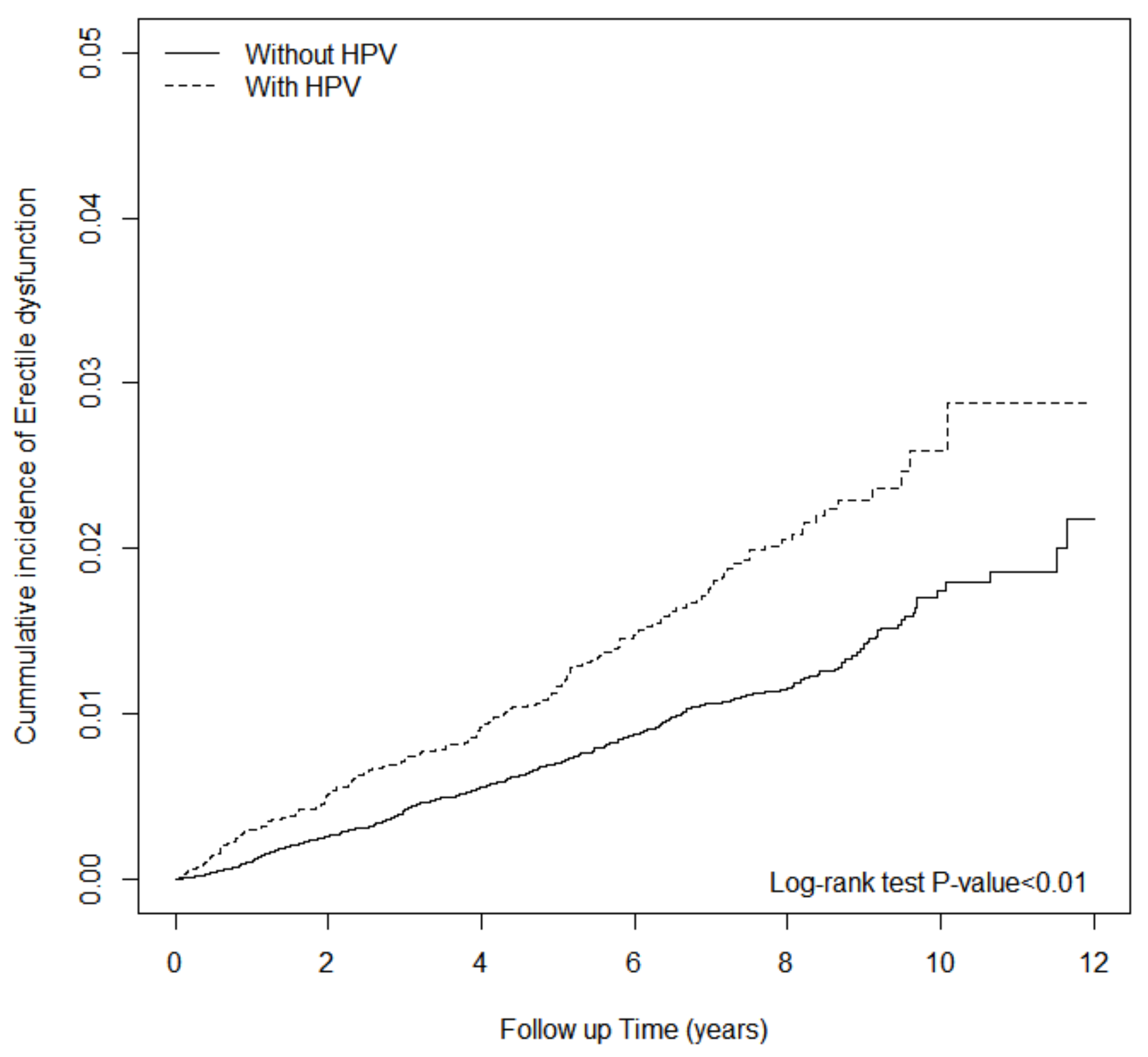
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Kocjan, B.; Bzhalava, D.; Forslund, O.; Dillner, J.; Poljak, M. Molecular methods for identification and characterization of novel papillomaviruses. Clin. Microbiol. Infect. 2015, 21, 808–816. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Bodily, J.; Laimins, L.A. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol. 2011, 19, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E. Reduction in Human Papillomavirus (HPV) Prevalence among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar] [CrossRef]
- Hariri, S.; Mph, M.L.J.; Bennett, N.M.; Bauer, H.M.; Park, I.U.; Schafer, S.; Niccolai, L.M.; Unger, E.R.; Markowitz, L.E.; HPV-IMPACT Working Group. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer 2015, 121, 2775–2781. [Google Scholar] [CrossRef]
- Guo, F.; Cofie, L.E.; Berenson, A.B. Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction. Am. J. Prev. Med. 2018, 55, 197–204. [Google Scholar] [CrossRef]
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine 2017, 35, 5753–5755. [Google Scholar] [CrossRef]
- Ozawa, K.; Hineno, A.; Kinoshita, T.; Ishihara, S.; Ikeda, S.-I. Suspected Adverse Effects After Human Papillomavirus Vaccination: A Temporal Relationship Between Vaccine Administration and the Appearance of Symptoms in Japan. Drug Saf. 2017, 40, 1219–1229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, J.J.; Beltran, T.H.; Song, J.W.; Klaric, J.; Choi, Y.S. Prevalence of Genital Human Papillomavirus Infection and Human Papillomavirus Vaccination Rates Among US Adult Men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol. 2017, 3, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, C.-W.; Ma, Y.-M.; Zhou, L.-Y.; Wang, S.-Y. Correlation between HPV sperm infection and male infertility. Asian J. Androl. 2013, 15, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Noventa, M.; De Toni, L.; Gizzo, S.; Garolla, A. HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology 2015, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-P.; Chen, C.-W.; Sheen, Y.-S.; Tsai, T.-F. Genotype distribution of human papillomavirus in anogenital warts of male patients in Taiwan. Dermatol. Sin. 2012, 30, 85–89. [Google Scholar] [CrossRef]
- Kucukunal, A.; Altunay, I.K.; Mercan, S. Sexual Dysfunction in Men Suffering from Genital Warts. J. Sex. Med. 2013, 10, 1585–1591. [Google Scholar] [CrossRef]
- Benet, A.E.; Melman, A. The Epidemiology of Erectile Dysfunction. Urol. Clin. N. Am. 1995, 22, 699–709. [Google Scholar] [CrossRef]
- Feldman, H.A.; Goldstein, I.; Hatzichristou, D.G.; Krane, R.J.; McKinlay, J.B. Impotence and Its Medical and Psychosocial Correlates: Results of the Massachusetts Male Aging Study. J. Urol. 1994, 151, 54–61. [Google Scholar] [CrossRef]
- Hwang, T.I.; Tsai, T.-F.; Lin, Y.-C.; Chiang, H.-S.; Chang, L.S. A Survey of Erectile Dysfunction in Taiwan: Use of the Erection Hardness Score and Quality of Erection Questionnaire. J. Sex. Med. 2010, 7, 2817–2824. [Google Scholar] [CrossRef]
- Traish, A.M.; Goldstein, I.; Kim, N. Testosterone and Erectile Function: From Basic Research to a New Clinical Paradigm for Managing Men with Androgen Insufficiency and Erectile Dysfunction. Eur. Urol. 2007, 52, 54–70. [Google Scholar] [CrossRef]
- Winegar, A.L.; Shepherd, M.D.; Lawson, K.A.; Richards, K.M. Comparison of the claim percent gross margin earned by Texas community independent pharmacies for dual-eligible beneficiary claims before and after Medicare Part D. J. Am. Pharm. Assoc. 2009, 49, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Margonato, A.; Macchi, A.; Galli, S.; Ravagnani, P.M.; Montorsi, P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur. Urol. 2003, 44, 360–365. [Google Scholar] [CrossRef]
- Chiurlia, E.; D’Amico, R.; Ratti, C.; Granata, A.R.; Romagnoli, R.; Modena, M.G. Subclinical Coronary Artery Atherosclerosis in Patients with Erectile Dysfunction. J. Am. Coll. Cardiol. 2005, 46, 1503–1506. [Google Scholar] [CrossRef]
- Ponholzer, A.; Temml, C.; Obermayr, R.; Wehrberger, C.; Madersbacher, S. Is erectile dysfunction an indicator for increased risk of coronary heart disease and stroke? Eur. Urol. 2005, 48, 512–518. [Google Scholar] [CrossRef]
- Thompson, I.M. Erectile Dysfunction and Subsequent Cardiovascular Disease. JAMA 2005, 294, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Rokkas, K.; Ioakeimidis, N.; Aggeli, C.; Michaelides, A.; Roussakis, G.; Fassoulakis, C.; Askitis, A.; Stefanadis, C. Prevalence of Asymptomatic Coronary Artery Disease in Men with Vasculogenic Erectile Dysfunction: A Prospective Angiographic Study. Eur. Urol. 2005, 48, 996–1003. [Google Scholar] [CrossRef]
- Kaiser, D.R.; Billups, K.; Mason, C.; Wetterling, R.; Lundberg, J.L.; Bank, A.J. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J. Am. Coll. Cardiol. 2004, 43, 179–184. [Google Scholar] [CrossRef][Green Version]
- Montorsi, P.; Montorsi, F.; Schulman, C.C. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur. Urol. 2003, 44, 352–354. [Google Scholar] [CrossRef]
- Solomon, H.; Man, J.W.; Jackson, G. Erectile dysfunction and the cardiovascular patient: Endothelial dysfunction is the common denominator. Heart 2003, 89, 251–253. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Chung, W.H.; Hsueh, Y.-J.; Chen, S.-Y.; Tokunaga, K.; Kinoshita, S.; Ma, D.H.K.; Ueta, M. Human leucocyte antigen association of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications in Han Chinese. Br. J. Ophthalmol. 2021, 106, 610–615. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Saeed, H.N.; Chodosh, J.; Wang, C.-W.; Chung, Y.-C.; Wei, L.-C.; Kuo, M.-T.; Liang, C.-M.; Chang, J.W.-C.; Chung, W.-H.; et al. Ocular manifestations of anti-neoplastic immune checkpoint inhibitor-associated Stevens-Johnson syndrome/toxic epidermal necrolysis in cancer patients. Ocul. Surf. 2021, 22, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-W.; Kuo, C.-L.; Wang, L.-T.; Ma, K.S.-K.; Huang, W.-Y.; Liu, F.-C.; Yang, K.D.; Yang, B.-H. Case Report: In Situ Vaccination by Autologous CD16+ Dendritic Cells and Anti-PD-L 1 Antibody Synergized With Radiotherapy To Boost T Cells-Mediated Antitumor Efficacy In A Psoriatic Patient With Cutaneous Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 752563. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Wei, J.C.; Chung, W.H. Correspondence to ‘Hypersensitivity reactions with allopurinol and febuxostat: A study using the Medicare claims data’. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Ma, K.S. Correspondence to ‘Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression’. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Ioakeimidis, N.; Rokkas, K.; Vasiliadou, C.; Alexopoulos, N.; Stefanadi, E.; Askitis, A.; Stefanadis, C. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur. Heart. J. 2006, 27, 2640–2648. [Google Scholar] [CrossRef]
- Billups, K.L.; Bank, A.J.; Padma-Nathan, H.; Katz, S.; Williams, R. Erectile dysfunction is a marker for cardiovascular disease: Results of the minority health institute expert advisory panel. J. Sex. Med. 2005, 2, 40–50, discussion 50–52. [Google Scholar] [CrossRef]
- Ma, K.S.; Lee, C.C.; Liu, K.J.; Wei, J.C.; Lee, Y.T.; Wang, L.T. Safety and Seroconversion of Immunotherapies against SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis of Clinical Trials. Pathogens 2021, 10, 1537. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Tang, P.-U.; Lee, G.H.; Chiang, T.-H.; Ma, K.S.-K.; Fang, C.-T. Prevalence of Nontuberculous Mycobacterium Infections versus Tuberculosis among Autopsied HIV Patients in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2020, 104, 628–633. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Chiang, C.-H.; Chen, Y.-W.; Wang, L.-T. Correspondence to ‘Bacterial citrullinated epitopes generated by Porphyromonas gingivalis infection-a missing link for ACPA production’. Ann. Rheum. Dis. 2021, 79, 1194–1202. [Google Scholar] [CrossRef]
- Wu, M.-C.; Ma, K.S.-K.; Wang, Y.-H.; Wei, J.C.-C. Impact of tonsillectomy on irritable bowel syndrome: A nationwide population-based cohort study. PLoS ONE 2020, 15, e0238242. [Google Scholar] [CrossRef]
- Luan, Y.Z.; Chen, B.S.; Ma, K.S. 16S rDNA Gene Sequencing and Virulence of Oral Microbiome in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Lio, C.F.; Chen, H.-T.; Wang, Y.-T.T.; Ma, K.S.-K.; Chou, Y.T.; Chang, F.-C.; Tsai, S.-Y. Discordance of vancomycin minimum inhibitory concentration for methicillin-resistant Staphylococcus aureus at 2 μg/mL between Vitek II, E-test, and Broth Microdilution. PeerJ 2020, 8, e8963. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Ma, K.S.-K.; Chen, H.-H.; Huang, J.-Y.; Wei, J.C.-C. Relationship between Helicobacter pylori infection and psoriasis: A nationwide population-based longitudinal cohort study. Medicine 2020, 99, e20632. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Lee, B.-S.; Jhang, Y.-T.; Ma, K.S.-K.; Huang, C.-P.; Fu, K.-L.; Lai, C.-H.; Tseng, W.-Y.; Kuo, M.Y.-P.; Chen, Y.-W. Er:YAG laser irradiation enhances bacterial and lipopolysaccharide clearance and human gingival fibroblast adhesion on titanium discs. Sci. Rep. 2021, 11, 23954. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, M.; Viik-Kajander, M.; Palosuo, T.; Toivanen, P.; Leinonen, M.; Saikku, P.; Tenkanen, L.; Manninen, V.; Hovi, T.; MänttäriM. Infections, inflammation, and the risk of coronary heart disease. Circulation 2000, 101, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Nieto, F.J.; Horne, B.D.; Anderson, J.L.; Muhlestein, J.B.; Epstein, S.E. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation 2001, 103, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Chiang, C.H.; Pickering, J.W.; Stoyanov, K.M.; Chew, D.P.; Neumann, J.T.; Ojeda, F.; Sörensen, N.A.; Su, K.Y.; Kavsak, P.; et al. Performance of the European Society of Cardiology 0/1-Hour, 0/2-Hour, and 0/3-Hour Algorithms for Rapid Triage of Acute Myocardial Infarction: An International Collaborative Meta-analysis. Ann. Intern. Med. 2022, 175, 101–113. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Liou, Y.-J.; Huang, P.-H.; Lin, P.-S.; Chen, Y.-W.; Chang, R.-F. Identifying Medically-compromised Patients with Periodontitis-Associated Cardiovascular Diseases Using Convolutional Neural Network-facilitated Multilabel Classification of Panoramic Radiographs. In Proceedings of the 2021 International Conference on Applied Artificial Intelligence (ICAPAI), Halden, Norway, 19–21 May 2021. [Google Scholar]
- Ma, K.S.-K.; Chiang, C.-H.; Lopez, A.A.V.; Wang, L.-T. Cohort study of periodontitis-associated signaling pathways in myocardial infarction following atherosclerotic cardiovascular diseases. Metabolism 2021, 116, 154478. [Google Scholar] [CrossRef]
- Kuo, H.K.; Fujise, K. Human papillomavirus and cardiovascular disease among U.S. women in the National Health and Nutrition Examination Survey 2003 to 2006. J. Am. Coll. Cardiol. 2011, 58, 2001–2006. [Google Scholar] [CrossRef]
- Muñoz-Grajales, C.; Pineda, J.C. Pathophysiological Relationship between Infections and Systemic Vasculitis. Autoimmune Dis. 2015, 2015, 286783. [Google Scholar] [CrossRef]
- Aksu, K.; Keser, G.; Günaydin, G.; Ozbek, S.S.; Colakoğlu, Z.; Gümüşdiş, G.; Doganavşargil, E. Erectile dysfunction in Behçet‘s disease without neurological involvement: Two case reports. Rheumatology 2000, 39, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Kaul, N.; Bhat, A.; Singh, R.; Singh, I. Erectile. Erectile Dysfunction in Behcet‘s Disease. Indian J. Dermatol. 2017, 62, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Pfeifer, J.D.; Lewis, J.S., Jr. Association between human papillomavirus DNA and temporal arteritis. BMC Musculoskelet. Disord. 2012, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Lin, C.-S.; Hong, C.-F.; Lee, D.-J.; Sun, C.; Lin, H.-H. Design of a Clinical Decision Support System for Predicting Erectile Dysfunction in Men Using NHIRD Dataset. IEEE J. Biomed. Health Inform. 2019, 23, 2127–2137. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chueh, S.-C.J.; Wu, V.-C.; Chen, L.; Lin, Y.-H.; Hu, Y.-H.; Wu, K.-D.; Tsai, Y.-C. Risk of severe erectile dysfunction in primary hyperaldosteronism: A population-based propensity score matching cohort study. Surgery 2019, 165, 622–628. [Google Scholar] [CrossRef]
- Lipsitch, M.; Tchetgen Tchetgen, E.; Cohen, T. Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 2010, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Ehrenstein, V.; Vandenbroucke, J.P. Confounding in observational studies based on large health care databases: Problems and potential solutions—A primer for the clinician. Clin. Epidemiol. 2017, 9, 185–193. [Google Scholar] [CrossRef]
- Hitchcock, J.R.; Cook, C.N.; Bobat, S.; Ross, E.; Flores-Langarica, A.; Lowe, K.L.; Khan, M.; Dominguez-Medina, C.C.; Lax, S.; Carvalho-Gaspar, M.; et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J. Clin. Investig. 2015, 125, 4429–4446. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Wei, J.C.-C.; Lin, M.-C.; Hung, Y.-M.; Hung, C.-H. Risk of subsequent ischemic stroke in patients with nontyphoidal salmonellosis: A nationwide population-based cohort study. J. Infect. 2020, 81, 396–402. [Google Scholar] [CrossRef]
- Zippe, C.D.; Raina, R.; Massanyi, E.Z.; Agarwal, A.; Jones, J.S.; Ulchaker, J.; Klein, E.A. Sexual function after male radical cystectomy in a sexually active population. Urology 2004, 64, 682–685, discussion 685–686. [Google Scholar] [CrossRef]
- Modh, R.A.; Mulhall, J.P.; Gilbert, S.M. Sexual dysfunction after cystectomy and urinary diversion. Nat. Rev. Urol. 2014, 11, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.-I.; Shu-Ling, H.; Huang, C.-Y.; Kao, C.-C.; Hung, S.-L.; Yang, H.-Y.; Tung, H.-Y. Sexual dysfunction in males following low anterior resection. J. Clin. Nurs. 2016, 25, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Sollie, S.; Challacombe, B.; Briggs, K.; Van Hemelrijck, M. The global prevalence of erectile dysfunction: A review. BJU Int. 2019, 124, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ranjeva, S.L.; Baskerville, E.B.; Dukic, V.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R.; Dwyer, G.; Cobey, S. Recurring infection with ecologically distinct HPV types can explain high prevalence and diversity. Proc. Natl. Acad. Sci. USA 2017, 114, 13573–13578. [Google Scholar] [CrossRef]
- Uysal, G.; Bas, S.; Gokulu, S.G.; Okcu, N.T.; Destegul, E. Sexual dysfunction in human papillomavirus positive females during reproductive age. Reprod. Syst. Sex. Disord. 2018, 7, 224. [Google Scholar]
- Rintala, M.A.M.; Pöllänen, P.P.; Nikkanen, V.P.; Grénman, S.E.; Syrjänen, S.M. Human papillomavirus DNA is found in the vas deferens. J. Infect. Dis. 2002, 185, 1664–1667. [Google Scholar] [CrossRef]
- Rintala, M.A.M.; E Greénman, S.; Pöllänen, P.P.; Suominen, J.J.; Syrjänen, S.M. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int. J. STD AIDS 2004, 15, 740–743. [Google Scholar] [CrossRef]
- Perino, A.; Giovannelli, L.; Schillaci, R.; Ruvolo, G.; Fiorentino, F.P.; Alimondi, P.; Cefalù, E.; Ammatuna, P. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: Impact on reproductive outcomes. Fertil. Steril. 2011, 95, 1845–1848. [Google Scholar] [CrossRef]
- Lue, T.F. Erectile dysfunction. N. Engl. J. Med. 2000, 342, 1802–1813. [Google Scholar] [CrossRef]
- Ma, K.S.; Lai, J.; Bds, J.J.V.; Chiu, L.; Van Dyke, T.E.; Wei, J.C. Fibromyalgia and periodontitis: Bidirectional associations in population-based 15-year retrospective cohorts. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Ma, K.; Hasturk, H.; Carreras, I.; Dedeoglu, A.; Veeravalli, J.; Huang, J.; Kantarci, A.; Wei, J. Dementia and the Risk of Periodontitis: A Population-Based Cohort Study. J. Dent. Res. 2022, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Wu, M.; Thota, E.; Wang, Y.; Alqaderi, H.E.; Wei, J.C. Tonsillectomy as a risk factor of periodontitis: A population-based cohort study. J. Periodontol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Thota, E.; Huang, J.; Huang, Y.; Wei, J.C. Onset of oral lichen planus following dental treatments: A nested case-control study. Oral. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.-K.; Ralda, M.M.I.; Veeravalli, J.J.; Wang, L.-T.; Thota, E.; Huang, J.-Y.; Kao, C.-T.; Wei, J.C.-C.; Resnick, C.M. Patients with juvenile idiopathic arthritis are at increased risk for obstructive sleep apnoea: A population-based cohort study. Eur. J. Orthod. 2022, 44, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Rylander, E. Human papillomavirus. Subclinical and atypical manifestations. Dermatol. Clin. 1998, 16, 817–822. [Google Scholar] [CrossRef]
- Plummer, M.; Schiffman, M.; Castle, P.E.; Maucort-Boulch, D.; Wheeler, C.M. ALTS (Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study) Group A 2-Year Prospective Study of Human Papillomavirus Persistence among Women with a Cytological Diagnosis of Atypical Squamous Cells of Undetermined Significance or Low-Grade Squamous Intraepithelial Lesion. J. Infect. Dis. 2007, 195, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.C.; Schiffman, M.; Herrero, R.; Wacholder, S.; Hildesheim, A.; Castle, P.E.; Solomon, D.; Burk, R.; On behalf of the Proyecto Epidemiologico Guanacaste Group. Rapid Clearance of Human Papillomavirus and Implications for Clinical Focus on Persistent Infections. JNCI J. Natl. Cancer Inst. 2008, 100, 513–517. [Google Scholar] [CrossRef]
| Variables | Non-HPV | HPV | SMD | ||
|---|---|---|---|---|---|
| (N = 53,184) | (N = 13,296) | ||||
| n | % | n | % | ||
| Age, year | |||||
| 18–30 | 19,768 | 37% | 4895 | 37% | 0.007 |
| 31–40 | 10,967 | 21% | 2710 | 20% | 0.006 |
| 41–50 | 9873 | 19% | 2399 | 18% | 0.01 |
| ≥51 | 12,576 | 24% | 3292 | 25% | 0.03 |
| mean, (SD) | 39.0 | (15.7) | 39.4 | (16.3) | 0.03 |
| Comorbidities | |||||
| hypertension | 9821 | 18% | 2492 | 19% | 0.007 |
| diabetes mellitus | 4398 | 8.0% | 1190 | 9.0% | 0.02 |
| hyperlipidemia | 9419 | 18% | 2458 | 18% | 0.02 |
| Stroke | 2243 | 4.0% | 654 | 5.0% | 0.03 |
| CAD | 640 | 1.0% | 217 | 2.0% | 0.04 |
| CKD | 548 | 1.0% | 187 | 1.0% | 0.03 |
| COPD | 4987 | 9% | 1351 | 10% | 0.03 |
| Alcohol-related illness | 1838 | 3.0% | 481 | 4.0% | 0.009 |
| HIV | 80 | 0.0% | 32 | 0.0% | 0.02 |
| AID | 754 | 1.0% | 238 | 2.0% | 0.03 |
| Medication | |||||
| α-blocker | 7283 | 14% | 1909 | 14% | 0.02 |
| β-blocker | 3776 | 7% | 1017 | 8% | 0.02 |
| CCB | 9842 | 19% | 2472 | 19% | 0.002 |
| diuretics | 6470 | 12% | 1673 | 13% | 0.01 |
| Hazard Ratio (95% CI) | |
|---|---|
| Primary analysis | |
| Model 1 (adjusted comorbidities + comedications) | 1.64 (1.38, 1.95) *** |
| Model 2 (adjusted comorbidities + comedications + relevant operation) | 1.63 (1.37, 1.94) *** |
| Sensitivity analyses | |
| HPV excluding stroke, cancer, AID | 1.59 (1.30, 1.95) *** |
| Alternative exposure (NTS) | 0.69 (0.31, 1.53) |
| Variables | Erectile Dysfunction | (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| n | PY | IR | cHR | (95% CI) | aHR ꝉ | ||
| non-HPV | 432 | 286,137 | 1.51 | 1.00 | - | 1.00 | - |
| HPV | 181 | 71,478 | 2.53 | 1.69 | (1.42, 2.00) *** | 1.63 | (1.37, 1.94) *** |
| Age, year | |||||||
| 18–30 | 78 | 141,370 | 0.55 | 1.00 | - | 1.00 | - |
| 31–40 | 93 | 75,174 | 1.24 | 2.25 | (1.67, 3.04) *** | 1.89 | (1.40, 2.56) *** |
| 41–50 | 176 | 65,317 | 2.69 | 4.93 | (3.78, 6.44) *** | 3.17 | (2.39, 4.2) *** |
| ≥51 | 266 | 75,754 | 3.51 | 6.49 | (5.04, 8.36) *** | 2.49 | (1.83, 3.39) *** |
| Comorbidities | |||||||
| hypertension | |||||||
| No | 416 | 298,632 | 1.39 | 1.00 | - | 1.00 | - |
| Yes | 197 | 58,983 | 3.34 | 2.43 | (2.05, 2.88) *** | 0.78 | (0.60, 1.00) * |
| diabetes mellitus | |||||||
| No | 501 | 331,883 | 1.51 | 1.00 | - | 1.00 | - |
| Yes | 112 | 25,732 | 4.35 | 2.92 | (2.38, 3.59) *** | 1.21 | (0.96, 1.52) |
| hyperlipidemia | |||||||
| No | 375 | 298,695 | 1.26 | 1.00 | - | 1.00 | - |
| Yes | 238 | 58,919 | 4.04 | 3.25 | (2.76, 3.82) *** | 1.63 | (1.34, 1.97) *** |
| Stroke | |||||||
| No | 581 | 344,885 | 1.68 | 1.00 | - | 1.00 | - |
| Yes | 32 | 12,729 | 2.51 | 1.52 | (1.06, 2.17) * | 0.60 | (0.41, 0.86) ** |
| CAD | |||||||
| No | 602 | 353,790 | 1.7 | 1.00 | - | ||
| Yes | 11 | 3825 | 2.88 | 1.70 | (0.94, 3.09) | ||
| CKD | |||||||
| No | 606 | 354,713 | 1.71 | 1.00 | - | ||
| Yes | 7 | 2902 | 2.41 | 1.45 | (0.69, 3.05) | ||
| COPD | |||||||
| No | 509 | 325,821 | 1.56 | 1.00 | - | 1.00 | - |
| Yes | 104 | 31,794 | 3.27 | 2.10 | (1.70, 2.59) *** | 1.03 | (0.82, 1.29) |
| Alcohol-related illness | |||||||
| No | 580 | 347,685 | 1.67 | 1.00 | - | 1.00 | - |
| Yes | 33 | 9930 | 3.32 | 2.05 | (1.44, 2.91) *** | 1.43 | (1.01, 2.05) * |
| HIV | |||||||
| No | 613 | 357,109 | 1.72 | ||||
| Yes | 0 | 506 | 0 | ||||
| AID | |||||||
| No | 590 | 352,918 | 1.67 | 1.00 | - | 1.00 | - |
| Yes | 23 | 4696 | 4.9 | 2.95 | (1.94, 4.47) *** | 1.66 | (1.09, 2.53) * |
| Spinal cord injury | |||||||
| No | 613 | 357,445 | 1.71 | ||||
| Yes | 0 | 170 | 0 | ||||
| Pelvic fracture | |||||||
| No | 613 | 357,599 | 1.71 | ||||
| Yes | 0 | 16 | 0 | ||||
| Burn of unspecified degree of genitalia | |||||||
| No | 613 | 357,605 | 1.71 | ||||
| Yes | 0 | 9 | 0 | ||||
| Foreign body entering through orifice | |||||||
| No | 523 | 321,822 | 1.63 | 1.00 | - | 1.00 | - |
| Yes | 90 | 35,793 | 2.51 | 1.57 | (1.25, 1.96) *** | 1.20 | (0.96, 1.50) |
| Injury to nerves and spinal cord | |||||||
| No | 604 | 354,341 | 1.7 | 1.00 | - | ||
| Yes | 9 | 3273 | 2.75 | 1.64 | (0.85, 3.17) | ||
| Poisoning | |||||||
| No | 610 | 354,612 | 1.72 | 1.00 | - | ||
| Yes | 3 | 3003 | 1 | 0.58 | (0.19, 1.82) | ||
| Medication | |||||||
| a-blocker | |||||||
| No | 316 | 307,984 | 1.03 | 1.00 | - | 1.00 | - |
| Yes | 297 | 49,631 | 5.98 | 5.81 | (4.96, 6.81) *** | 4.07 | (3.33, 4.97) *** |
| b-blocker | |||||||
| No | 502 | 330,756 | 1.52 | 1.00 | - | 1.00 | - |
| Yes | 111 | 26,859 | 4.13 | 2.70 | (2.19, 3.31) *** | 1.30 | (1.03, 1.64) * |
| CCB | |||||||
| No | 388 | 291,236 | 1.33 | 1.00 | - | 1.00 | - |
| Yes | 225 | 66,378 | 3.39 | 2.53 | (2.15, 2.98) *** | 1.02 | (0.80, 1.30) |
| diuretics | |||||||
| No | 474 | 314,505 | 1.51 | 1.00 | - | 1.00 | - |
| Yes | 139 | 43,110 | 3.22 | 2.12 | (1.76, 2.57) *** | 0.74 | (0.59, 0.93) * |
| Treatment | |||||||
| Radical cystectomy | |||||||
| No | 613 | 357,555 | 1.71 | ||||
| Yes | 0 | 60 | 0 | ||||
| LAR + APR | |||||||
| No | 610 | 357,350 | 1.71 | 1.00 | - | 1.00 | - |
| Yes | 3 | 265 | 11.3 | 6.56 | (2.11, 20.41) ** | 2.70 | (0.86, 8.43) |
| Variables | Non-HPV | HPV | p for Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | n | PY | IR | cHR | (95% CI) | aHR ꝉ | (95% CI) | ||
| Age, year | 0.40 | ||||||||||
| 18–30 | 61 | 113,415 | 0.54 | 17 | 27,955 | 0.61 | 1.14 | (0.66, 1.94) | 1.14 | (0.67, 1.95) | |
| 31–40 | 67 | 60,335 | 1.11 | 26 | 14,839 | 1.75 | 1.61 | (1.03, 2.54) * | 1.65 | (1.05, 2.6) * | |
| 41–50 | 127 | 52,646 | 2.41 | 49 | 12,671 | 3.87 | 1.61 | (1.16, 2.24) ** | 1.63 | (1.17, 2.27) ** | |
| ≥51 | 177 | 59,740 | 2.96 | 89 | 16,013 | 5.56 | 1.87 | (1.45, 2.42) *** | 1.85 | (1.43, 2.39) *** | |
| Comorbidities | |||||||||||
| hypertension | 0.10 | ||||||||||
| No | 303 | 239,303 | 1.27 | 113 | 59,329 | 1.9 | 1.51 | (1.22, 1.87) *** | 1.5 | (1.21, 1.87) *** | |
| Yes | 129 | 46,833 | 2.75 | 68 | 12,150 | 5.6 | 2.05 | (1.53, 2.76) *** | 1.99 | (1.48, 2.68) *** | |
| diabetes mellitus | 0.05 | ||||||||||
| No | 364 | 266,014 | 1.37 | 137 | 65,869 | 2.08 | 1.52 | (1.25, 1.86) *** | 1.5 | (1.23, 1.82) *** | |
| Yes | 68 | 20,122 | 3.38 | 44 | 5610 | 7.84 | 2.38 | (1.63, 3.49) *** | 2.39 | (1.63, 3.51) *** | |
| hyperlipidemia | 0.89 | ||||||||||
| No | 265 | 239,430 | 1.11 | 110 | 59,265 | 1.86 | 1.68 | (1.34, 2.1) *** | 1.69 | (1.36, 2.12) *** | |
| Yes | 167 | 46,706 | 3.58 | 71 | 12,213 | 5.81 | 1.65 | (1.25, 2.18) *** | 1.58 | (1.19, 2.09) ** | |
| Stroke | 0.001 | ||||||||||
| No | 420 | 276,453 | 1.52 | 161 | 68,433 | 2.35 | 1.56 | (1.3, 1.87) *** | 1.53 | (1.28, 1.84) *** | |
| Yes | 12 | 9684 | 1.24 | 20 | 3046 | 6.57 | 5.18 | (2.53, 10.6) *** | 4.82 | (2.34, 9.92) *** | |
| CAD | 0.46 | ||||||||||
| No | 424 | 283,349 | 1.5 | 178 | 70,441 | 2.53 | 1.7 | (1.42, 2.02) *** | 1.67 | (1.4, 1.99) *** | |
| Yes | 8 | 2787 | 2.87 | 3 | 1037 | 2.89 | 1.07 | (0.28, 4.07) | 1.3 | (0.32, 5.23) | |
| CKD | 0.27 | ||||||||||
| No | 429 | 283,990 | 1.51 | 177 | 70,723 | 2.5 | 1.66 | (1.4, 1.98) *** | 1.63 | (1.36, 1.94) *** | |
| Yes | 3 | 2147 | 1.4 | 4 | 755 | 5.3 | 3.64 | (0.81, 16.27) | 4.99 | (0.94, 26.6) | |
| COPD | 0.03 | ||||||||||
| No | 370 | 261,178 | 1.42 | 139 | 64,642 | 2.15 | 1.52 | (1.25, 1.85) *** | 1.5 | (1.23, 1.82) *** | |
| Yes | 62 | 24,958 | 2.48 | 42 | 6836 | 6.14 | 2.56 | (1.73, 3.81) *** | 2.51 | (1.69, 3.74) *** | |
| Alcohol-related illness | 0.17 | ||||||||||
| No | 413 | 278,340 | 1.48 | 167 | 69,345 | 2.41 | 1.63 | (1.36, 1.95) *** | 1.6 | (1.34, 1.92) *** | |
| Yes | 19 | 7796 | 2.44 | 14 | 2134 | 6.56 | 2.68 | (1.34, 5.34) ** | 2.52 | (1.25, 5.07) ** | |
| HIV | 1.00 | ||||||||||
| No | 432 | 285,790 | 1.51 | 181 | 71,319 | 2.54 | 1.69 | (1.42, 2.01) *** | 1.64 | (1.38, 1.96) *** | |
| Yes | 0 | 347 | 0 | 0 | 159 | 0 | |||||
| AID | 0.17 | ||||||||||
| No | 420 | 282,571 | 1.49 | 170 | 70,347 | 2.42 | 1.63 | (1.37, 1.95) *** | 1.62 | (1.35, 1.93) *** | |
| Yes | 12 | 3566 | 3.37 | 11 | 1131 | 9.73 | 2.85 | (1.26, 6.46) * | 2.44 | (1.07, 5.6) * | |
| Medication | |||||||||||
| a-blocker | 0.47 | ||||||||||
| No | 228 | 246,731 | 0.92 | 88 | 61,253 | 1.44 | 1.56 | (1.22, 1.99) *** | 1.55 | (1.21, 1.98) *** | |
| Yes | 204 | 39,405 | 5.18 | 93 | 10,226 | 9.09 | 1.76 | (1.37, 2.25) *** | 1.75 | (1.37, 2.24) *** | |
| b-blocker | 0.82 | ||||||||||
| No | 355 | 264,742 | 1.34 | 147 | 66,014 | 2.23 | 1.66 | (1.37, 2.02) *** | 1.63 | (1.35, 1.98) *** | |
| Yes | 77 | 21,394 | 3.6 | 34 | 5464 | 6.22 | 1.75 | (1.16, 2.62) ** | 1.7 | (1.13, 2.56) * | |
| CCB | 0.07 | ||||||||||
| No | 283 | 232,996 | 1.21 | 105 | 58,240 | 1.8 | 1.49 | (1.19, 1.86) *** | 1.45 | (1.16, 1.82) ** | |
| Yes | 149 | 53,140 | 2.8 | 76 | 13,238 | 5.74 | 2.06 | (1.56, 2.72) *** | 2.02 | (1.53, 2.67) *** | |
| Diuretics | 0.25 | ||||||||||
| No | 340 | 251,848 | 1.35 | 134 | 62,657 | 2.14 | 1.58 | (1.3, 1.93) *** | 1.54 | (1.26, 1.88) *** | |
| Yes | 92 | 34,289 | 2.68 | 47 | 8821 | 5.33 | 2.06 | (1.45, 2.93) *** | 2.07 | (1.45, 2.96) *** | |
| Non-HPV | HPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-Up, Year | n | PY | IR | N | PY | IR | cHR | (95% CI) | aHR ꝉ | (95% CI) |
| <1 | 58 | 52,691 | 0.11 | 40 | 13,210 | 0.30 | 1.67 | (1.41, 1.99) *** | 1.64 | (1.38, 1.95) *** |
| 1–5 | 238 | 262,705 | 0.09 | 86 | 65,757 | 0.13 | 1.51 | (1.24, 1.83) *** | 1.48 | (1.22, 1.80) *** |
| >5 | 136 | 212,530 | 0.06 | 55 | 53,049 | 0.10 | 1.67 | (1.22, 2.29) ** | 1.7 | (1.24, 2.34) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, S.-E.; Ma, K.S.-K.; Kao, P.-E.; Wei, J.C.-C.; Yip, H.-T.; Chou, M.-C.; Hung, Y.-M.; Chin, N.-C. Human Papillomavirus Infection and the Risk of Erectile Dysfunction: A Nationwide Population-Based Matched Cohort Study. J. Pers. Med. 2022, 12, 699. https://doi.org/10.3390/jpm12050699
Juang S-E, Ma KS-K, Kao P-E, Wei JC-C, Yip H-T, Chou M-C, Hung Y-M, Chin N-C. Human Papillomavirus Infection and the Risk of Erectile Dysfunction: A Nationwide Population-Based Matched Cohort Study. Journal of Personalized Medicine. 2022; 12(5):699. https://doi.org/10.3390/jpm12050699
Chicago/Turabian StyleJuang, Sin-Ei, Kevin Sheng-Kai Ma, Pei-En Kao, James Cheng-Chung Wei, Hei-Tung Yip, Mei-Chia Chou, Yao-Min Hung, and Ning-Chien Chin. 2022. "Human Papillomavirus Infection and the Risk of Erectile Dysfunction: A Nationwide Population-Based Matched Cohort Study" Journal of Personalized Medicine 12, no. 5: 699. https://doi.org/10.3390/jpm12050699
APA StyleJuang, S.-E., Ma, K. S.-K., Kao, P.-E., Wei, J. C.-C., Yip, H.-T., Chou, M.-C., Hung, Y.-M., & Chin, N.-C. (2022). Human Papillomavirus Infection and the Risk of Erectile Dysfunction: A Nationwide Population-Based Matched Cohort Study. Journal of Personalized Medicine, 12(5), 699. https://doi.org/10.3390/jpm12050699






