Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Data Collection
2.3. Molecular Characterization
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Molecular Features
3.3. Standard Prognosticators
3.4. Molecular Prognosticators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frederick, L.; Wang, X.Y.; Eley, G.; James, C.D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000, 60, 1383–1387. [Google Scholar] [PubMed]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in glioblastoma: Prognostic significance revisited. Neoplasia 2011, 13, 1113–1121. [Google Scholar] [CrossRef]
- Chen, J.R.; Xu, H.Z.; Yao, Y.; Qin, Z.Y. Prognostic value of epidermal growth factor receptor amplification and EGFRvIII in glioblastoma: Meta-analysis. Acta Neurol. Scand. 2015, 132, 310–322. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; IARC: Lyon, France, 2021. [Google Scholar]
- Pelloski, C.E.; Ballman, K.V.; Furth, A.F.; Zhang, L.; Lin, E.; Sulman, E.P.; Bhat, K.; McDonald, J.M.; Yung, W.K.; Colman, H.; et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 2007, 25, 2288–2594. [Google Scholar] [CrossRef]
- D’alessandris, Q.G.; Martini, M.; Cenci, T.; Di Bonaventura, R.; Lauretti, L.; Stumpo, V.; Olivi, A.; Larocca, L.M.; Pallini, R.; Montano, N. Tailored therapy for recurrent glioblastoma. Report of a personalized molecular approach. J. Neurosurg. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. (Eds.) WHO Classification of Tumours of the Central Nervous System; IARC: Lyon, France, 2007. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. (Eds.) WHO Classification of Tumours of the Central Nervous System, Revised 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Central Nervous System Cancers. Version 2.2021. 8 September 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 14 March 2022).
- D’Alessandris, Q.G.; Biffoni, M.; Martini, M.; Runci, D.; Buccarelli, M.; Cenci, T.; Signore, M.; Stancato, L.; Olivi, A.; De Maria, R.; et al. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro-Oncology 2017, 19, 1097–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horbinski, C.; Kofler, J.; Kelly, L.M.; Murdoch, G.H.; Nikiforova, M.N. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J. Neuropathol. Exp. Neurol. 2009, 68, 1319–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of gender on the survival of patients with glioblastoma. Biosci. Rep. 2018, 38, BSR20180752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegi, M.E.; Stupp, R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter-still a dilemma? Neuro-Oncology 2015, 17, 1425–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finocchiaro, G.; Gentner, B.; Farina, F.; Capotondo, A.; Eoli, M.; Anghileri, E.; Carabba, M.G.; Cuccarini, V.; DI Meco, F.; Legnani, F.; et al. A phase I-IIa study of genetically modified Tie-2 expressing monocytes in patients with glioblastoma multiforme (TEM-GBM Study). J. Clin. Oncol. 2021, 39 (Suppl. 15), 2532. [Google Scholar] [CrossRef]
- Struve, N.; Binder, Z.A.; Stead, L.F.; Brend, T.; Bagley, S.J.; Faulkner, C.; Ott, L.; Müller-Goebel, J.; Weik, A.S.; Hoffer, K.; et al. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 2020, 39, 3041–3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conforti, F.; Pala, L.; Bagnardi, V.; Viale, G.; De Pas, T.; Pagan, E.; Gelber, R.D.; Goldhirsch, A. Sex-based differences of the tumor mutational burden and T-cell inflammation of the tumor microenvironment. Ann. Oncol. 2019, 30, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, L. (Department of Oncology and Molecular Medicine, Istituto Superiore di Sanità). 2022; manuscript in preparation.


| Parameter | Result |
|---|---|
| n | 355 |
| Age (mean ± SD) | 62.5 ± 10.6 years |
| Sex, M:F (%) | 219:134 (62–38%) |
| Symptom duration (mean ± SD) | 1.5 ± 1.6 months |
| Tumor diameter (mean ± SD) | 4.7 ± 1.5 cm |
| Postoperative KPS (median, range) | 70 (90–20) |
| Tumor location (%) | |
| frontal | 109 (30.8%) |
| temporal | 150 (42.4%) |
| parietal | 54 (15.3%) |
| other | 29 (8.2%) |
| multicentric | 13 (3.7%) |
| Extent of resection | |
| GTR | 244 (68.7%) |
| STR | 93 (26.2%) |
| biopsy | 18 (5.1%) |
| Median OS | 13 months |
| Parameter | Result |
|---|---|
| EGFRvIII | |
| positive | 184 (51.8%) |
| negative | 171 (48.2%) |
| MGMT promoter | |
| methylated | 198 (56.3%) |
| unmethylated | 154 (43.8%) |
| VEGF | |
| hyperexpressed | 235 (87%) |
| not hyperexpressed | 35 (13%) |
| Proliferation index, median (range) | 35% (4–70) |
| Parameter | Median OS | p-Value |
|---|---|---|
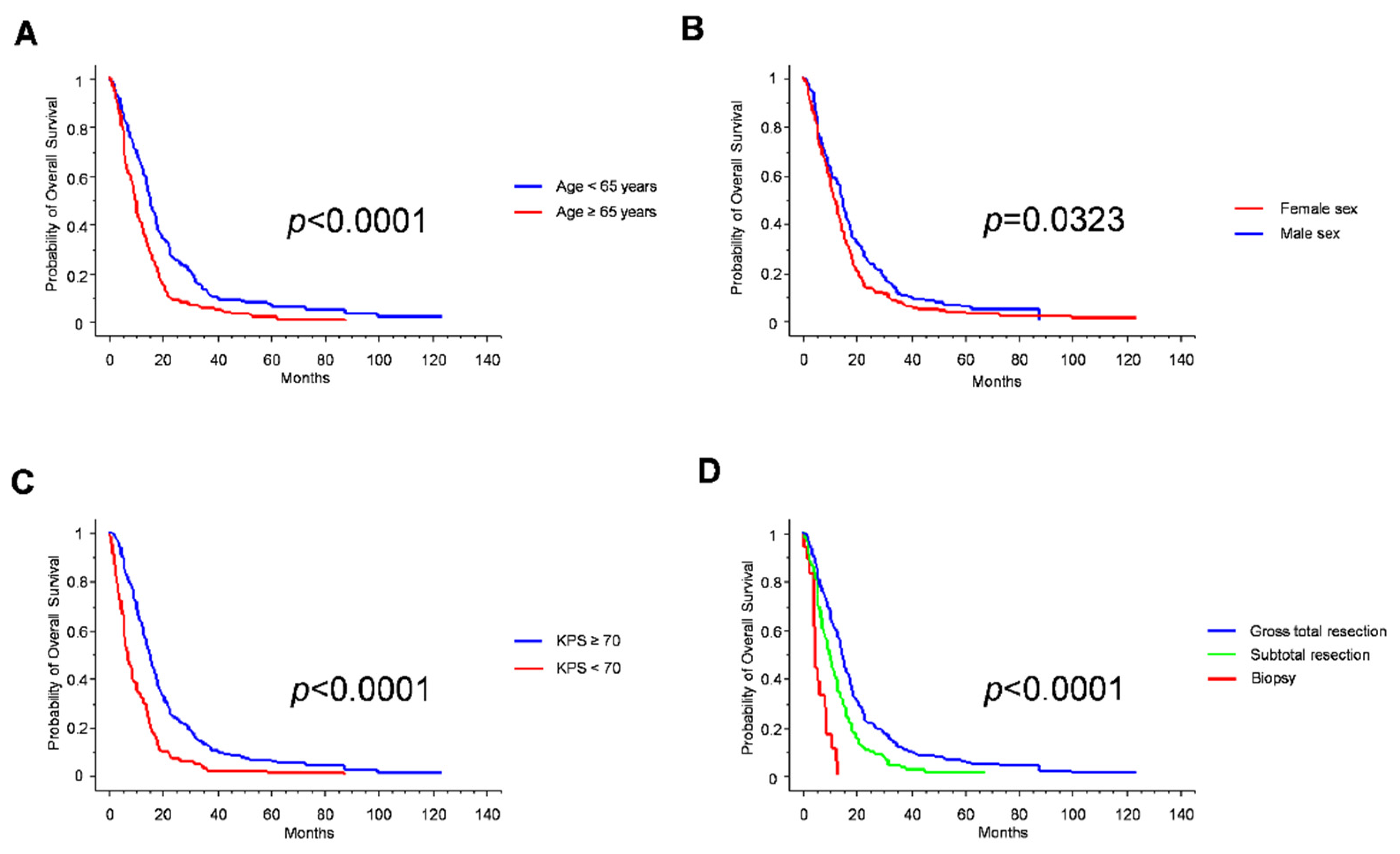
| Whole series | 13 months | NA |
| Age | <0.0001 | |
| ≥65 years | 15.5 months | |
| <65 years | 10 months | |
| Sex | 0.0323 | |
| Male | 12 months | |
| Female | 15 months | |
| KPS | <0.0001 | |
| ≥70 | 15.5 months | |
| <70 | 7.5 months | |
| EOR | <0.0001 | |
| GTR | 14.5 months | |
| STR | 10 months | |
| biopsy | 4.5 months | |
| EGFRvIII | 0.6559 | |
| positive | 13 months | |
| negative | 13 months | |
| MGMT promoter | 0.0555 | |
| methylated | 13 months | |
| unmethylated | 12.5 months | |
| Proliferative index | 0.2804 | |
| ≥30% | 13 months | |
| <30% | 13 months | |
| VEGF | 0.8747 | |
| hyperexpressed | 13 months | |
| not hyperexpressed | 11 months |
| Parameter | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age | 0.564 | 0.449–0.709 | <0.0001 |
| Sex | 0.624 | 0.492–0.791 | <0.0001 |
| KPS | 0.45 | 0.348–0.581 | <0.0001 |
| Extent of resection | 1.970 | 1.543–2.514 | <0.0001 |
| EGFRvIII | 0.813 | 0.650–1.016 | 0.0687 |
| MGMT | 0.941 | 0.748–1.184 | 0.6038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauretti, L.; Cenci, T.; Montano, N.; Offi, M.; Giordano, M.; Caccavella, V.M.; Mangraviti, A.; Agostini, L.; Olivi, A.; Gabriele, L.; et al. Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis. J. Pers. Med. 2022, 12, 685. https://doi.org/10.3390/jpm12050685
Lauretti L, Cenci T, Montano N, Offi M, Giordano M, Caccavella VM, Mangraviti A, Agostini L, Olivi A, Gabriele L, et al. Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis. Journal of Personalized Medicine. 2022; 12(5):685. https://doi.org/10.3390/jpm12050685
Chicago/Turabian StyleLauretti, Liverana, Tonia Cenci, Nicola Montano, Martina Offi, Martina Giordano, Valerio M. Caccavella, Antonella Mangraviti, Ludovico Agostini, Alessandro Olivi, Lucia Gabriele, and et al. 2022. "Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis" Journal of Personalized Medicine 12, no. 5: 685. https://doi.org/10.3390/jpm12050685
APA StyleLauretti, L., Cenci, T., Montano, N., Offi, M., Giordano, M., Caccavella, V. M., Mangraviti, A., Agostini, L., Olivi, A., Gabriele, L., Larocca, L. M., Pallini, R., Martini, M., & D’Alessandris, Q. G. (2022). Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis. Journal of Personalized Medicine, 12(5), 685. https://doi.org/10.3390/jpm12050685











