Association between Gout and Dyslipidemia: A Nested Case–Control Study Using a National Health Screening Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Gout
2.3. Definition of Dyslipidemia
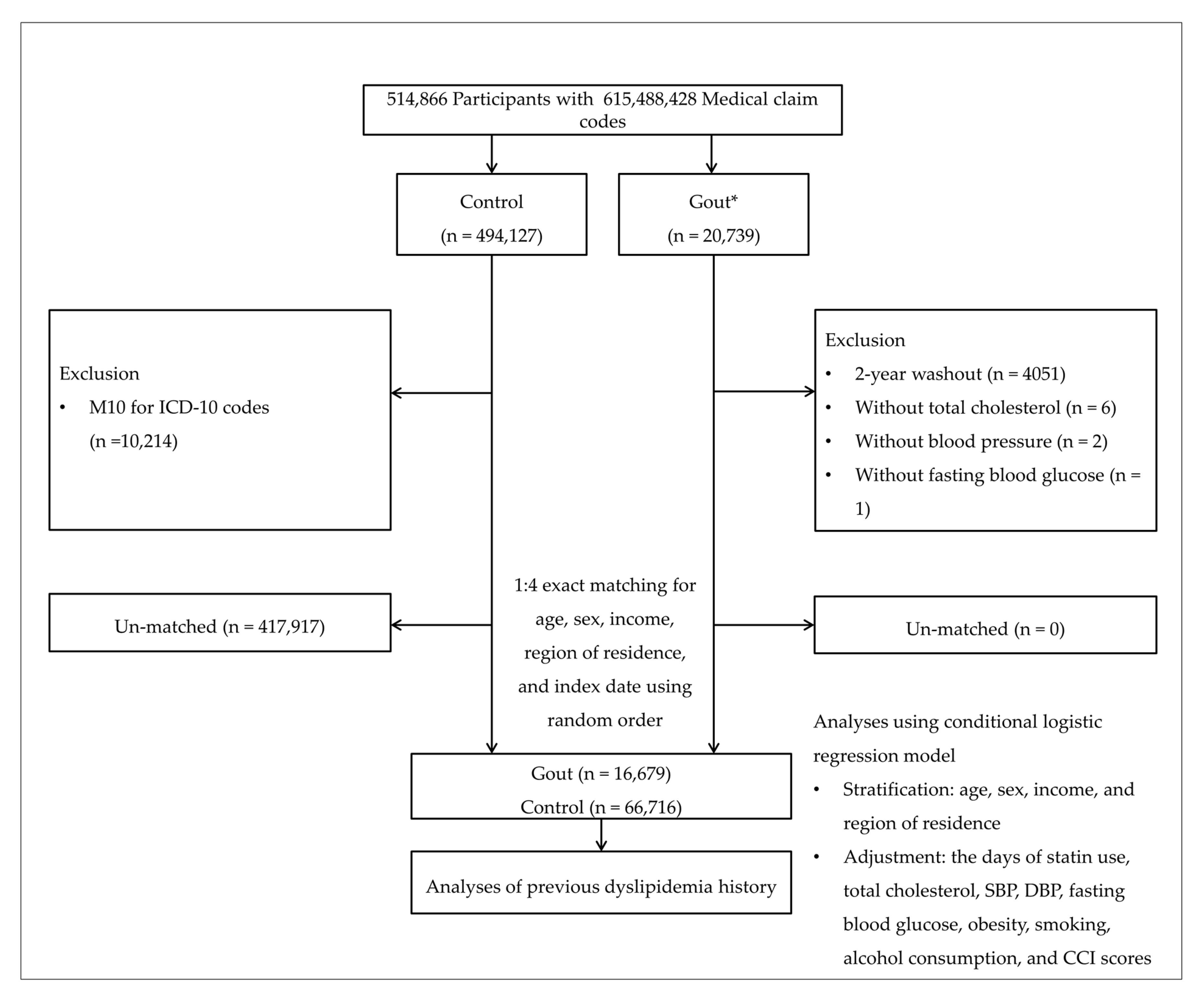
2.4. Participant Selection
2.5. Covariates
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73–77. [CrossRef]
- Carroll, M.D.; Fryar, C.D.; Nguyen, D.T. Total and High-density Lipoprotein Cholesterol in Adults: United States, 2015–2016. NCHS Data Brief 2017, 290, 1–8. [Google Scholar]
- Cho, S.M.J.; Lee, H.; Lee, H.H.; Baek, J.; Heo, J.E.; Joo, H.J.; Hong, S.J.; Kim, H.C. Dyslipidemia Fact Sheets in Korea 2020: An Analysis of Nationwide Population-based Data. J. Lipid Atheroscler. 2021, 10, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; McEvoy, J.W.; Khan, S.U.; Wood, D.A.; Graham, I.M.; Blumenthal, R.S.; Mishra, A.K.; Michos, E.D. Comparison of Transatlantic Approaches to Lipid Management: The AHA/ACC/Multisociety Guidelines vs. the ESC/EAS Guidelines. Mayo Clin. Proc. 2020, 95, 998–1014. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Dai, X.; Zhou, B.; Su, Y.; Xie, Z.; Liu, D. Association between Lipid Profiles and Serum Urate: A Cross-Sectional Study in Southwestern China. Int. J. Endocrinol. 2021, 2021, 2741131. [Google Scholar] [CrossRef]
- Chen, S.; Yang, H.; Chen, Y.; Wang, J.; Xu, L.; Miao, M.; Xu, C. Association between serum uric acid levels and dyslipidemia in Chinese adults: A cross-sectional study and further meta-analysis. Medicine 2020, 99, e19088. [Google Scholar] [CrossRef]
- Son, M.; Seo, J.; Yang, S. Association between dyslipidemia and serum uric acid levels in Korean adults: Korea National Health and Nutrition Examination Survey 2016–2017. PLoS ONE 2020, 15, e0228684. [Google Scholar] [CrossRef]
- Ali, N.; Rahman, S.; Islam, S.; Haque, T.; Molla, N.H.; Sumon, A.H.; Kathak, R.R.; Asaduzzaman, M.; Islam, F.; Mohanto, N.C.; et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc. Disord. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwabara, M.; Borghi, C.; Cicero, A.F.G.; Hisatome, I.; Niwa, K.; Ohno, M.; Johnson, R.J.; Lanaspa, M.A. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int. J. Cardiol. 2018, 261, 183–188. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Wang, C.C.; Kao, T.W.; Chan, J.Y.; Yang, Y.H.; Chang, Y.W.; Chen, W.L. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res. Int. 2015, 2015, 127596. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, H.; Ma, Q.; Zhou, X.; Xu, W.; Huang, L.; Hu, J.; Zhang, Y. Associations of non-high density lipoprotein cholesterol and traditional blood lipid profiles with hyperuricemia among middle-aged and elderly Chinese people: A community-based cross-sectional study. Lipids Health Dis. 2014, 13, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Kwak, S.G.; Lee, H.; Kim, S.K.; Choe, J.Y.; Park, S.H. Prevalence and incidence of gout in Korea: Data from the national health claims database 2007–2015. Rheumatol. Int. 2017, 37, 1499–1506. [Google Scholar] [CrossRef]
- Kwon, J.W.; Ha, J.W.; Lee, T.S.; Moon, S.H.; Lee, H.M.; Park, Y. Comparison of the Prevalence of Low Back Pain and Related Spinal Diseases among Smokers and Nonsmokers: Using Korean National Health Insurance Database. Clin. Orthop. Surg. 2020, 12, 200–208. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Yoo, D.M.; Chang, J.; Lee, H.J.; Park, B.; Choi, H.G. Hearing Impairment Increases Economic Inequality. Clin. Exp. Otorhinolaryngol. 2021, 14, 278–286. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for the Western Pacific. In The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Oputa, T.J.; Dupley, L.; Bourne, J.T. One Hundred Twenty-Day Mortality Rates for Hip Fracture Patients with COVID-19 Infection. Clin. Orthop. Surg. 2021, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Hughes, K.; Flynn, T.J.; Merriman, T.R. Mendelian randomization provides no evidence for a causal role of serum urate in increasing serum triglyceride levels. Circ. Cardiovasc. Genet. 2014, 7, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Montagnana, M.; Luca Salvagno, G.; Targher, G.; Cesare Guidi, G. Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin. Cardiol. 2010, 33, E76–E80. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chen, C.L.; Shen, M.L.; Kamatani, N. Trends in the manifestations of gout in Taiwan. Rheumatology 2003, 42, 1529–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Song, G.G.; Ji, J.D.; Lee, Y.H.; Kim, J.H.; Seo, Y.H.; Choi, S.J. Metabolic syndrome: Prevalence and risk factors in Korean gout patients. Korean J. Intern. Med. 2018, 33, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Comorbidities in patients with gout prior to and following diagnosis: Case-control study. Ann. Rheum. Dis. 2016, 75, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.J.; Wu, T.Y.; Lin, C.L.; Su, C.Y.; Li, J.R.; Chung, Y.L.; Tien, N.; Lim, Y.P. Effects of Urate-Lowering Therapy on Risk of Hyperlipidemia in Gout by a Population-Based Cohort Study and on In Vitro Hepatic Lipogenesis-Related Gene Expression. Mediat. Inflamm. 2020, 2020, 8890300. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am. J. Med. 2012, 125, 679–687.e671. [Google Scholar] [CrossRef]
- Thompson, M.D.; Wu, Y.Y.; Cooney, R.V.; Wilkens, L.R.; Haiman, C.A.; Pirkle, C.M. Modifiable Factors and Incident Gout across Ethnicity within a Large Multiethnic Cohort of Older Adults. J. Rheumatol. 2022, 1, 394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Zhang, Y.; Zeng, C. Recent advances in fructose intake and risk of hyperuricemia. Biomed. Pharmacother. 2020, 131, 110795. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.C.; Yang, W.H.; Haldeman, J.M.; Grenier-Larouche, T.; An, J.; Lapworth, A.L.; Astapova, I.; et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018, 27, 1281–1293.e1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, K.B.; Bae, S. Patterns of statin utilisation for new users and market dynamics in South Korea: A 13-year retrospective cohort study. BMJ Open 2019, 9, e026603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, E.; Cho, S.; Kim, D.J.; Choi, J.; Han, E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 2020, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Yada, T.; Nakata, M.; Shiraishi, T.; Kakei, M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br. J. Pharmacol. 1999, 126, 1205–1213. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Sarno, G.; Gastaldelli, A.; Savastano, S.; Ascione, A.; Colao, A.; Orio, F. The good and bad effects of statins on insulin sensitivity and secretion. Endocr. Res. 2014, 39, 137–143. [Google Scholar] [CrossRef]
- Kopprasch, S.; Srirangan, D.; Bergmann, S.; Graessler, J.; Schwarz, P.E.; Bornstein, S.R. Association between systemic oxidative stress and insulin resistance/sensitivity indices–the PREDIAS study. Clin. Endocrinol. 2016, 84, 48–54. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Reiner, Ž.; Simental-Mendía, L.E.; Sahebkar, A. Impact of Statin Therapy on Plasma Uric Acid Concentrations: A Systematic Review and Meta-Analysis. Drugs 2016, 76, 947–956. [Google Scholar] [CrossRef]
- Hill, M.F.; Bordoni, B. Hyperlipidemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, Japan, 2021. [Google Scholar]
| Characteristics | Total Participants | ||
|---|---|---|---|
| Gout | Control | p-Value | |
| Age (years old, n, %) | 1.000 | ||
| 40–44 | 355 (2.1) | 1420 (2.1) | |
| 45–49 | 1743 (10.5) | 6972 (10.5) | |
| 50–54 | 3171 (19.0) | 12,684 (19.0) | |
| 55–59 | 3141 (18.8) | 12,564 (18.8) | |
| 60–64 | 2623 (15.7) | 10,492 (15.7) | |
| 65–69 | 2320 (13.9) | 9280 (13.9) | |
| 70–74 | 1772 (10.6) | 7088 (10.6) | |
| 75–79 | 1061 (6.4) | 4244 (6.4) | |
| 80–84 | 403 (2.4) | 1612 (2.4) | |
| 85+ | 90 (0.5) | 360 (0.5) | |
| Sex (n, %) | 1.000 | ||
| Male | 13,278 (79.6) | 53,112 (79.6) | |
| Female | 3401 (20.4) | 13,604 (20.4) | |
| Income (n, %) | 1.000 | ||
| 1 (lowest) | 2354 (14.1) | 9416 (14.1) | |
| 2 | 2084 (12.5) | 8336 (12.5) | |
| 3 | 2551 (15.3) | 10,204 (15.3) | |
| 4 | 3509 (21.0) | 14,036 (21.0) | |
| 5 (highest) | 6181 (37.1) | 24,724 (37.1) | |
| Region of residence (n, %) | 1.000 | ||
| Urban | 7091 (42.5) | 28,364 (42.5) | |
| Rural | 9588 (57.5) | 38,352 (57.5) | |
| Total cholesterol (mg/dL, mean, SD) | 199.8 (40.2) | 196.5 (37.7) | <0.001 † |
| SBP (mmHg) | 129.7 (17.1) | 127.4 (16.4) | <0.001 † |
| DBP (mmHg) | 80.5 (11.1) | 79.1 (10.7) | <0.001 † |
| Fasting blood glucose (mg/dL) | 101.7 (28.1) | 102.1 (31.7) | 0.118 |
| Obesity (n, %) ‡ | <0.001 * | ||
| Underweight | 218 (1.3) | 1667 (2.5) | |
| Normal | 4237 (25.4) | 23,280 (34.9) | |
| Overweight | 4585 (27.5) | 18,681 (28.0) | |
| Obesity grade I | 6948 (41.7) | 21,485 (32.2) | |
| Obesity grade II | 691 (4.1) | 1603 (2.4) | |
| Smoking status (n, %) | <0.001 * | ||
| Non-smoker | 9545 (57.2) | 37,431 (56.1) | |
| Past smoker | 3376 (20.2) | 12,330 (18.5) | |
| Current smoker | 3758 (22.5) | 16,955 (25.4) | |
| Alcohol consumption (n, %) | <0.001 * | ||
| <1 time a week | 8665 (52.0) | 37,811 (56.7) | |
| ≥1 time a week | 8014 (48.1) | 28,905 (43.3) | |
| CCI score (score, n, %) | <0.001 * | ||
| 0 | 10,486 (62.9) | 45,574 (68.3) | |
| 1 | 2578 (15.5) | 9111 (13.7) | |
| 2 | 1558 (9.3) | 5289 (7.9) | |
| 3 | 854 (5.1) | 2873 (4.3) | |
| ≥ 4 | 1203 (7.2) | 3869 (5.8) | |
| The days of statin use (day, mean, SD) | 79.4 (195.5) | 57.0 (169.7) | <0.001 † |
| Dyslipidemia (n, %) | 5523 (33.1) | 16,034 (24.0) | <0.001 * |
| Characteristics | Odds Ratios for Gout | |||||
|---|---|---|---|---|---|---|
| Crude † | p-Value | Partial †,‡ | p-Value | Full †,§ | p-Value | |
| Total participants (n = 83,395) | ||||||
| Dyslipidemia | 1.59 (1.54–1.66) | <0.001 * | 1.50 (1.44–1.57) | <0.001 * | 1.43 (1.37–1.49) | <0.001 * |
| Non-dyslipidemia | 1 | 1 | 1 | |||
| Age < 60 years, men (n = 34,370) | ||||||
| Dyslipidemia | 1.74 (1.64–1.85) | <0.001 * | 1.62 (1.51–1.74) | <0.001 * | 1.51 (1.41–1.63) | <0.001 * |
| Non-dyslipidemia | 1 | 1 | 1 | |||
| Age < 60 years, women (n = 7680) | ||||||
| Dyslipidemia | 1.56 (1.37–1.78) | <0.001 * | 1.49 (1.28–1.74) | <0.001 * | 1.47 (1.26–1.71) | <0.001 * |
| Non-dyslipidemia | 1 | 1 | 1 | |||
| Age ≥ 60 years, men (n = 32,020) | ||||||
| Dyslipidemia | 1.54 (1.46–1.64) | <0.001 * | 1.49 (1.40–1.60) | <0.001 * | 1.42 (1.32–1.51) | <0.001 * |
| Non-dyslipidemia | 1 | 1 | 1 | |||
| Age ≥ 60 years old, women (n = 9325) | ||||||
| Dyslipidemia | 1.41 (1.27–1.56) | <0.001 * | 1.25 (1.11–1.40) | <0.001 * | 1.23 (1.09–1.39) | 0.001 |
| Non-dyslipidemia | 1 | 1 | 1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.G.; Kwon, B.-C.; Kwon, M.J.; Kim, J.H.; Kim, J.-H.; Park, B.; Lee, J.W. Association between Gout and Dyslipidemia: A Nested Case–Control Study Using a National Health Screening Cohort. J. Pers. Med. 2022, 12, 605. https://doi.org/10.3390/jpm12040605
Choi HG, Kwon B-C, Kwon MJ, Kim JH, Kim J-H, Park B, Lee JW. Association between Gout and Dyslipidemia: A Nested Case–Control Study Using a National Health Screening Cohort. Journal of Personalized Medicine. 2022; 12(4):605. https://doi.org/10.3390/jpm12040605
Chicago/Turabian StyleChoi, Hyo Geun, Bong-Cheol Kwon, Mi Jung Kwon, Ji Hee Kim, Joo-Hee Kim, Bumjung Park, and Jung Woo Lee. 2022. "Association between Gout and Dyslipidemia: A Nested Case–Control Study Using a National Health Screening Cohort" Journal of Personalized Medicine 12, no. 4: 605. https://doi.org/10.3390/jpm12040605
APA StyleChoi, H. G., Kwon, B.-C., Kwon, M. J., Kim, J. H., Kim, J.-H., Park, B., & Lee, J. W. (2022). Association between Gout and Dyslipidemia: A Nested Case–Control Study Using a National Health Screening Cohort. Journal of Personalized Medicine, 12(4), 605. https://doi.org/10.3390/jpm12040605






