Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis
Abstract
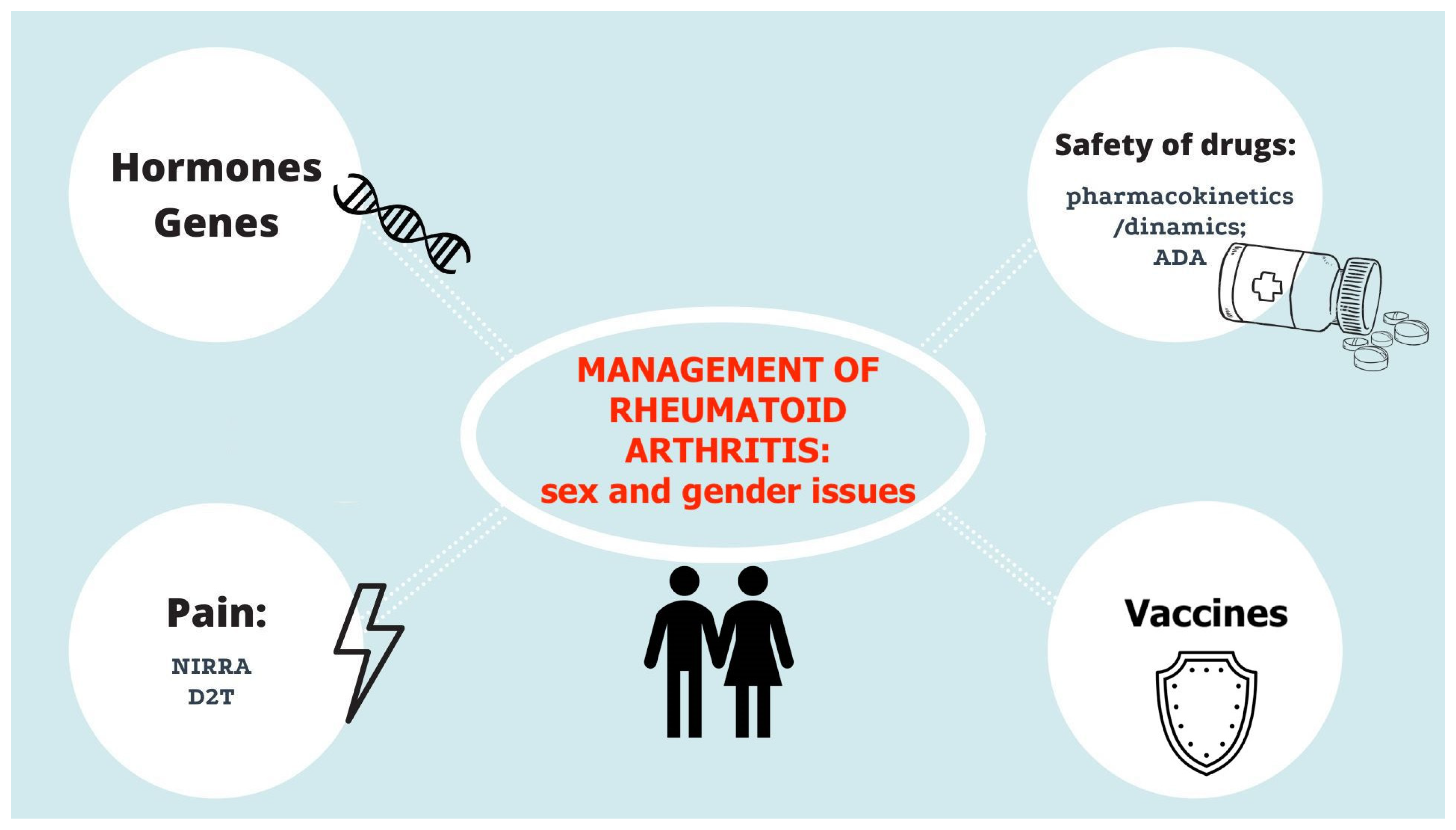
:1. Introduction
2. Rheumatoid Arthritis Recommendations: Sex/Gender Issues
3. Safety of Drugs
4. Early Rheumatoid Arthritis and Erosive Disease
5. Sex Interaction and Pro-Inflammatory Immune Pathways
6. Treat-to-Target Approach: The Importance of Disease Activity Assessment
7. Vaccination
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Unger, R. Toward a redefinition of sex and gender. Am. Psychol. 1979, 34, 1085–1094. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Chia, F.; Dans, L.; Harrison, A.; Hsieh, T.Y.; Jain, R.; Jung, S.M.; Kishimoto, M.; Kumar, A.; Leong, K.P.; et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Silvagni, E.; Sakellariou, G.; Bortoluzzi, A.; Giollo, A.; Ughi, N.; Vultaggio, L.; Scire, C.A. One year in review 2021: Novelties in the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2021, 39, 705–720. [Google Scholar]
- Romao, V.C.; Fonseca, J.E. Major Challenges in Rheumatology: Will We Ever Treat Smarter, Instead of Just Harder? Front. Med. 2019, 6, 144. [Google Scholar] [CrossRef]
- Hughes, G.C.; Choubey, D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 2014, 10, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, W.J.; Olsen, N.J. Sexual dimorphism of RA manifestations: Genes, hormones and behavior. Nat. Rev. Rheumatol. 2011, 7, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Cattalini, M.; Soliani, M.; Caparello, M.C.; Cimaz, R. Sex Differences in Pediatric Rheumatology. Clin. Rev. Allergy Immunol. 2019, 56, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Amin, R.M.; Thorne, J.E. The role of gender in juvenile idiopathic arthritis-associated uveitis. J. Ophthalmol. 2014, 2014, 461078. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, C.R.; Ahn, Y.Y.; Smith, E.; Macaluso, B.; Lariviere, V. Factors affecting sex-related reporting in medical research: A cross-disciplinary bibliometric analysis. Lancet 2019, 393, 550–559. [Google Scholar] [CrossRef] [Green Version]
- NIH Policy on the Inclusion of Women and Minority Groups in Research. Available online: https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable (accessed on 8 January 2022).
- International Council for Harmonisation Guidance. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-genderconsiderations-conduct-clinicaltrials-step-5_en.pdf (accessed on 8 January 2022).
- SAGER Guidelines. Available online: https://ease.org.uk/communities/gender-policycommittee/the-sagerguidelines/ (accessed on 8 January 2022).
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.; Symmons, D.P.; McCarey, D.; Dijkmans, B.A.; Nicola, P.; Kvien, T.K.; McInnes, I.B.; Haentzschel, H.; Gonzalez-Gay, M.A.; Provan, S.; et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis. 2010, 69, 325–331. [Google Scholar] [CrossRef] [Green Version]
- van der Goes, M.C.; Jacobs, J.W.; Boers, M.; Andrews, T.; Blom-Bakkers, M.A.; Buttgereit, F.; Caeyers, N.; Cutolo, M.; Da Silva, J.A.; Guillevin, L.; et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann. Rheum. Dis. 2010, 69, 1913–1919. [Google Scholar] [CrossRef]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharm. 2009, 48, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Jeong, S.; Jung, E.; Kim, K.S.; Jeon, I.; Lee, Y.; Cho, J.Y.; Oh, W.Y.; Chung, J.Y. Effect of CYP3A4 metabolism on sex differences in the pharmacokinetics and pharmacodynamics of zolpidem. Sci. Rep. 2021, 11, 19150. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Bejan-Angoulvant, T.; Passot, C.; Mulleman, D.; Paintaud, G. Clinical Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies Approved to Treat Rheumatoid Arthritis. Clin. Pharm. 2015, 54, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Ducourau, E.; Fuzibet, P.; Vignault, C.; Watier, H.; Lequerre, T.; Le Loet, X.; Vittecoq, O.; Goupille, P.; Mulleman, D.; et al. Pharmacokinetics and concentration-effect relationship of adalimumab in rheumatoid arthritis. Br. J. Clin. Pharmacol. 2015, 79, 286–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.M.; Bruno, R.; Combs, D.; Davies, B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J. Clin. Pharmacol. 2005, 45, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Moots, R.J.; Xavier, R.M.; Mok, C.C.; Rahman, M.U.; Tsai, W.C.; Al-Maini, M.H.; Pavelka, K.; Mahgoub, E.; Kotak, S.; Korth-Bradley, J.; et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study. PLoS ONE 2017, 12, e0175207. [Google Scholar]
- Quistrebert, J.; Hassler, S.; Bachelet, D.; Mbogning, C.; Musters, A.; Tak, P.P.; Wijbrandts, C.A.; Herenius, M.; Bergstra, S.A.; Akdemir, G.; et al. Incidence and risk factors for adalimumab and infliximab anti-drug antibodies in rheumatoid arthritis: A European retrospective multicohort analysis. Semin. Arthritis Rheum. 2019, 48, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K.; Geborek, P.; Svenson, M.; Larsson, L.; Kapetanovic, M.C.; Saxne, T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006, 54, 3782–3789. [Google Scholar] [CrossRef]
- Korswagen, L.A.; Bartelds, G.M.; Krieckaert, C.L.; Turkstra, F.; Nurmohamed, M.T.; van Schaardenburg, D.; Wijbrandts, C.A.; Tak, P.P.; Lems, W.F.; Dijkmans, B.A.; et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: A case series and cohort study. Arthritis Rheum. 2011, 63, 877–883. [Google Scholar] [CrossRef]
- Pascual-Salcedo, D.; Plasencia, C.; Ramiro, S.; Nuno, L.; Bonilla, G.; Nagore, D.; Ruiz Del Agua, A.; Martinez, A.; Aarden, L.; Martin-Mola, E.; et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology 2011, 50, 1445–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambardzumyan, K.; Hermanrud, C.; Marits, P.; Vivar, N.; Ernestam, S.; Wallman, J.K.; van Vollenhoven, R.F.; Fogdell-Hahn, A.; Saevarsdottir, S.; SWEFOT study group. Association of female sex and positive rheumatoid factor with low serum infliximab and anti-drug antibodies, related to treatment failure in early rheumatoid arthritis: Results from the SWEFOT trial population. Scand. J. Rheumatol. 2019, 48, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehab, M. Relationship Between Patient Sex and Serum Tumor Necrosis Factor Antagonist Drug and Anti-drug Antibody Concentrations in Inflammatory Bowel Disease; A Nationwide Cohort Study. Front. Med. 2021, 2753. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.E.; Karlsson, J.A.; Englund, M.; Petersson, I.F.; Saxne, T.; Geborek, P. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: An observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res. 2010, 62, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex Differ. 2020, 11, 24. [Google Scholar] [CrossRef]
- Souto, A.; Maneiro, J.R.; Gomez-Reino, J.J. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: A systematic review and meta-analysis of drug registries and health care databases. Rheumatology 2016, 55, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, F.R. Gender does not influence clinical response to JAK inhibitors in rheumatoid arthritis: An italian multicentre analysis. Ann. Rheum. Dis. 2020, 79, 1016–1017. [Google Scholar] [CrossRef]
- Jin, Y.; Desai, R.J.; Liu, J.; Choi, N.K.; Kim, S.C. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, M.E.; Silvagni, E.; Carrara, G.; Zanetti, A.; Govoni, M.; Scire, C.A.; Bortoluzzi, A. Role of comorbidities on therapeutic persistence of biological agents in rheumatoid arthritis: Results from the RECord-linkage On Rheumatic Disease study on administrative healthcare databases. Scand. J. Rheumatol. 2021, 50, 333–342. [Google Scholar] [CrossRef]
- Sokka, T.; Toloza, S.; Cutolo, M.; Kautiainen, H.; Makinen, H.; Gogus, F.; Skakic, V.; Badsha, H.; Peets, T.; Baranauskaite, A.; et al. Women, men, and rheumatoid arthritis: Analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res. Ther. 2009, 11, R7. [Google Scholar]
- Millett, C.E.; Phillips, B.E.; Saunders, E.F.H. The Sex-specific Effects of LPS on Depressive-like Behavior and Oxidative Stress in the Hippocampus of the Mouse. Neuroscience 2019, 399, 77–88. [Google Scholar] [CrossRef]
- Redeker, I.; Albrecht, K.; Kekow, J.; Burmester, G.R.; Braun, J.; Schafer, M.; Zink, A.; Strangfeld, A. Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: Data from the German RABBIT register. Ann. Rheum. Dis. 2022, 81, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Parhar, K.; Huang, B.; Vadlamudi, N. Risk Factors for Herpes Zoster Infection: A Meta-Analysis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020; Volume 7, p. ofaa005. [Google Scholar]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Diez-Domingo, J.; Orrico-Sanchez, A. Herpes zoster risk and burden of disease in immunocompromised populations: A population-based study using health system integrated databases, 2009–2014. BMC Infect. Dis. 2020, 20, 905. [Google Scholar] [CrossRef] [PubMed]
- Semb, A.G.; Ikdahl, E.; Wibetoe, G.; Crowson, C.; Rollefstad, S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat. Rev. Rheumatol. 2020, 16, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Targońska-Stępniak, B. Gender Differences in Cardiovascular Risk Profile in Rheumatoid Arthritis Patients with Low Disease Activity. Biomed. Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrich, D.C.; van de Wetering, E.H.M.; Rennings, A.J.; Arts, E.E.; Meek, I.L.; den Broeder, A.A.; Fransen, J.; Popa, C.D. Younger age and female gender are determinants of underestimated cardiovascular risk in rheumatoid arthritis patients: A prospective cohort study. Arthritis Res. Ther. 2021, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Bove, R. Autoimmune diseases and reproductive aging. Clin. Immunol. 2013, 149, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argnani, L.; Zanetti, A.; Carrara, G.; Silvagni, E.; Guerrini, G.; Zambon, A.; Scire, C.A. Rheumatoid Arthritis and Cardiovascular Risk: Retrospective Matched-Cohort Analysis Based on the RECORD Study of the Italian Society for Rheumatology. Front. Med. 2021, 8, 745601. [Google Scholar] [CrossRef]
- Kim, S.C.; Schneeweiss, S.; Liu, J.; Solomon, D.H. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res. 2013, 65, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Mansour, R.; Azrielant, S.; Watad, A.; Tiosano, S.; Yavne, Y.; Comaneshter, D.; Cohen, A.D.; Amital, H. Venous thromboembolism events among RA patients. Mediterr. J. Rheumatol. 2019, 30, 38–43. [Google Scholar] [CrossRef]
- Coffey, C.M.; Davis, J.M., III; Crowson, C.S. The impact of gender on time to rheumatoid arthritis classification: A retrospective analysis of a population-based cohort. Rheumatol. Int. 2019, 39, 2025–2030. [Google Scholar] [CrossRef]
- Jawaheer, D.; Olsen, J.; Hetland, M.L. Sex differences in response to anti-tumor necrosis factor therapy in early and established rheumatoid arthritis -- results from the DANBIO registry. J. Rheumatol. 2012, 39, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.C.; Cordero, M.; Dyer, A.; Chang, R.W. Current tumor necrosis factor-alpha inhibitor use is associated with a higher probability of remissions in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1662–1665. [Google Scholar] [PubMed]
- Makinen, H.; Hannonen, P.; Sokka, T. Definitions of remission for rheumatoid arthritis and review of selected clinical cohorts and randomised clinical trials for the rate of remission. Clin. Exp. Rheumatol. 2006, 24 (Suppl. 43), S22. [Google Scholar]
- Forslind, K.; Hafstrom, I.; Ahlmen, M.; Svensson, B.; Group, B.S. Sex: A major predictor of remission in early rheumatoid arthritis? Ann. Rheum. Dis. 2007, 66, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, M.L.; Forslind, K.; Hafstrom, I. Comparing Five Year Out-Come in Two Cohorts of Patients with Early Rheumatoid Arthritis—A BARFOT Study. Open Rheumatol. J. 2015, 9, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyand, C.M.; Schmidt, D.; Wagner, U.; Goronzy, J.J. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998, 41, 817–822. [Google Scholar] [CrossRef]
- Wolfe, F.; Sharp, J.T. Radiographic outcome of recent-onset rheumatoid arthritis: A 19-year study of radiographic progression. Arthritis Rheum. 1998, 41, 1571–1582. [Google Scholar] [CrossRef]
- Jawaheer, D.; Maranian, P.; Park, G.; Lahiff, M.; Amjadi, S.S.; Paulus, H.E. Disease progression and treatment responses in a prospective DMARD-naive seropositive early rheumatoid arthritis cohort: Does gender matter? J. Rheumatol. 2010, 37, 2475–2485. [Google Scholar] [CrossRef] [Green Version]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Laffont, S.; Rouquie, N.; Azar, P.; Seillet, C.; Plumas, J.; Aspord, C.; Guery, J.C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J. Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef] [Green Version]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quinones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilev, G.; Manolova, I.; Ivanova, M.; Stanilov, I.; Miteva, L.; Stanilova, S. The role of IL-18 in addition to Th17 cytokines in rheumatoid arthritis development and treatment in women. Sci. Rep. 2021, 11, 15391. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, U.; Kahlfuss, S.; Yang, J.; Ivanova, E.; Koralov, S.B.; Feske, S. Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metab. 2019, 29, 1104–1118.e6. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M.; Hendrey, M.R.; Malley, J.D.; Learch, T.J.; Davis, J.C., Jr.; Reveille, J.D.; Weisman, M.H. Clinical and immunogenetic prognostic factors for radiographic severity in ankylosing spondylitis. Arthritis Rheum. 2009, 61, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traish, A.; Bolanos, J.; Nair, S.; Saad, F.; Morgentaler, A. Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J. Clin. Med. 2018, 7, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutolo, M.; Straub, R.H.; Bijlsma, J.W. Neuroendocrine-immune interactions in synovitis. Nat. Clin. Pr. Rheumatol. 2007, 3, 627–634. [Google Scholar] [CrossRef]
- Krishnan, E.; Tugwell, P.; Fries, J.F. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI). Arthritis Res. Ther. 2004, 6, R505–R513. [Google Scholar] [CrossRef] [Green Version]
- Hakkinen, A.; Kautiainen, H.; Hannonen, P.; Ylinen, J.; Makinen, H.; Sokka, T. Muscle strength, pain, and disease activity explain individual subdimensions of the Health Assessment Questionnaire disability index, especially in women with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, T.; Miwa, Y.; Nishimi, A.; Nishimi, S.; Saito, M.; Oguro, N.; Miura, Y.; Ishii, S.; Takahashi, R.; Kasama, T.; et al. Sex Differences in the Effects of a Biological Drug for Rheumatoid Arthritis on Depressive State. Open Rheumatol. J. 2015, 9, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Schrepf, A.; Kaplan, C.M.; Ichesco, E.; Larkin, T.; Harte, S.E.; Harris, R.E.; Murray, A.D.; Waiter, G.D.; Clauw, D.J.; Basu, N. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat. Commun. 2018, 9, 2243. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Affaitati, G.; Ceccarelli, I.; Fiorenzani, P.; Lerza, R.; Rossi, C.; Pace, M.C.; Chiefari, M.; Aurilio, C.; Giamberardino, M.A. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur. J. Pain 2010, 14, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Dahan, A.; Kest, B.; Zacny, J.; Stijnen, T.; Aarts, L.; Sarton, E. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 2010, 151, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Gazerani, P.; Aloisi, A.M.; Ueda, H. Editorial: Differences in Pain Biology, Perception, and Coping Strategies: Towards Sex and Gender Specific Treatments. Front. Neurosci. 2021, 15, 697285. [Google Scholar] [CrossRef]
- Keogh, E. The gender context of pain. Health Psychol. Rev. 2021, 15, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sonvg, X.; Ji, P.; Wang, Y.; Maynard, J.; Yim, S.; Sahajwalla, C.; Xu, M.; Kim, M.J.; Zhao, L. Impact of Sex on Clinical Response in Rheumatoid Arthritis Patients Treated With Biologics at Approved Dosing Regimens. J. Clin. Pharmacol. 2020, 60 (Suppl. 2), S103–S109. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Chimenti, M.S.; Tonelli, M.; Sunzini, F.; Kroegler, B.; Perricone, R. Factors Predicting 2 Years of Remission and Low Disease Activity in Rheumatoid Arthritis Patients Treated with TNF-inhibitors. Isr. Med. Assoc. J. 2017, 19, 467–472. [Google Scholar]
- Yu, C.; Jin, S.; Wang, Y.; Jiang, N.; Wu, C.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: A systematic review and meta-analysis. Clin. Rheumatol. 2019, 38, 727–738. [Google Scholar] [CrossRef]
- Couderc, M.; Gottenberg, J.E.; Mariette, X.; Pereira, B.; Bardin, T.; Cantagrel, A.; Combe, B.; Dougados, M.; Flipo, R.M.; Le Loet, X.; et al. Influence of gender on response to rituximab in patients with rheumatoid arthritis: Results from the Autoimmunity and Rituximab registry. Rheumatology 2014, 53, 1788–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nourisson, C.; Soubrier, M.; Mulliez, A.; Baillet, A.; Bardin, T.; Cantagrel, A.; Combe, B.; Dougados, M.; Flipo, R.M.; Schaeverbeke, T.; et al. Impact of gender on the response and tolerance to abatacept in patients with rheumatoid arthritis: Results from the ‘ORA’ registry. RMD Open 2017, 3, e000515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, P.D.H.; Pauling, J.D.; McHugh, N.; Shaddick, G.; Hyrich, K.; Group, B.-R.C. Predictors, demographics and frequency of sustained remission and low disease activity in anti-tumour necrosis factor-treated rheumatoid arthritis patients. Rheumatology 2019, 58, 2162–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstra, S.A.; Allaart, C.F.; Ramiro, S.; Chopra, A.; Govind, N.; Silva, C.; Murphy, E.A.; Landewe, R.B.M. Sex-associated Treatment Differences and Their Outcomes in Rheumatoid Arthritis: Results from the METEOR Register. J. Rheumatol. 2018, 45, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.; Kedves, M.; Hamar, A.; van der Goes, M.C.; Kent, A.; Bakkers, M.; Blaas, E.; Senolt, L.; et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Fathi, A.; Addo, M.M.; Dahlke, C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front. Immunol. 2020, 11, 601170. [Google Scholar] [CrossRef]
- Fink, A.L.; Engle, K.; Ursin, R.L.; Tang, W.Y.; Klein, S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12477–12482. [Google Scholar] [CrossRef] [Green Version]
- Fehervari, Z. Vaccine sex differences. Nat. Immunol. 2019, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F. Sex differences in injection site reactions with human vaccines. Hum. Vaccines 2009, 5, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L.; Klein, S.L. Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W. EULAR December 2020 View points on SARS-CoV-2 vaccination in patients with RMDs. Ann. Rheum. Dis. 2021, 80, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Womens Health 2021, 2, 761511. [Google Scholar] [CrossRef] [PubMed]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in Efficacy in COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-14 December 2020–18 January 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Iguchi, T.; Umeda, H.; Kojima, M.; Kanno, Y.; Tanaka, Y.; Kinoshita, N.; Sato, D. Cumulative Adverse Event Reporting of Anaphylaxis After mRNA COVID-19 Vaccine (Pfizer-BioNTech) Injections in Japan: The First-Month Report. Drug Saf. 2021, 44, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; MacLure, K.; Elkout, H.; Stewart, D. Thrombotic Adverse Events Reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 Vaccines: Comparison of Occurrence and Clinical Outcomes in the EudraVigilance Database. Vaccines 2021, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Terracina, K.A.; Tan, F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e469–e470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. https://doi.org/10.3390/jpm12030499
Maranini B, Bortoluzzi A, Silvagni E, Govoni M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. Journal of Personalized Medicine. 2022; 12(3):499. https://doi.org/10.3390/jpm12030499
Chicago/Turabian StyleMaranini, Beatrice, Alessandra Bortoluzzi, Ettore Silvagni, and Marcello Govoni. 2022. "Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis" Journal of Personalized Medicine 12, no. 3: 499. https://doi.org/10.3390/jpm12030499
APA StyleMaranini, B., Bortoluzzi, A., Silvagni, E., & Govoni, M. (2022). Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. Journal of Personalized Medicine, 12(3), 499. https://doi.org/10.3390/jpm12030499





