Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Technique
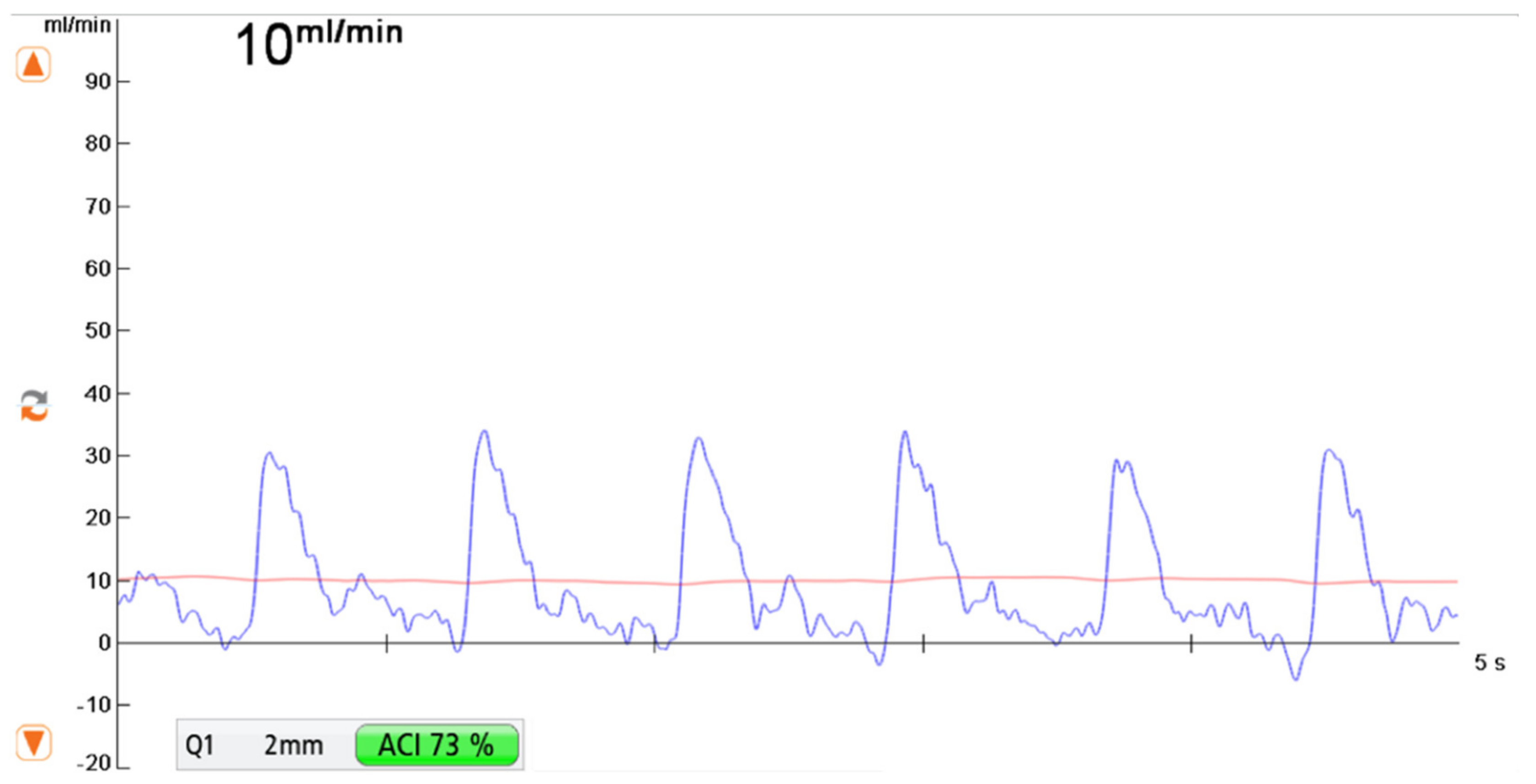
2.2. Transit-Time Flow Measurement (TTFM)
- -
- Measurement F was taken after flap elevation and isolation on its vascular pedicle prior to free flap transfer
- -
- Measurement R was performed at the recipient vessel prior to anastomosis.
- -
- Measurement AA was taken at the vascular pedicle after anastomosis and flap reperfusion.
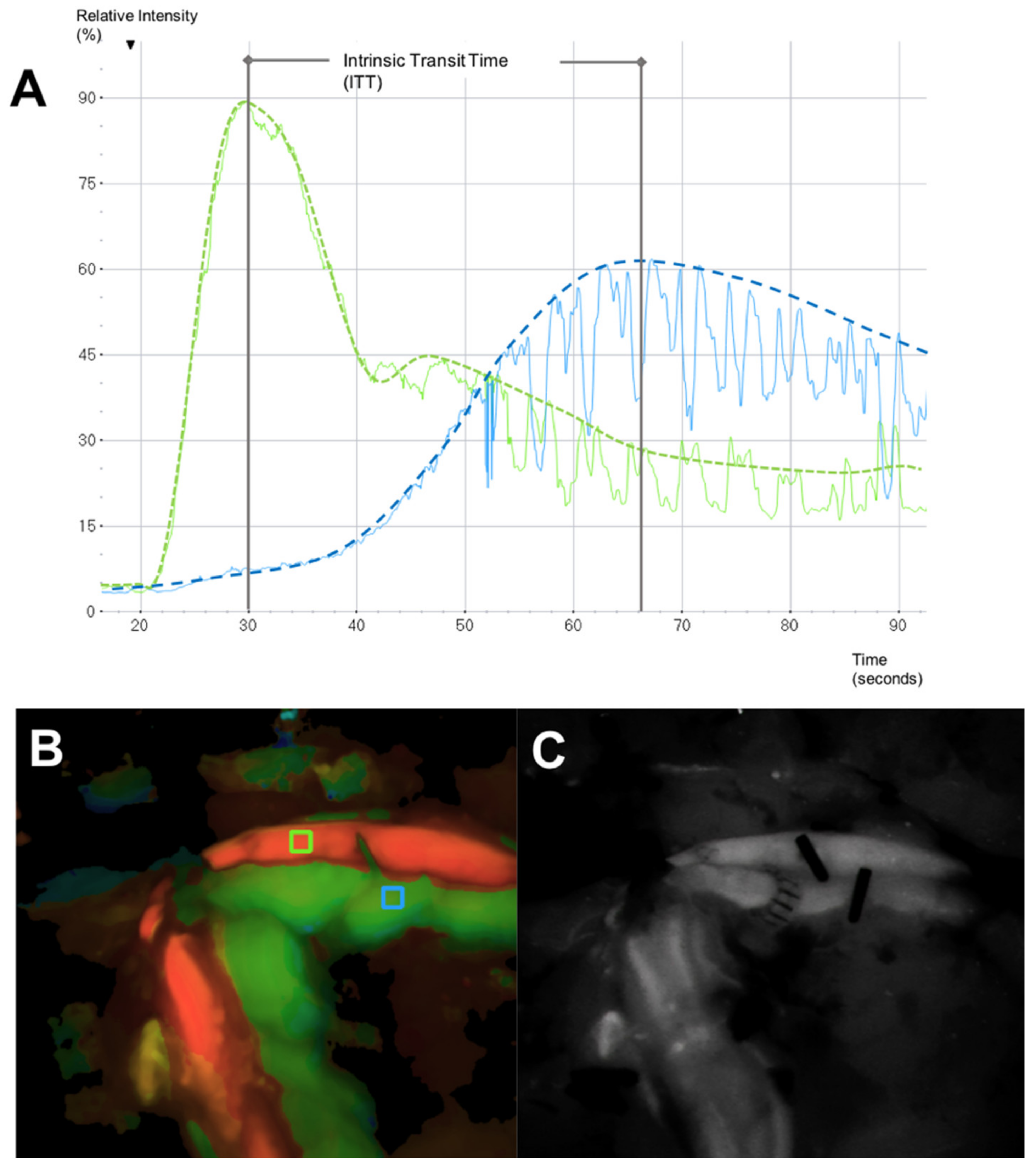
2.3. Microvascular Indocyanine Green Angiography (mICG-A)
2.4. Vascular Resistance
2.5. Data Analysis and Synthesis
3. Results
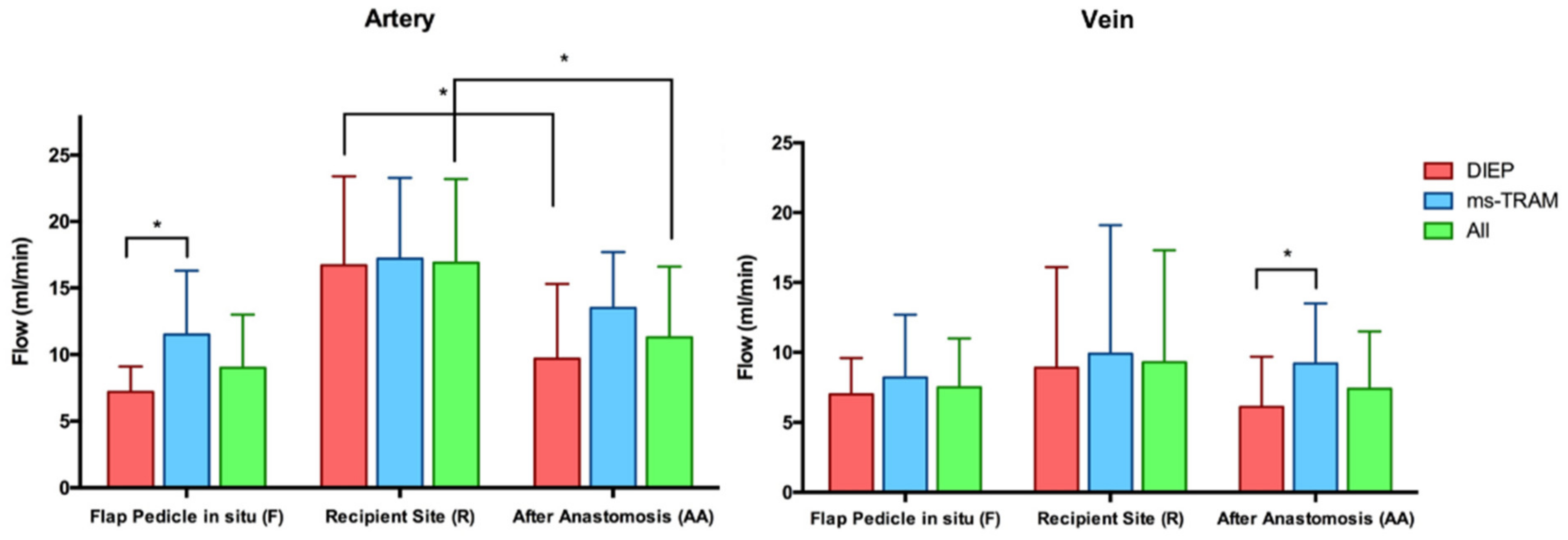
3.1. Blood Flow Volume (mL/min)
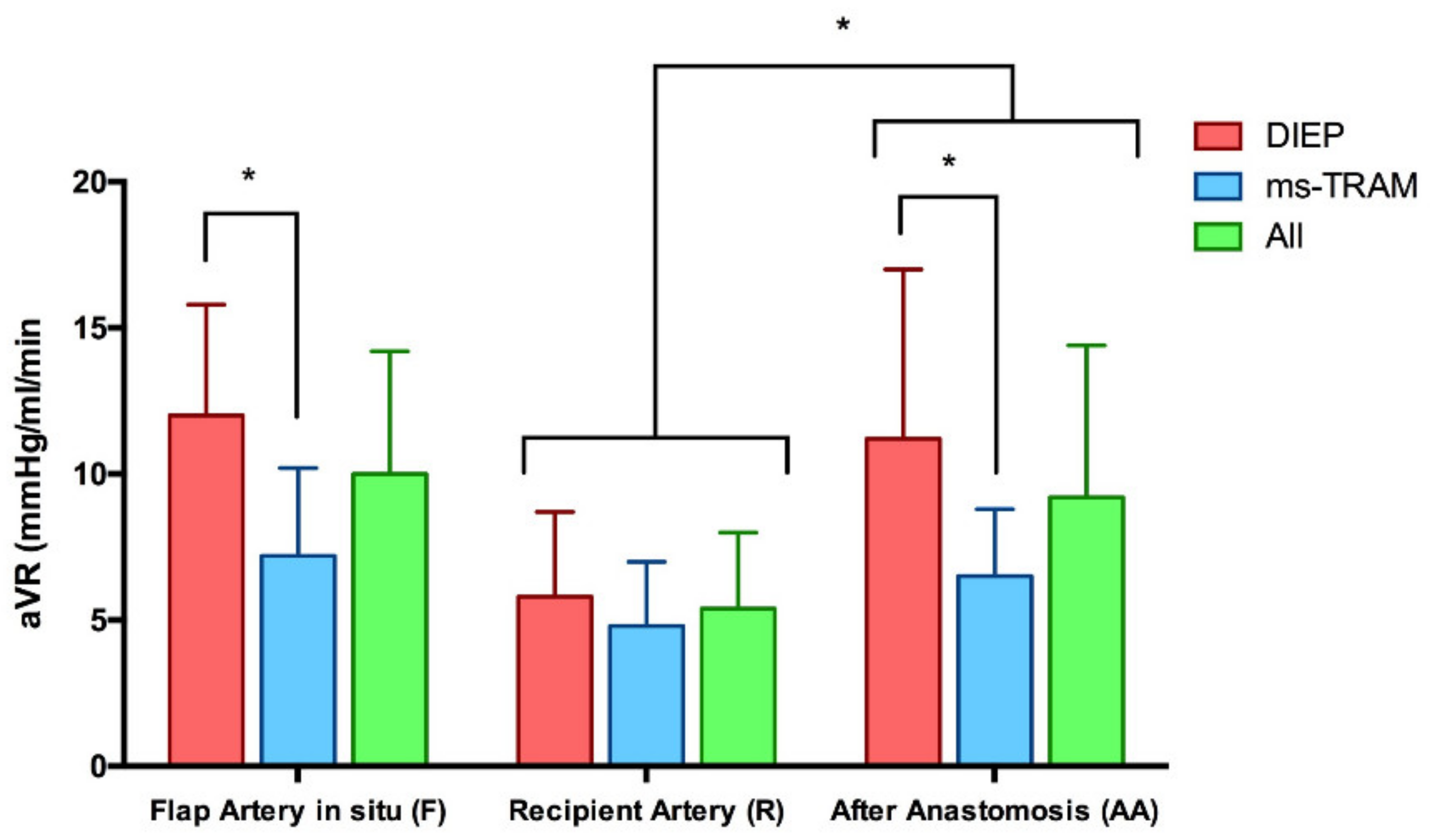
3.2. Vascular Resistance (mmHg/mL/min)
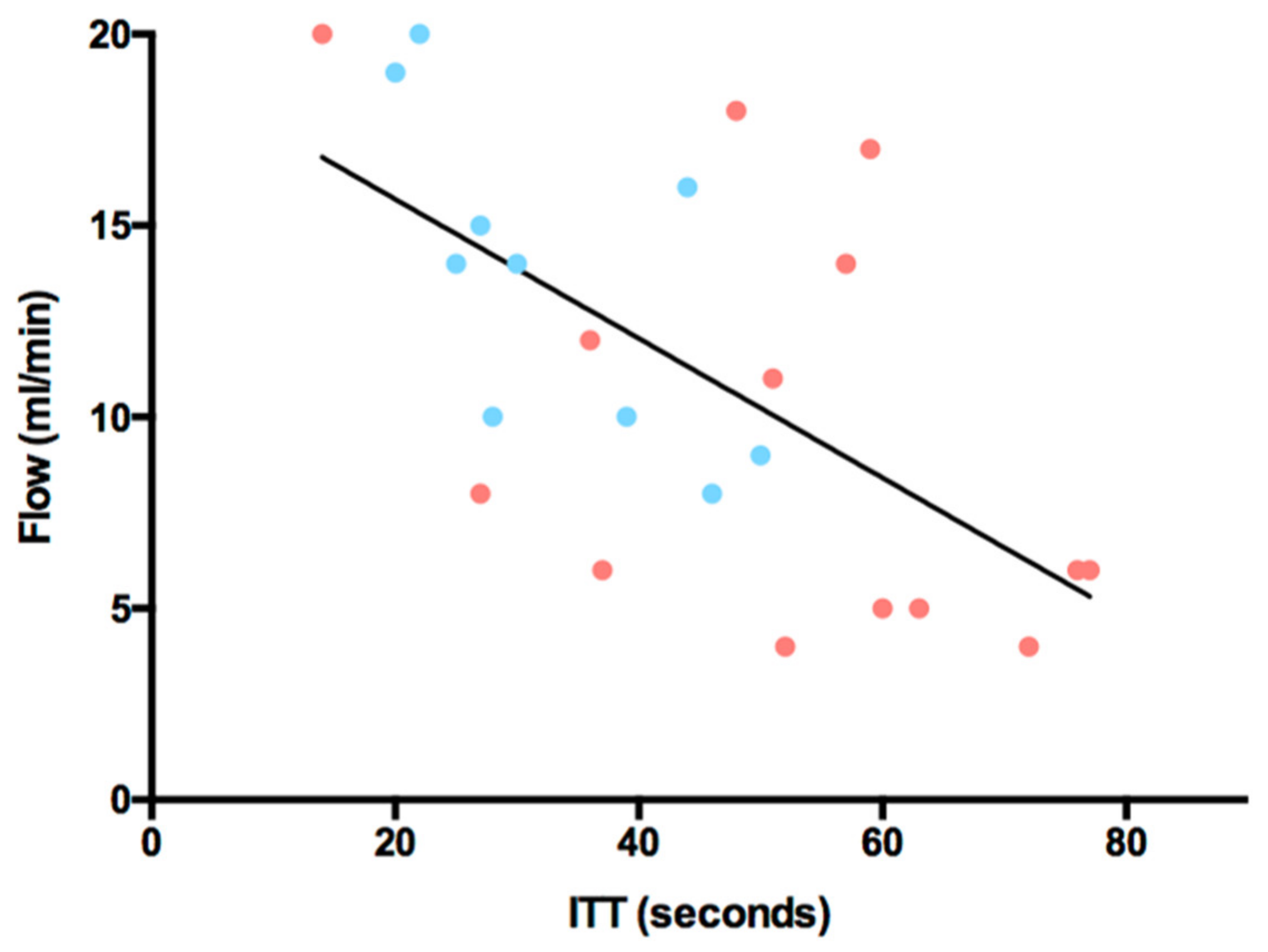
3.3. Intrinsic Transit Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenhardt, S.U.; Momeni, A.; von Fritschen, U.; Horch, R.E.; Stark, G.B.; Bannasch, H.; Harder, Y.; Heitmann, C.; Kremer, T.; Rieger, U.M.; et al. Breast reconstruction with the free TRAM or DIEP flap—What is the current standard? Consensus Statement of the German Speaking Working Group for Microsurgery of the Peripheral Nerves and Vessels. Handchirurgie Mikrochirurgie Plastische Chirurgie 2018, 50, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Cai, A.; Suckau, J.; Arkudas, A.; Beier, J.P.; Momeni, A.; Horch, R.E. Autologous Breast Reconstruction with Transverse Rectus Abdominis Musculocutaneous (TRAM) or Deep Inferior Epigastric Perforator (DIEP) Flaps: An Analysis of the 100 Most Cited Articles. Med. Sci. Monit. 2019, 25, 3520–3536. [Google Scholar] [CrossRef] [PubMed]
- Fritschen, U.V.; Grill, B.; Wagner, J.; Schuster, H.; Sukhova, I.; Giunta, R.E.; Heitmann, C.; Andree, C.; Horch, R.E.; Kneser, U.; et al. Quality assurance in breast reconstruction—Establishment of a prospective national online registry for microsurgical breast reconstructions. Handchirurgie Mikrochirurgie Plastische Chirurgie 2020, 52, 58–66. [Google Scholar] [CrossRef]
- Kengelbach-Weigand, A.; Tasbihi, K.; Strissel, P.L.; Schmid, R.; Marques, J.M.; Beier, J.P.; Beckmann, M.W.; Strick, R.; Horch, R.E.; Boos, A.M. Plasticity of patient-matched normal mammary epithelial cells is dependent on autologous adipose-derived stem cells. Sci. Rep. 2019, 9, 10722. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Mou, S.; Xiong, L.; Wang, Z.; Wang, R.; Weigand, A.; Yuan, Q.; Horch, R.E.; Sun, J.; Yang, J. Adipose-derived mesenchymal stem cells formed acinar-like structure when stimulated with breast epithelial cells in three-dimensional culture. PLoS ONE 2018, 13, e0204077. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Lee, S.; Kim, J. Meta-analysis of flap perfusion and donor site complications for breast reconstruction using pedicled versus free TRAM and DIEP flaps. Breast 2018, 38, 45–51. [Google Scholar] [CrossRef]
- Beier, J.P.; Horch, R.E.; Arkudas, A.; Dragu, A.; Schmitz, M.; Kneser, U. Decision-making in DIEP and ms-TRAM flaps: The potential role for a combined laser Doppler spectrophotometry system. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2013, 66, 73–79. [Google Scholar] [CrossRef]
- Wechselberger, G.; Rumer, A.; Schoeller, T.; Schwabegger, A.; Ninkovic, M.; Anderl, H. Free-flap monitoring with tissue-oxygen measurement. J. Reconstr. Microsurg. 1997, 13, 125–130. [Google Scholar] [CrossRef]
- Machens, H.G.; Mailänder, P.; Pasel, J.; Lutz, B.S.; Funke, M.; Siemers, F.; Berger, A.C. Flap perfusion after free musculocutaneous tissue transfer: The impact of postoperative complications. Plast. Reconstr. Surg. 2000, 105, 2395–2399. [Google Scholar] [CrossRef]
- Udesen, A.; Løntoft, E.; Kristensen, S.R. Monitoring of free TRAM flaps with microdialysis. J. Reconstr. Microsurg. 2000, 16, 101–106. [Google Scholar] [CrossRef]
- Ludolph, I.; Arkudas, A.; Schmitz, M.; Boos, A.M.; Taeger, C.D.; Rother, U.; Horch, R.E.; Beier, J.P. Cracking the perfusion code?: Laser-assisted Indocyanine Green angiography and combined laser Doppler spectrophotometry for intraoperative evaluation of tissue perfusion in autologous breast reconstruction with DIEP or ms-TRAM flaps. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2016, 69, 1382–1388. [Google Scholar] [CrossRef]
- Ludolph, I.; Horch, R.E.; Arkudas, A.; Schmitz, M. Enhancing Safety in Reconstructive Microsurgery Using Intraoperative Indocyanine Green Angiography. Front. Surg. 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Yuen, J.C.; Feng, Z. Monitoring free flaps using the laser Doppler flowmeter: Five-year experience. Plast. Reconstr. Surg. 2000, 105, 55–61. [Google Scholar] [CrossRef]
- Salmi, A.M.; Tierala, E.K.; Tukiainen, E.J.; Asko-Seljavaara, S.L. Blood flow in free muscle flaps measured by color Doppler ultrasonography. Microsurgery 1995, 16, 666–672. [Google Scholar] [CrossRef]
- Figus, A.; Mosahebi, A.; Ramakrishnan, V. Microcirculation in DIEP flaps: A study of the haemodynamics using laser Doppler flowmetry and lightguide reflectance spectrophotometry. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2006, 59, 604–612, discussion 613. [Google Scholar] [CrossRef]
- Heitland, A.S.; Markowicz, M.; Koellensperger, E.; Schoth, F.; Feller, A.M.; Pallua, N. Duplex ultrasound imaging in free transverse rectus abdominis muscle, deep inferior epigastric artery perforator, and superior gluteal artery perforator flaps: Early and long-term comparison of perfusion changes in free flaps following breast reconstruction. Ann. Plast. Surg. 2005, 55, 117–121. [Google Scholar] [CrossRef]
- Sekido, M.; Yamamoto, Y.; Sugihara, T. Arterial blood flow changes after free tissue transfer in head and neck reconstruction. Plast. Reconstr. Surg. 2005, 115, 1547–1552. [Google Scholar] [CrossRef]
- Nasir, S.; Baykal, B.; Altuntas, S.; Aydin, M.A. Hemodynamic differences in blood flow between free skin and muscles flaps: Prospective study. J. Reconstr. Microsurg. 2009, 25, 355–360. [Google Scholar] [CrossRef]
- Malagon, P.; Carrasco, C.; Vila, J.; Priego, D.; Higueras, C. Intraoperative hemodynamic changes in the arterial blood flow measured by transit time flowmetry (TTFM) during breast reconstruction with free diep flap. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2020, 73, 1775–1784. [Google Scholar] [CrossRef]
- Groom, R.; Tryzelaar, J.; Forest, R.; Niimi, K.; Cecere, G.; Donegan, D.; Katz, S.; Weldner, P.; Quinn, R.; Braxton, J.; et al. Intra-operative quality assessment of coronary artery bypass grafts. Perfusion 2001, 16, 511–518. [Google Scholar] [CrossRef]
- D’Ancona, G.; Karamanoukian, H.L.; Ricci, M.; Schmid, S.; Bergsland, J.; Salerno, T.A. Graft revision after transit time flow measurement in off-pump coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2000, 17, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Albäck, A.; Mäkisalo, H.; Nordin, A.; Lepäntalo, M. Validity and reproducibility of transit time flowmetry. Ann. Chir. Gynaecol. 1996, 85, 325–331. [Google Scholar]
- Laustsen, J.; Pedersen, E.M.; Terp, K.; Steinbrüchel, D.; Kure, H.H.; Paulsen, P.K.; Jørgensen, H.; Paaske, W.P. Validation of a new transit time ultrasound flowmeter in man. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 1996, 12, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Beldi, G.; Bosshard, A.; Hess, O.M.; Althaus, U.; Walpoth, B.H. Transit time flow measurement: Experimental validation and comparison of three different systems. Ann. Thorac. Surg. 2000, 70, 212–217. [Google Scholar] [CrossRef]
- Holm, C.; Mayr, M.; Höfter, E.; Dornseifer, U.; Ninkovic, M. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: A preliminary study. Microsurgery 2009, 29, 509–514. [Google Scholar] [CrossRef]
- Holzbach, T.; Artunian, N.; Spanholtz, T.A.; Volkmer, E.; Engelhardt, T.O.; Giunta, R.E. Microscope-integrated intraoperative indocyanine green angiography in plastic surgery. Handchirurgie Mikrochirurgie Plastische Chirurgie 2012, 44, 84–88. [Google Scholar] [CrossRef]
- Holm, C.; Dornseifer, U.; Sturtz, G.; Basso, G.; Schuster, T.; Ninkovic, M. The intrinsic transit time of free microvascular flaps: Clinical and prognostic implications. Microsurgery 2010, 30, 91–96. [Google Scholar] [CrossRef]
- Mahabir, R.C.; Williamson, J.S.; Carr, N.J.; Courtemanche, D.J. Vascular resistance in human muscle flaps. Ann. Plast. Surg. 2001, 47, 148–152. [Google Scholar] [CrossRef]
- Warren, J.V.; Gorlin, R. Calculation of vascular resistance. Methods Med. Res. 1958, 7, 98–99. [Google Scholar] [PubMed]
- Sasmor, M.T.; Reus, W.F.; Straker, D.J.; Colen, L.B. Vascular resistance considerations in free-tissue transfer. J. Reconstr. Microsurg. 1992, 8, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Takanari, K.; Kamei, Y.; Toriyama, K.; Yagi, S.; Torii, S. Differences in blood flow volume and vascular resistance between free flaps: Assessment in 58 cases. J. Reconstr. Microsurg. 2009, 25, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahabedian, M.Y.; Momen, B.; Galdino, G.; Manson, P.N. Breast Reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast. Reconstr. Surg. 2002, 110, 466–475, discussion 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, D.; Horch, R.E.; Ludolph, I.; Schmitz, M.; Beier, J.P.; Arkudas, A. Interdisciplinary Treatment of Breast Cancer After Mastectomy with Autologous Breast Reconstruction Using Abdominal Free Flaps in a University Teaching Hospital—A Standardized and Safe Procedure. Front. Oncol. 2020, 10, 177. [Google Scholar] [CrossRef]
- Hembd, A.S.; Yan, J.; Zhu, H.; Haddock, N.T.; Teotia, S.S. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast. Reconstr. Surg. 2020, 146, 1e–10e. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Tokumoto, H.; Kubota, Y.; Kuriyama, M.; Sasahara, Y.; Yamaji, Y.; Satoh, K. Regional Oxygen Saturation Index: A Novel Criterion for Free Flap Assessment Using Tissue Oximetry. Plast. Reconstr. Surg. 2016, 138, 510e–518e. [Google Scholar] [CrossRef]
- Rother, U.; Muller-Mohnssen, H.; Lang, W.; Ludolph, I.; Arkudas, A.; Horch, R.E.; Regus, S.; Meyer, A. Wound closure by means of free flap and arteriovenous loop: Development of flap autonomy in the long-term follow-up. Int. Wound J. 2020, 17, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Ritschl, L.M.; Schmidt, L.H.; Fichter, A.M.; Hapfelmeier, A.; Wolff, K.D.; Mucke, T. Multimodal analysis using flowmeter analysis, laser-Doppler spectrophotometry, and indocyanine green videoangiography for the detection of venous compromise in flaps in rats. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2018, 46, 905–915. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Suominen, S.; Tukiainen, E.; Kuokkanen, H.; Suominen, E.; Vuola, J.; Asko-Seljavaara, S. Evaluation of blood flow in free microvascular flaps. J. Reconstr. Microsurg. 2001, 17, 163–167. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Kuokkanen, H.; von Smitten, K.; Asko-Seljavaara, S. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann. Plast. Surg. 2001, 46, 590–593. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Giordano, S.; Tukiainen, E. Intraoperative hemodynamic evaluation of the latissimus dorsi muscle flap: A prospective study. J. Reconstr. Microsurg. 2012, 28, 273–278. [Google Scholar] [CrossRef]
- Selber, J.C.; Garvey, P.B.; Clemens, M.W.; Chang, E.I.; Zhang, H.; Hanasono, M.M. A prospective study of transit-time flow volume measurement for intraoperative evaluation and optimization of free flaps. Plast. Reconstr. Surg. 2013, 131, 270–281. [Google Scholar] [CrossRef]
- Preidl, R.H.; Schlittenbauer, T.; Weber, M.; Neukam, F.W.; Wehrhan, F. Assessment of free microvascular flap perfusion by intraoperative fluorescence angiography in craniomaxillofacial surgery. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2015, 43, 643–648. [Google Scholar] [CrossRef]
- Eguchi, T.; Kawaguchi, K.; Basugi, A.; Kanai, I.; Hamada, Y. Intraoperative real-time assessment of blood flow using indocyanine green angiography after anastomoses in free-flap reconstructions. Br. J. Oral Maxillofac. Surg. 2017, 55, 628–630. [Google Scholar] [CrossRef]
- Hitier, M.; Cracowski, J.L.; Hamou, C.; Righini, C.; Bettega, G. Indocyanine green fluorescence angiography for free flap monitoring: A pilot study. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2016, 44, 1833–1841. [Google Scholar] [CrossRef]
- Aschoff, L. Thrombosis. Lectures on Pathology; Paul, B., Ed.; Hoeber, Inc.: New York, NY, USA, 1924; pp. 253–278. [Google Scholar]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]

| Patient Number | Age | Type of Flap | Flap Weight | Ischemia Time | Intrinsic Transit Time | Hemodynamic Postoperative Complications |
|---|---|---|---|---|---|---|
| (Years) | (g) | (min) | (s) | |||
| 1 | 46 | DIEP | 459 | 70 | 37 | - |
| 2 | 55 | ms-TRAM | 340 | 49 | 46 | - |
| DIEP | 314 | 53 | 36 | - | ||
| 3 | 57 | DIEP | 1036 | 45 | 27 | - |
| 4 | 46 | ms-TRAM | 344 | 46 | 50 | - |
| 5 | 39 | ms-TRAM | 1169 | 35 | 22 | - |
| 6 | 62 | DIEP | 309 | 37 | 60 | - |
| 7 | 49 | DIEP | 370 | 56 | 14 | - |
| ms-TRAM | 310 | 64 | 25 | - | ||
| 8 | 47 | DIEP | 514 | 42 | 76 | - |
| 9 | 57 | ms-TRAM | 328 | 56 | 30 | - |
| 10 | 55 | DIEP | 591 | 54 | 59 | - |
| 11 | 51 | DIEP | 352 | 50 | 77 | Arterial Thrombosis |
| 12 | 55 | ms-TRAM | 464 | 42 | 28 | - |
| ms-TRAM | 517 | 32 | 27 | - | ||
| 13 | 44 | DIEP | 365 | 44 | 72 | - |
| 14 | 56 | DIEP | 310 | 48 | 48 | - |
| 15 | 49 | DIEP | 688 | 44 | 63 | - |
| 16 | 43 | DIEP | 299 | 41 | 51 | - |
| 17 | 64 | ms-TRAM | 691 | 41 | 20 | - |
| ms-TRAM | 810 | 40 | 44 | - | ||
| 18 | 68 | DIEP | 713 | 43 | 57 | - |
| 19 | 42 | ms-TRAM | 631 | 38 | 39 | - |
| 20 | 51 | DIEP | 410 | 42 | 52 | - |
| Flow in mL/in (Mean ± SD) | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flap Pedicle In Situ (F) | Recipient Vessel (R) | After Anastomosis (AA) | ||||||||||
| Type of Flap | No. of Flaps | Artery | Vein | Ratio A/V | Artery | Vein | Artery | Vein | Ratio A/V | Artery vs. Vein | F vs. AA | R vs. AA |
| All | 24 | 9 ± 4 | 7.5 ± 3.5 | 1.4 ± 0.7 | 16.9 ± 6.3 | 9.4 ± 8 | 11.3 ± 5.3 | 7.4 ± 4.1 | 1.8 ± 1.3 | 0.07 (F); 0.0001 (AA) | 0.1 (A); 0.9 (V) | 0.002 (A); 0.4 (V) |
| DIEP | 14 | 7.2 ± 1.9 | 7 ± 2.6 | 1.1 ± 0.3 | 16.7 ± 6.7 | 8.9 ± 7.2 | 9.7 ± 5.6 | 6.1 ± 3.6 | 1.9 ± 1.6 | 0.5 (F); 0.002 (AA) | 0.2 (A); 0.5 (V) | 0.01 (A); 0.2 (V) |
| ms-TRAM | 10 | 11.5 ± 4.8 | 8.2 ± 4.5 | 1.8 ± 1 | 17.2 ± 6.1 | 9.9 ± 9.2 | 13.5 ± 4.2 | 9.2 ± 4.3 | 1.7 ± 0.8 | 0.04 (F); 0.02 (AA) | 0.1 (A); 0.5 (V) | 0.1 (A); 0.9 (V) |
| p-value (DIEP vs. ms-TRAM) | 0.02 | 0.5 | 0.06 | 0.85 | 0.96 | 0.07 | 0.04 | 0.8 | ||||
| Arterial Vascular Resistance (aVR) in mmHg/mL/min (Mean ± SD) | p-Value | ||||
|---|---|---|---|---|---|
| Type of Flap | Flap Artery (FA) | Recipient Artery (RA) | After Anastomosis (AA) | FA vs. AA | RA vs. AA |
| All | 10 ± 4.2 | 5.4 ± 2.6 | 9.2 ± 5.2 | 0.5 | 0.0002 |
| DIEP | 12 ± 3.8 | 5.8 ± 2.9 | 11.2 ± 5.8 | 0.7 | 0.005 |
| ms-TRAM | 7.2 ± 3 | 4.8 ± 2.2 | 6.5 ± 2.3 | 0.5 | 0.02 |
| p-value (DIEP vs. ms-TRAM) | 0.004 | 0.4 | 0.02 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geierlehner, A.; Horch, R.E.; Ludolph, I.; Arkudas, A. Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography. J. Pers. Med. 2022, 12, 482. https://doi.org/10.3390/jpm12030482
Geierlehner A, Horch RE, Ludolph I, Arkudas A. Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography. Journal of Personalized Medicine. 2022; 12(3):482. https://doi.org/10.3390/jpm12030482
Chicago/Turabian StyleGeierlehner, Alexander, Raymund E. Horch, Ingo Ludolph, and Andreas Arkudas. 2022. "Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography" Journal of Personalized Medicine 12, no. 3: 482. https://doi.org/10.3390/jpm12030482
APA StyleGeierlehner, A., Horch, R. E., Ludolph, I., & Arkudas, A. (2022). Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography. Journal of Personalized Medicine, 12(3), 482. https://doi.org/10.3390/jpm12030482








