A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
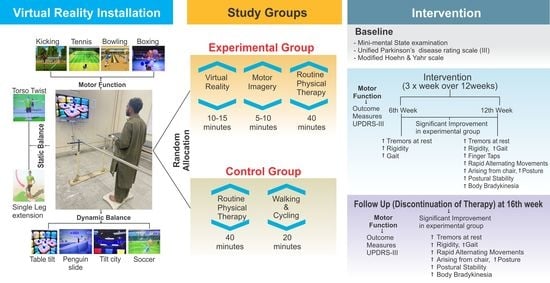
2.1. Study Design and Setting
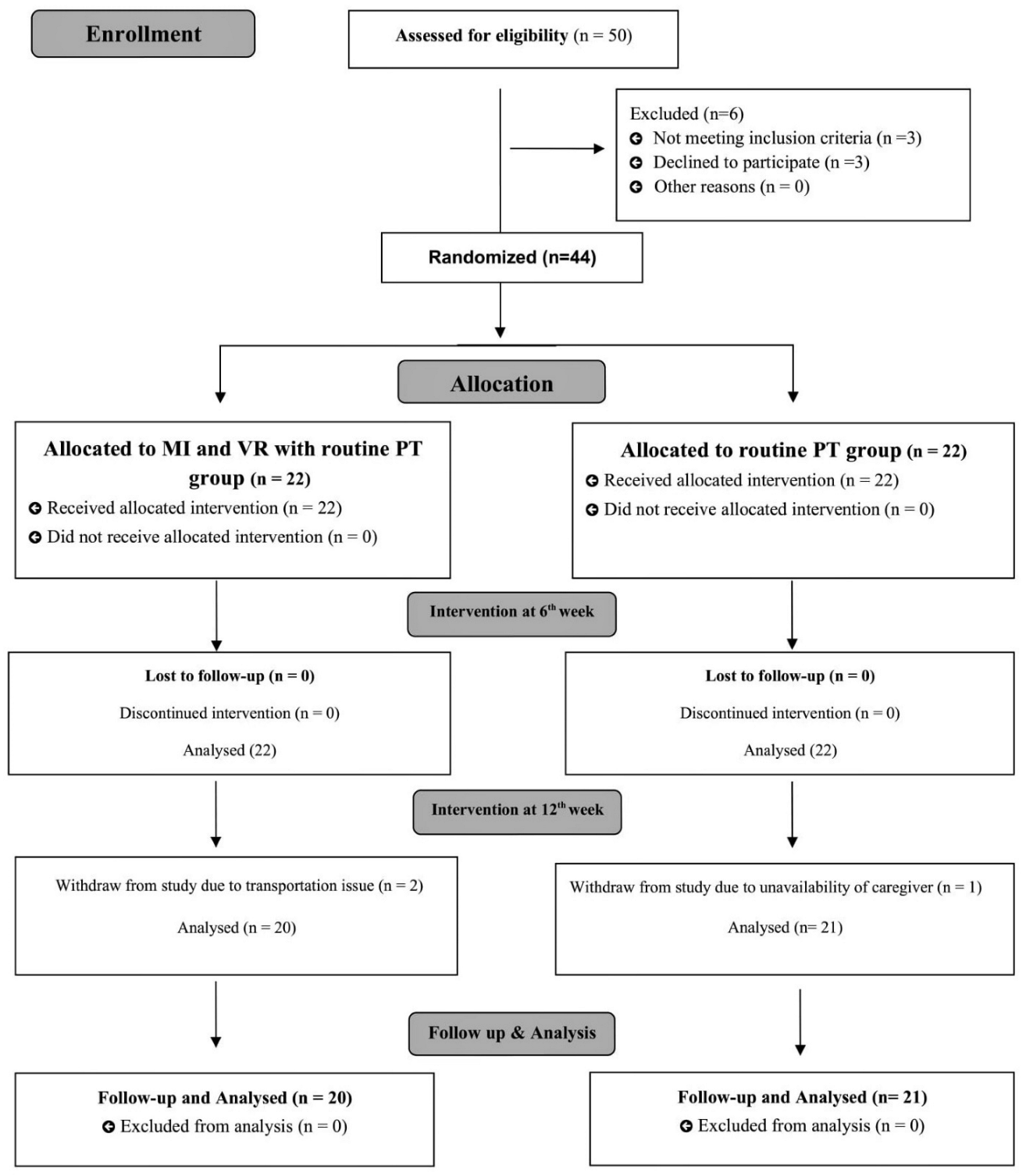
2.2. Study Participants
2.3. Sample Size Calculation
2.4. Groups and Intervention Procedures
2.5. Randomization and Blinding
2.6. Interventions
2.6.1. VR Rehabilitation Protocol
2.6.2. MI Rehabilitation Protocol
2.6.3. Routine PT Treatment
2.7. Outcome Measures
Assessment of Sub-Components of Motor Function
2.8. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Gillani, S.A.; Hanif, A.; Iram, H. Effects of Virtual Reality with Motor Imagery Techniques in Patients with Parkinson’s Disease: Study Protocol for a Randomized Controlled Trial. Neurodegener. Dis. 2020, 20, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Gilani, S.A.; Iram, H.; Farooq, M. Psychometric Properties of the Urdu Translation of Berg Balance Scale in People with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2022, 19, 2346. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.-K.; Chung, Y.-C.; Bellard, D.; Swan, L.; Gobreial, N.; Romano, A.; Glatt, R.; Bonaguidi, M.A.; Lee, D.J.; Jin, Y. Gamified Dual-Task Training for Individuals with Parkinson Disease: An Exploratory Study on Feasibility, Safety, and Efficacy. Int. J. Environ. Res. Public Health 2021, 18, 12384. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, V.C.B.; de Macêdo, J.G.F.; da Silva, L.P.; da Silva, I.B.; de Sales, M.D.G.W.; Coriolano, D.M. The role of rehabilitation with virtual reality in functional ability and quality of life of individuals with Parkinson’s disease. CEP 2017, 53140, 160. [Google Scholar] [CrossRef][Green Version]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Levac, D.E.; Sveistrup, H. Motor learning and virtual reality. In Virtual Reality for Physical and Motor Rehabilitation; Springer: Berlin/Heidelberg, Germany, 2014; pp. 25–46. [Google Scholar]
- Kashif, M.; Jones, S.; Haider Darain, H.I.; Raqib, A.; Butt, A.A. Factors influencing the community integration of patients following traumatic spinal cord injury: A systematic review. JPMA 2019, 69, 1337–1343. [Google Scholar]
- Cheng, Y.-C.; Su, C.-H. Evidence Supports PA Prescription for Parkinson’s Disease: Motor Symptoms and Non-Motor Features: A Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 2894. [Google Scholar] [CrossRef]
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2008, 23, 631–640. [Google Scholar] [CrossRef]
- Schootemeijer, S.; van der Kolk, N.M.; Ellis, T.; Mirelman, A.; Nieuwboer, A.; Nieuwhof, F.; Schwarzschild, M.A.; de Vries, N.M.; Bloem, B.R. Barriers and Motivators to Engage in Exercise for Persons with Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1293–1299. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Holden, M.K. Virtual environments for motor rehabilitation. Cyberpsychol. Behav. 2005, 8, 187–211. [Google Scholar] [CrossRef]
- Deutsch, J.E.; Borbely, M.; Filler, J.; Huhn, K.; Guarrera-Bowlby, P. Use of a low-cost, commercially available gaming console (Wii) for rehabilitation of an adolescent with cerebral palsy. Phys. Ther. 2008, 88, 1196–1207. [Google Scholar] [CrossRef]
- Molina, K.I.; Ricci, N.A.; de Moraes, S.A.; Perracini, M.R. Virtual reality using games for improving physical functioning in older adults: A systematic review. J. Neuroeng. Rehabil. 2014, 11, 156. [Google Scholar] [CrossRef]
- Cuthbert, J.P.; Staniszewski, K.; Hays, K.; Gerber, D.; Natale, A.; O’dell, D. Virtual reality-based therapy for the treatment of balance deficits in patients receiving inpatient rehabilitation for traumatic brain injury. Brain Inj. 2014, 28, 181–188. [Google Scholar] [CrossRef]
- Meldrum, D.; Herdman, S.; Vance, R.; Murray, D.; Malone, K.; Duffy, D.; Glennon, A.; McConn-Walsh, R. Effectiveness of conventional versus virtual reality–based balance exercises in vestibular rehabilitation for unilateral peripheral vestibular loss: Results of a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 1319–1328.e1. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Lee, D.-K.; Song, H.-S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef]
- Ravi, D.; Kumar, N.; Singhi, P. Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: An updated evidence-based systematic review. Physiotherapy 2017, 103, 245–258. [Google Scholar] [CrossRef]
- Shizgal, P.; Arvanitogiannis, A. Gambling on dopamine. Science 2003, 299, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.D.; Attree, E.A.; Brooks, B.M.; Parslow, D.M.; Penn, P.R. Training in virtual environments: Transfer to real world tasks and equivalence to real task training. Ergonomics 2000, 43, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Saiote, C.; Tacchino, A.; Brichetto, G.; Roccatagliata, L.; Bommarito, G.; Cordano, C.; Battaglia, M.; Mancardi, G.L.; Inglese, M. Resting-state functional connectivity and motor imagery brain activation. Hum. Brain Mapp. 2016, 37, 3847–3857. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J. Cogn. Neurosci. 1999, 11, 491–501. [Google Scholar] [CrossRef]
- Nicholson, V.; Watts, N.; Chani, Y.; Keogh, J.W. Motor imagery training improves balance and mobility outcomes in older adults: A systematic review. J. Physiother. 2019, 65, 200–207. [Google Scholar] [CrossRef]
- Timmermans, A.A.; Seelen, H.A.; Willmann, R.D.; Kingma, H. echnology-assisted training of arm-hand skills in stroke: Concepts on reacquisition of motor control and therapist guidelines for rehabilitation technology design. J. Neuroeng. Rehabil. 2009, 6, 1–18. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef]
- Mirelman, A.; Maidan, I.; Deutsch, J.E. Virtual reality and motor imagery: Promising tools for assessment and therapy in Parkinson’s disease. Mov. Disord. 2013, 28, 1597–1608. [Google Scholar] [CrossRef]
- Morris, M.E. Movement disorders in people with Parkinson disease: A model for physical therapy. Phys. Ther. 2000, 80, 578–597. [Google Scholar] [CrossRef]
- Keus, S.H.; Bloem, B.R.; Hendriks, E.J.; Bredero-Cohen, A.B.; Munneke, M.; Group, P.R.D. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov. Disord. 2007, 22, 451–460. [Google Scholar] [CrossRef]
- Descombes, S.; Bonnet, A.; Gasser, U.; Thalamas, C.; Dingemanse, J.; Arnulf, I.; Bareille, M.; Agid, Y.; Rascol, O. Dual-release formulation, a novel principle in L-dopa treatment of Parkinson’s disease. Neurology 2001, 56, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-Y.; Lin, K.-H.; Hu, M.-H.; Wu, R.-M.; Lu, T.-W.; Lin, C.-H. Effects of virtual reality–augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: A randomized controlled trial. Phys. Ther. 2011, 91, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.; Orawiec, R.; Duda-Kłodowska, D.; Opala, G. Assessment of postural instability in patients with Parkinson’s disease. Exp. Brain Res. 2007, 183, 107–114. [Google Scholar] [CrossRef]
- Yang, W.-C.; Wang, H.-K.; Wu, R.-M.; Lo, C.-S.; Lin, K.-H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: A randomized controlled trial. J. Formos. Med. Assoc. 2016, 115, 734–743. [Google Scholar] [CrossRef]
- Pompeu, J.E.; dos Santos Mendes, F.A.; da Silva, K.G.; Lobo, A.M.; de Paula Oliveira, T.; Zomignani, A.P.; Piemonte, M.E.P. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy 2012, 98, 196–204. [Google Scholar] [CrossRef]
- dos Santos Mendes, F.A.; Pompeu, J.E.; Lobo, A.M.; da Silva, K.G.; de Paula Oliveira, T.; Zomignani, A.P.; Piemonte, M.E.P. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease–effect of motor and cognitive demands of games: A longitudinal, controlled clinical study. Physiotherapy 2012, 98, 217–223. [Google Scholar] [CrossRef]
- Herz, N.B.; Mehta, S.H.; Sethi, K.D.; Jackson, P.; Hall, P.; Morgan, J.C. Nintendo Wii rehabilitation (“Wii-hab”) provides benefits in Parkinson’s disease. Parkinsonism Relat. Disord. 2013, 19, 1039–1042. [Google Scholar] [CrossRef]
- Cianci, H. Parkinson’s Disease: Fitness Counts; National Parkinson Foundation: New York, NY, USA, 2004. [Google Scholar]
- Martínez-Martín, P.; Gil-Nagel, A.; Gracia, L.M.; Gómez, J.B.; Martinez-Sarries, J.; Bermejo, F.; Group, C.M. Unified Parkinson’s disease rating scale characteristics and structure. Mov. Disord. 1994, 9, 76–83. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Goetz, C.G.; Lang, A.E.; Cubo, E. Factor analysis of the motor section of the unified Parkinson’s disease rating scale during the offstate. Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 585–589. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The unified Parkinson’s disease rating scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Gillani, S.A. The combined effects of virtual reality with motor imagery techniques in patients with Parkinson’s disease. Pak. J. Med. Assoc. 2022; aheadprint. [Google Scholar] [CrossRef]
- Lokhandwala, M.; Kulkarni, V.; Nair, M. Comparison of Combined Effects of Physical and Mental Practice with Physical Practice alone on Functional Independence in Parkinson (PD) Patients. Int. J. Physiother. Res. 2019, 7, 3207–3213. [Google Scholar] [CrossRef]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; García-Sancho, C.; Sanmartín, G.; Flores, J.; Cudeiro, J.; Arias, P. Effects of movement imitation training in Parkinson’s disease: A virtual reality pilot study. Parkinsonism Relat. Disord. 2016, 26, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Avanzino, L.; Marchese, R.; Pelosin, E. Action observation and motor imagery: Innovative cognitive tools in the rehabilitation of Parkinson’s disease. Parkinsons Dis. 2015, 2015, 124214. [Google Scholar] [CrossRef]
- Cornacchioli, J.; Galambos, A.; Rentouli, S.; Canciello, R.; Marongiu, R.; Cabrera, D.; Njie, E.G. Virtual Reality Tremor Reduction in Parkinson’s Disease. Preprints 2020, 2020020452. [Google Scholar] [CrossRef][Green Version]
- Helmich, R.C.; Bloem, B.R.; Toni, I. Motor imagery evokes increased somatosensory activity in Parkinson’s disease patients with tremor. Hum. Brain Mapp. 2012, 33, 1763–1779. [Google Scholar] [CrossRef]
- Severiano, M.I.R.; Zeigelboim, B.S.; Teive, H.A.G.; Santos, G.J.B.; Fonseca, V.R. Effect of virtual reality in Parkinson’s disease: A prospective observational study. Arq. De Neuro-Psiquiatr. 2018, 76, 78–84. [Google Scholar] [CrossRef]
- Triegaardt, J.; Han, T.S.; Sada, C.; Sharma, S.; Sharma, P. The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: Meta-analysis and systematic review in 1031 participants. Neurol. Sci. 2020, 41, 529–536. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos Interdiscip. J. Nonlinear Sci. 2009, 19, 026113. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M.; Chan, P. Motor automaticity in Parkinson’s disease. Neurobiol. Dis. 2015, 82, 226–234. [Google Scholar] [CrossRef]
- Bloem, B.R.; Beckley, D.J.; Van Dijk, J.G.; Zwinderman, A.H.; Remler, M.P.; Roos, R.A. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1996, 11, 509–521. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa I sa D ouble-E dged S word for B alance and G ait in P eople W ith P arkinson’s D isease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- de Melo, G.E.L.; Kleiner, A.F.R.; Lopes, J.B.P.; Dumont, A.J.L.; Lazzari, R.D.; Galli, M.; Oliveira, C.S. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. NeuroRehabilitation 2018, 42, 473–480. [Google Scholar] [CrossRef]
- Abraham, A.; Duncan, R.P.; Earhart, G.M. The Role of Mental Imagery in Parkinson’s Disease Rehabilitation. Brain Sci. 2021, 11, 185. [Google Scholar] [CrossRef]
- Ustinova, K.; Chernikova, L.; Bilimenko, A.; Telenkov, A.; Epstein, N. Effect of robotic locomotor training in an individual with Parkinson’s disease: A case report. Disabil. Rehabil. Assist. Technol. 2011, 6, 77–85. [Google Scholar] [CrossRef]
- Nallegowda, M.; Singh, U.; Handa, G.; Khanna, M.; Wadhwa, S.; Yadav, S.L.; Kumar, G.; Behari, M. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson’s disease: A pilot study. Am. J. Phys. Med. 2004, 83, 898–908. [Google Scholar] [CrossRef]

| Randomized (n = 44) | p-Value | |
|---|---|---|
| Variables | Experimental/Control Group | |
| (n = 22)/(n = 22) | ||
| Age (years) | 63.86 ± 4.57/62.32 ± 4.61 | 0.936 |
| Gender | ||
| Female | 9 (41%)/10 (45.45%) | |
| Male | 13 (59%)/12 (54.55%) | |
| Height (cm) | 160.36 ± 3.70/164.36 ± 2.68 | 0.397 |
| Weight (kg) | 59.59 ± 4.90/60.73 ± 5.43 | 0.71 |
| Disease duration (years) | 6.23 ± 1.85/6.55 ± 1.68 | 0.887 |
| Age at onset of PD | 56.00 ± 4.06/55.50 ± 4.53 | 0.912 |
| Age at diagnosis of PD | 59.55 ± 3.91/60.05 ± 4.13 | 0.443 |
| H&Y Stage | 2.11 ± 0.74/2.25 ± 0.67 | 0.72 |
| MMSE | 26.41 ± 1.91/25.27 ± 4.38 | 0.029 |
| UPDRS-III baseline | 32.45 ± 3.98/31.86 ± 4.62 | 0.742 |
| Motor Function | Groups | Baseline | Assessment at 6th Week | Assessment at 12th Week | Follow Up at 16th Week |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Speech | Exp. | 2.00 ± 0.000 | 1.86 ± 0.351 | 1.27 ± 0.631 | 1.76 ± 0.429 |
| Control | 1.95 ± 0.213 | 1.82 ± 0.395 | 1.36 ± 0.658 | 1.51 ± 0.740 | |
| Z | −1 | −0.407 | −0.523 | −0.634 | |
| p Value | 0.317 | 0.684 | 0.601 | 0.526 | |
| Facial Expression | Exp. | 1.95 ± 0.213 | 1.91 ± 0.294 | 1.64 ± 0.492 | 1.68 ± 0.477 |
| Control | 1.95 ± 0.213 | 1.82 ± 0.395 | 1.64 ± 0.492 | 1.59 ± 0.590 | |
| Z | 0 | −0.869 | 0 | −0.412 | |
| p Value | 1 | 0.385 | 1 | 0.68 | |
| Action or Postural Tremor | Exp. | 2.00 ± 0.53 | 1.27 ± 0.882 | 0.77 ± 0.611 | 1.18 ± 0.664 |
| Control | 1.77 ± 1.02 | 1.45 ± 0.962 | 1.18 ± 0.795 | 1.40 ± 0.734 | |
| Z | −1.392 | −0.482 | −1.779 | −1.02 | |
| p Value | 0.164 | 0.63 | 0.075 | 0.308 | |
| Finger Taps | Exp. | 2.23 ± 0.528 | 1.81 ± 0.501 | 1.18 ± 0.795 | 1.45 ± 0.670 |
| Control | 2.18 ± 0.501 | 1.95 ± 0.722 | 1.86 ± 0.710 | 1.72 ± 0.882 | |
| Z | −0.309 | −0.956 | −1.472 | −1.391 | |
| p Value | 0.757 | 0.339 | 0.141 | 0.164 | |
| Hand Movements | Exp. | 1.54 ± 0.509 | 1.13 ± 0.833 | 0.72 ± 0.882 | 0.86 ± 0.940 |
| Control | 1.45 ± 0.738 | 1.18 ± 0.852 | 1.09 ± 0.811 | 1.00 ± 0.872 | |
| Z | −0.395 | −0.075 | −1.47 | −0.524 | |
| p Value | 0.693 | 0.94 | 0.142 | 0.6 | |
| Rapid Alternating Movements | Exp. | 1.78 ± 0.428 | 1.40 ± 0.59 | 1.00 ± 0.690 | 1.09 ± 0.610 |
| Control | 1.86 ± 0.467 | 1.59 ± 0.503 | 1.40 ± 0.59033 | 1.50 ± 0.51 | |
| Z | −0.629 | −1.007 | −1.989 | −2.209 | |
| p Value | 0.53 | 0.314 | 0.047 | 0.027 | |
| Leg Agility | Exp. | 1.91 ± 0.683 | 1.27 ± 0.882 | 0.863 ± 0.83 | 1.00 ± 0.872 |
| Control | 1.90 ± 0.81 | 1.63 ± 1.04 | 1.36 ± 1.09 | 1.32 ± 1.13 | |
| Z | −0.129 | −1.229 | −1.522 | −0.909 | |
| p Value | 0.898 | 0.219 | 0.128 | 0.363 | |
| Arising from a Chair | Exp. | 1.18 ± 0.395 | 0.91 ± 0.526 | 0.50 ± 0.598 | 0.50 ± 0.598 |
| Control | 1.23 ± 0.528 | 1.09 ± 0.684 | 1.05 ± 0.722 | 1.00 ± 0.690 | |
| Z | −0.405 | −0.989 | −2.515 | −2.407 | |
| p Value | 0.685 | 0.323 | 0.012 | 0.016 | |
| Gait | Exp. | 1.86 ± 0.468 | 1.23 ± 0.429 | 0.86 ± 0.560 | 1.00 ± 0.690 |
| Control | 1.73 ± 0.456 | 1.55 ± 0.510 | 1.50 ± 0.598 | 1.45 ± 0.671 | |
| Z | −0.935 | −2.143 | −3.273 | −2.171 | |
| p Value | 0.35 | 0.032 | 0.001 | 0.03 |
| Motor Function | Groups | Baseline | Assessment at 6th Week | Assessment at 12th Week | Follow Up at 16th Week | Friedman Test | |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | X2 | p | ||
| Speech | Exp. | 2.00 ± 0.000 | 1.86 ± 0.351 | 1.27 ± 0.631 | 1.76 ± 0.429 | 28.933 | <0.001 |
| Control | 1.95 ± 0.213 | 1.82 ± 0.395 | 1.36 ± 0.658 | 1.51 ± 0.740 | 20.068 | <0.001 | |
| Facial Expression | Exp. | 1.95 ± 0.213 | 1.91 ± 0.294 | 1.64 ± 0.492 | 1.68 ± 0.477 | 17.077 | 0.001 |
| Control | 1.95 ± 0.213 | 1.82 ± 0.395 | 1.64 ± 0.492 | 1.59 ± 0.590 | 12.636 | 0.005 | |
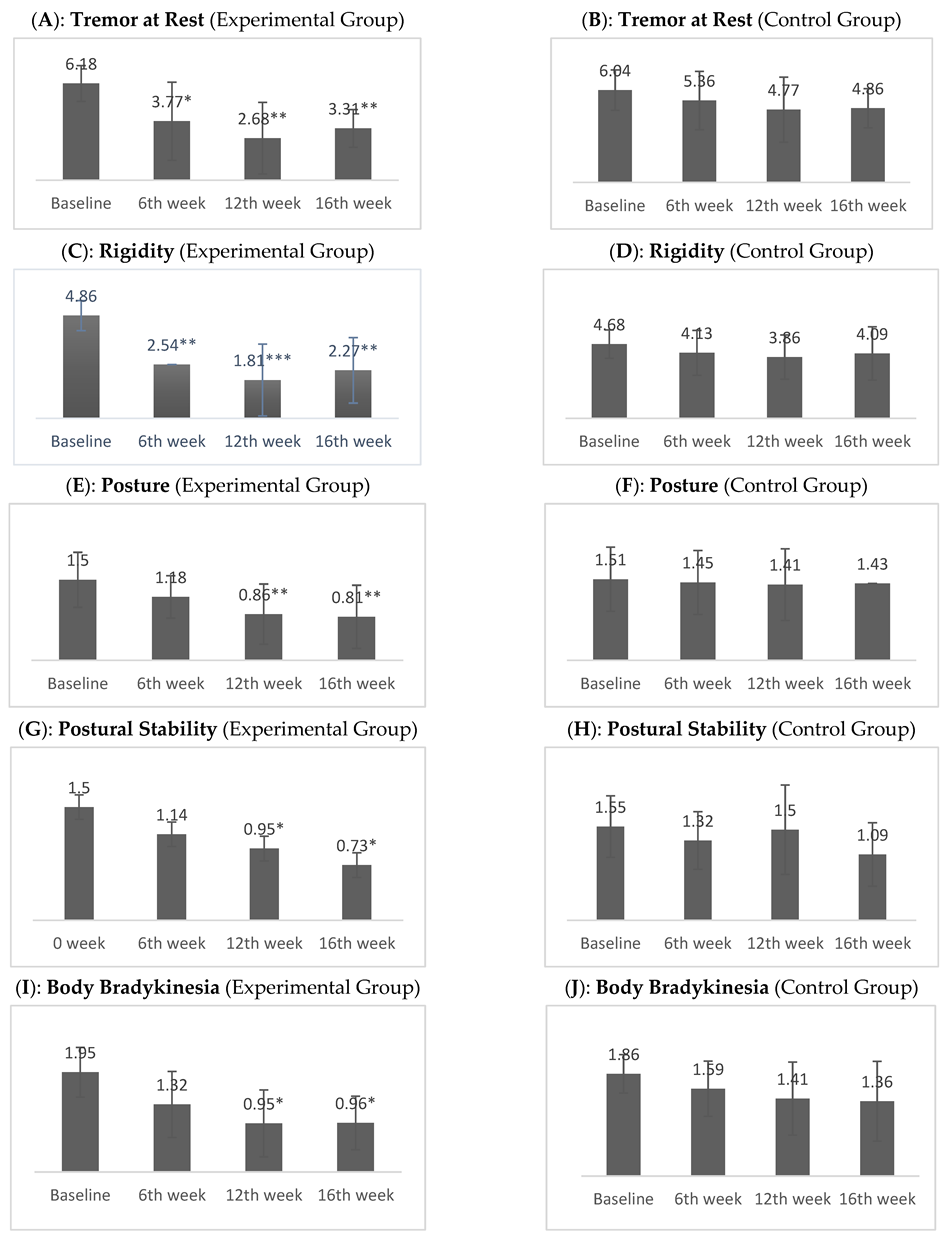
| Tremor at Rest | Exp. | 6.18 ± 1.14 | 3.77 ± 2.50 | 1.68 ± 1.08 | 3.31 ± 1.21 | 46.523 | <0.001 |
| Control | 6.04 ± 1.32 | 5.36 ± 1.91 | 4.90 ± 1.97 | 4.86 ± 1.28 | 16.804 | 0.001 | |
| Action or Postural Tremor | Exp. | 2.00 ± 0.53 | 1.27 ± 0.882 | 0.77 ± 0.611 | 1.20 ± 0.664 | 35.318 | <0.001 |
| Control | 1.77 ± 1.02 | 1.45 ± 0.962 | 1.18 ± 0.795 | 1.40 ± 0.734 | 7.487 | 0.058 | |
| Rigidity | Exp. | 4.86 ± 0.710 | 2.54 ± 1.68 | 1.81± 1.70 | 2.27 ± 1.55 | 43.219 | <0.001 |
| Control | 4.68 ± 0.893 | 4.13 ± 1.42 | 3.86 ± 1.39 | 4.09 ± 1.68 | 15.518 | 0.001 | |
| Finger Taps | Exp. | 2.23 ± 0.528 | 1.81 ± 0.501 | 1.18 ± 0.795 | 1.45 ± 0.670 | 33.444 | <0.001 |
| Control | 2.18 ± 0.501 | 1.95 ± 0.722 | 1.86 ± 0.710 | 1.72 ± 0.882 | 8.202 | 0.042 | |
| Hand Movements | Exp. | 1.54 ± 0.509 | 1.13 ± 0.833 | 0.72 ± 0.882 | 0.86 ± 0.940 | 30.429 | <0.001 |
| Control | 1.45 ± 0.738 | 1.18 ± 0.852 | 1.09 ± 0.811 | 1.00 ± 0.872 | 11.949 | 0.008 | |
| Rapid Alternating Movements | Exp. | 1.78 ± 0.428 | 1.40 ± 0.59 | 1.00 ± 0.690 | 1.09 ± 0.610 | 31.33 | <0.001 |
| Control | 1.86 ± 0.467 | 1.59 ± 0.503 | 1.40 ± 0.590 | 1.50 ± 0.51 | 20.085 | <0.001 | |
| Leg agility | Exp. | 1.91 ± 0.683 | 1.27 ± 0.882 | 0.863 ± 0.83 | 1.00 ± 0.872 | 32.385 | <0.001 |
| Control | 1.90 ± 0.81 | 1.63 ± 1.04 | 1.36 ± 1.09 | 1.32 ± 1.13 | 20.542 | <0.001 | |
| Arising from a Chair | Exp. | 1.18 ± 0.395 | 0.91 ± 0.526 | 0.50 ± 0.598 | 0.50 ± 0.598 | 27.117 | <0.001 |
| Control | 1.23 ± 0.528 | 1.09 ± 0.684 | 1.05 ± 0.722 | 1.00 ± 0.690 | 4.017 | 0.26 | |
| Posture | Exp. | 1.50 ± 0.512 | 1.18 ± 0.395 | 0.86 ± 0.560 | 0.82 ± 0.588 | 32.941 | <0.001 |
| Control | 1.50 ± 0.598 | 1.45 ± 0.596 | 1.41 ± 0.666 | 1.45 ± 0.596 | 2.4 | 0.494 | |
| Gait | Exp. | 1.86 ± 0.468 | 1.23 ± 0.429 | 0.86 ± 0.560 | 1.00 ± 0.690 | 36.609 | <0.001 |
| Control | 1.73 ± 0.456 | 1.55 ± 0.510 | 1.50 ± 0.598 | 1.45 ± 0.671 | 11.308 | 0.01 | |
| Postural Stability | Exp. | 1.50 ± 0.512 | 1.14 ± 0.468 | 0.95 ± 0.653 | 0.73 ± 0.631 | 22.983 | <0.001 |
| Control | 1.55 ± 0.510 | 1.32 ± 0.477 | 1.50 ± 0.740 | 1.09 ± 0.526 | 10.220 | 0.017 | |
| Body Bradykinesia | Exp. | 1.95 ± 0.486 | 1.32 ± 0.646 | 0.95 ± 0.653 | 91 ± 0.684 | 44.146 | <0.001 |
| Control | 1.86 ± 0.351 | 1.59 ± 0.503 | 1.41 ± 0.666 | 1.36 ± 0.727 | 21.682 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Syed, H.A.; Raza, A.; Sana, V. A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease. J. Pers. Med. 2022, 12, 450. https://doi.org/10.3390/jpm12030450
Kashif M, Ahmad A, Bandpei MAM, Syed HA, Raza A, Sana V. A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease. Journal of Personalized Medicine. 2022; 12(3):450. https://doi.org/10.3390/jpm12030450
Chicago/Turabian StyleKashif, Muhammad, Ashfaq Ahmad, Muhammad Ali Mohseni Bandpei, Hafiza Aroosa Syed, Ali Raza, and Vishal Sana. 2022. "A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease" Journal of Personalized Medicine 12, no. 3: 450. https://doi.org/10.3390/jpm12030450
APA StyleKashif, M., Ahmad, A., Bandpei, M. A. M., Syed, H. A., Raza, A., & Sana, V. (2022). A Randomized Controlled Trial of Motor Imagery Combined with Virtual Reality Techniques in Patients with Parkinson’s Disease. Journal of Personalized Medicine, 12(3), 450. https://doi.org/10.3390/jpm12030450