Are Risk Factors for Postoperative Significant Hemorrhage following Total Knee Arthroplasty Potentially Modifiable? A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
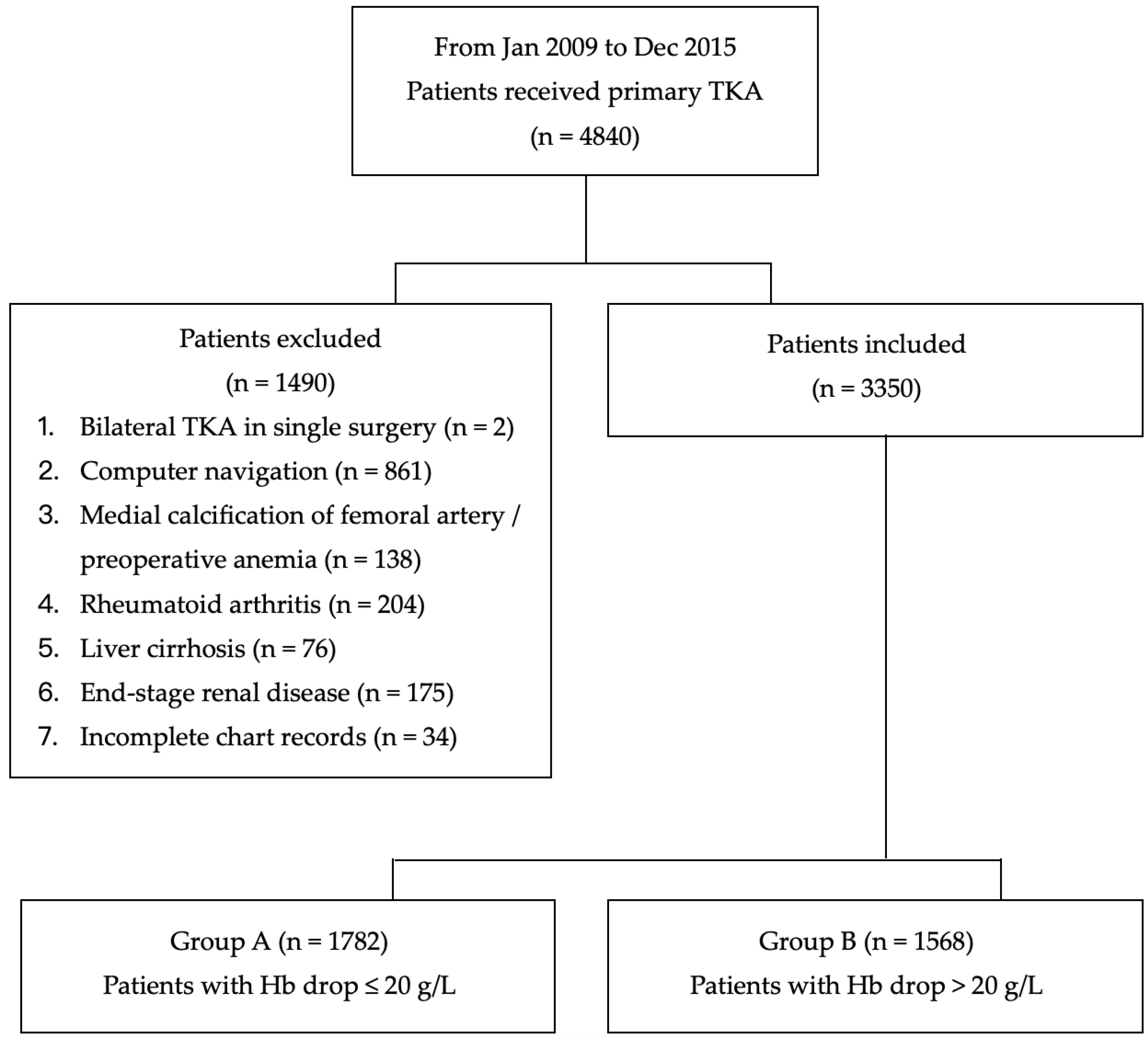
2. Materials and Methods
2.1. Definition of Significant Hemorrhage
2.2. Choice of Anesthesia
2.3. The Management of Perioperative Bleeding
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fransen, M.; Bridgett, L.; March, L.; Hoy, D.; Penserga, E.; Brooks, P. The epidemiology of osteoarthritis in Asia. Int. J. Rheum. Dis. 2011, 14, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Boutsiadis, A.; Reynolds, R.J.; Saffarini, M.; Panisset, J.C. Factors that influence blood loss and need for transfusion following total knee arthroplasty. Ann. Transl. Med. 2017, 5, 418. [Google Scholar] [CrossRef] [PubMed]
- Cushner, F.D.; Friedman, R.J. Blood loss in total knee arthroplasty. Clin. Orthop. Relat. Res. 1991, 261, 98–101. [Google Scholar] [CrossRef]
- Noticewala, M.S.; Nyce, J.D.; Wang, W.; Geller, J.A.; Macaulay, W. Predicting need for allogeneic transfusion after total knee arthroplasty. J. Arthroplast. 2012, 27, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Padmanabhan, V.; Mullaji, A. Blood loss in total knee arthroplasty: An analysis of risk factors. Int. Orthop. 2007, 31, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Petis, S.M.; Lanting, B.A.; Vasarhelyi, E.M.; Naudie, D.D.R.; Ralley, F.E.; Howard, J.L. Is There a Role for Preoperative Iron Supplementation in Patients Preparing for a Total Hip or Total Knee Arthroplasty? J. Arthroplast. 2017, 32, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Issa, K.; Kapadia, B.H.; Khanuja, H.S.; Harwin, S.F.; McInerney, V.K.; Mont, M.A. Intraoperative nonpharmacotherapeutic blood management strategies in total knee arthroplasty. J. Knee Surg. 2013, 26, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chareancholvanich, K.; Siriwattanasakul, P.; Narkbunnam, R.; Pornrattanamaneewong, C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: A prospective randomized controlled trial. BMC Musculoskelet. Disord. 2012, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Su, E.P.; Su, S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Jt. J. 2016, 98-B, 98–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bierbaum, B.E.; Callaghan, J.J.; Galante, J.O.; Rubash, H.E.; Tooms, R.E.; Welch, R.B. An analysis of blood management in patients having a total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 1999, 81, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Khalil, J.A.; Carli, A.; Huk, O.; Zukor, D.; Antoniou, J. Blood transfusion in primary total hip and knee arthroplasty. Incidence, risk factors, and thirty-day complication rates. J. Bone Jt. Surg. Am. 2014, 96, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Lasocki, S.; Krauspe, R.; von Heymann, C.; Mezzacasa, A.; Chainey, S.; Spahn, D.R. PREPARE: The prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: A multicentre, observational study. Eur. J. Anaesthesiol. 2015, 32, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, S.O.; Sim, J.H.; Hahm, K.D. Postoperative Anemia Is Associated with Acute Kidney Injury in Patients Undergoing Total Hip Replacement Arthroplasty: A Retrospective Study. Anesth. Analg. 2016, 122, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.; Ozrazgat-Baslanti, T.; Kuxhausen, A.; Thottakkara, P.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and Mortality Associated with Postoperative Acute Kidney Injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.V.; Spies, C.D.; Bichmann, A.; Sieg, M.; Mueller, A. Postoperative anaemia might be a risk factor for postoperative delirium and prolonged hospital stay: A secondary analysis of a prospective cohort study. PLoS ONE 2020, 15, e0229325. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015, 122, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Beris, P.; Munoz, M.; Garcia-Erce, J.A.; Thomas, D.; Maniatis, A.; Van der Linden, P. Perioperative anaemia management: Consensus statement on the role of intravenous iron. Br. J. Anaesth. 2008, 100, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [PubMed]
- Leal-Noval, S.R.; Munoz, M.; Asuero, M.; Contreras, E.; Garcia-Erce, J.A.; Llau, J.V.; Moral, V.; Paramo, J.A.; Quintana, M.; Spanish Expert Panel on Alternatives to Allogeneic Blood Transfusion. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: The 2013 update of the “Seville Document”. Blood Transfus 2013, 11, 585–610. [Google Scholar] [CrossRef][Green Version]
- Vaglio, S.; Prisco, D.; Biancofiore, G.; Rafanelli, D.; Antonioli, P.; Lisanti, M.; Andreani, L.; Basso, L.; Velati, C.; Grazzini, G.; et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016, 14, 23–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.L.; Park, J.H.; Han, S.B.; Cho, I.Y.; Jang, K.M. Allogeneic Blood Transfusion Is a Significant Risk Factor for Surgical-Site Infection Following Total Hip and Knee Arthroplasty: A Meta-Analysis. J. Arthroplast. 2017, 32, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, E. Safety of the blood supply. Clin. Orthop. Relat. Res. 1998, 357, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.H. Special report: Transfusion risks. Am. J. Clin. Pathol. 1987, 88, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.; Taconet, C.; Harrois, A.; Hamada, S.; Gauss, T.; Raux, M.; Duranteau, J.; Traumabase, G. How useful are hemoglobin concentration and its variations to predict significant hemorrhage in the early phase of trauma? A multicentric cohort study. Ann. Intensive Care 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Freeman, R.; Edmondson, M.; Rogers, B.A. The efficacy of tranexamic acid in hip hemiarthroplasty surgery: An observational cohort study. Injury 2015, 46, 1978–1982. [Google Scholar] [CrossRef] [PubMed]
- Yefet, E.; Yossef, A.; Suleiman, A.; Hatokay, A.; Nachum, Z. Hemoglobin drop following postpartum hemorrhage. Sci. Rep. 2020, 10, 21546. [Google Scholar] [CrossRef] [PubMed]
- Cammerer, U.; Dietrich, W.; Rampf, T.; Braun, S.L.; Richter, J.A. The predictive value of modified computerized thromboelastography and platelet function analysis for postoperative blood loss in routine cardiac surgery. Anesth. Analg. 2003, 96, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rasouli, M.R.; Mortazavi, S.M.; Tokarski, A.T.; Maltenfort, M.G.; Parvizi, J. Predictors of perioperative blood loss in total joint arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Tian, N.; Zhang, X.; Chen, M. Changes in perioperative hemoglobin and hematocrit in patients undergoing total knee arthroplasty: A prospective observational study of optimal timing of measurement. J. Int. Med. Res. 2020, 48, 300060520969303. [Google Scholar] [CrossRef]
- Hennessy, O.; McSorley, K. Is Routine Hemoglobin Monitoring Necessary after Elective Hip and Knee Arthroplasty? Arthroplast. Today 2020, 6, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.K.; Carson, J.A.; Poses, R.; McCoy, S.; Pello, M.; Alexander, J.; Popovich, J.; Norcross, E.; Camishion, R.C. Elective surgery without transfusion: Influence of preoperative hemoglobin level and blood loss on mortality. Am. J. Surg. 1990, 159, 320–324. [Google Scholar] [CrossRef]
- Glance, L.G.; Blumberg, N.; Eaton, M.P.; Lustik, S.J.; Osler, T.M.; Wissler, R.; Zollo, R.; Karcz, M.; Feng, C.; Dick, A.W. Preoperative thrombocytopenia and postoperative outcomes after noncardiac surgery. Anesthesiology 2014, 120, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Botker, H.E.; Hert, S.D.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.U.; Gluckman, T.J.; Hucker, W.J.; Januzzi, J.L., Jr.; Ortel, T.L.; Saxonhouse, S.J.; Spinler, S.A. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients with Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J. Am. Coll. Cardiol. 2017, 69, 871–898. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2016, 152, 1243–1275. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, W.; Beaulieu, Y.; Puliero, B.; Kiss, M.O.; Vendittoli, P.A. Bone resection for mechanically aligned total knee arthroplasty creates frequent gap modifications and imbalances. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S.; Pietrzak, J.R.T.; Haddad, F.S. Iatrogenic Bone and Soft Tissue Trauma in Robotic-Arm Assisted Total Knee Arthroplasty Compared with Conventional Jig-Based Total Knee Arthroplasty: A Prospective Cohort Study and Validation of a New Classification System. J. Arthroplast. 2018, 33, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Osei, D.A.; Rebehn, K.A.; Boyer, M.I. Soft-tissue Defects after Total Knee Arthroplasty: Management and Reconstruction. J. Am. Acad. Orthop. Surg. 2016, 24, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Chaudhry, S.; Rasouli, M.R.; Pulido, L.; Joshi, A.; Herman, J.H.; Rothman, R.H. Who needs autologous blood donation in joint replacement? J. Knee Surg. 2011, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Spangehl, M.J. Tourniquet Use in Total Knee Arthroplasty. J. Knee Surg. 2019, 32, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Q.; Wei, B.G.; Zhang, X.S.; Torsha, T.T.; Xiao, J.; Shi, Z.J. Blood loss of total knee arthroplasty in osteoarthritis: An analysis of influential factors. J. Orthop. Surg. Res. 2018, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Iyer, U.S.; Sardesai, A.; Chitnavis, J. Tourniquet failure during total knee replacement due to arterial calcification: Case report and review of the literature. J. Perioper. Pract. 2010, 20, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Bunker, T.D.; Ratliff, A.H. Uncontrollable bleeding under tourniquet. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 1905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeyaseelan, S.; Stevenson, T.M.; Pfitzner, J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia 1981, 36, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Sehat, K.R.; Evans, R.; Newman, J.H. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee 2000, 7, 151–155. [Google Scholar] [CrossRef]
- Churchill, W.H.; McGurk, S.; Chapman, R.H.; Wallace, E.L.; Bertholf, M.F.; Goodnough, L.T.; Kao, K.J.; Olson, J.D.; Woodson, R.D.; Surgenor, D.M. The Collaborative Hospital Transfusion Study: Variations in use of autologous blood account for hospital differences in red cell use during primary hip and knee surgery. Transfusion 1998, 38, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Romano, C.L.; Vassena, C.; Banfi, G. Behaviour of perioperative values of haemoglobin, haematocrit and red blood cells in elderly patients undergoing lower limb arthroplasty: A retrospective cohort study on non-transfused patients. Int. J. Immunopathol. Pharmacol. 2013, 26, 427–433. [Google Scholar] [CrossRef]
- Bong, M.R.; Patel, V.; Chang, E.; Issack, P.S.; Hebert, R.; Di Cesare, P.E. Risks associated with blood transfusion after total knee arthroplasty. J. Arthroplast. 2004, 19, 281–287. [Google Scholar] [CrossRef]
- Rosencher, N.; Kerkkamp, H.E.; Macheras, G.; Munuera, L.M.; Menichella, G.; Barton, D.M.; Cremers, S.; Abraham, I.L.; Investigation, O. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: Blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003, 43, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Nagrebetsky, A.; Al-Samkari, H.; Davis, N.M.; Kuter, D.J.; Wiener-Kronish, J.P. Perioperative thrombocytopenia: Evidence, evaluation, and emerging therapies. Br. J. Anaesth. 2019, 122, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.G., Jr. Hematopoietic function in the elderly. Arch. Intern. Med. 1988, 148, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, H.W. Aging of the hematopoietic system. Curr. Opin. Hematol. 2013, 20, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Alshryda, S.; Sarda, P.; Sukeik, M.; Nargol, A.; Blenkinsopp, J.; Mason, J.M. Tranexamic acid in total knee replacement: A systematic review and meta-analysis. J. Bone Jt. Surg. Br. 2011, 93, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Rosso, F.; Bruzzone, M.; Bonasia, D.E.; Dettoni, F.; Rossi, R. Use of tranexamic acid in total knee arthroplasty. Joints 2016, 4, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; Memtsoudis, S.G.; Sayeed, S.A.; et al. Tranexamic acid in total joint arthroplasty: The endorsed clinical practice guides of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. Reg. Anesth. Pain Med. 2019, 44, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.; Walker, N.; Schug, S.; McKee, A.; Kehlet, H.; van Zundert, A.; Sage, D.; Futter, M.; Saville, G.; Clark, T.; et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ 2000, 321, 1493. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Z.Y.; Hua, Y.Q.; Li, J.; Cai, Z.D. A comparison of regional and general anaesthesia for total replacement of the hip or knee: A meta-analysis. J. Bone Jt. Surg. Br. 2009, 91, 935–942. [Google Scholar] [CrossRef]
- Macfarlane, A.J.; Prasad, G.A.; Chan, V.W.; Brull, R. Does regional anaesthesia improve outcome after total hip arthroplasty? A systematic review. Br. J. Anaesth. 2009, 103, 335–345. [Google Scholar] [CrossRef]
- Memtsoudis, S.G.; Stundner, O.; Rasul, R.; Sun, X.; Chiu, Y.L.; Fleischut, P.; Danninger, T.; Mazumdar, M. Sleep apnea and total joint arthroplasty under various types of anesthesia: A population-based study of perioperative outcomes. Reg. Anesth. Pain Med. 2013, 38, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Pugely, A.J.; Martin, C.T.; Gao, Y.; Mendoza-Lattes, S.; Callaghan, J.J. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Stundner, O.; Chiu, Y.L.; Sun, X.; Mazumdar, M.; Fleischut, P.; Poultsides, L.; Gerner, P.; Fritsch, G.; Memtsoudis, S.G. Comparative perioperative outcomes associated with neuraxial versus general anesthesia for simultaneous bilateral total knee arthroplasty. Reg. Anesth. Pain Med. 2012, 37, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Juelsgaard, P.; Larsen, U.T.; Sorensen, J.V.; Madsen, F.; Soballe, K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: Reduced blood loss and transfusion. Reg. Anesth. Pain Med. 2001, 26, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Farley, K.X.; Erens, G.A.; Guild, G.N. 3rd. General vs. Spinal Anesthesia for Revision Total Knee Arthroplasty: Do Complication Rates Differ? J. Arthroplast. 2019, 34, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Palanne, R.; Rantasalo, M.; Vakkuri, A.; Madanat, R.; Olkkola, K.T.; Lahtinen, K.; Reponen, E.; Linko, R.; Vahlberg, T.; Skants, N. Effects of anaesthesia method and tourniquet use on recovery following total knee arthroplasty: A randomised controlled study. Br. J. Anaesth. 2020, 125, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.F.; Fan, Q.H.; Zhong, H.H.; Peng, S.; Song, H. The effects of tourniquet use on blood loss in primary total knee arthroplasty for patients with osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, N.; Chen, S.; Tan, Y.; Al-Aidaros, M.; Chen, L. The effects of a tourniquet used in total knee arthroplasty: A meta-analysis. J. Orthop. Surg. Res. 2014, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Chawla, A.; Underwood, M.; Price, A.J.; Metcalfe, A.; Hutchinson, C.; Warwick, J.; Seers, K.; Parsons, H.; Wall, P.D. Tourniquet use for knee replacement surgery. Cochrane Database Syst. Rev. 2020, 12, CD012874. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, K.; Xiao, L.; Tang, R. Clinical Outcomes after Computer-assisted Versus Conventional Total Knee Arthroplasty. Orthopedics 2012, 35, e647–e653. [Google Scholar] [CrossRef]
| Features | n (%)/Median (IQR) | Group A | Group B | p Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 2601 (77.6%) | 1437 (80.6%) | 1164 (74.2%) | <0.001 |
| Male | 749 (22.4%) | 345 (19.4%) | 404 (25.8%) | |
| Age | 70.0 (64.0–75.0) | 69. 0(64.0–74.0) | 71.0 (65.0–76.0) | <0.001 |
| ASA | ||||
| Ⅰ | 63 (1.9%) | 37 (2.1%) | 2 6(1.7%) | 0.019 |
| Ⅱ | 2042 (61.0%) | 1121 (62.9%) | 921 (58.7%) | |
| Ⅲ | 1245 (37.2%) | 624 (35.0%) | 621 (39.6%) | |
| BMI | ||||
| BMI < 18.5 | 8 (0.2%) | 3 (0.2%) | 5 (0.3%) | 0.372 |
| BMI = 18.5–24 | 565 (16.9%) | 289 (16.2%) | 276 (17.6%) | |
| BMI > 24 | 2777 (82.9%) | 1490 (83.6%) | 1287 (82.1%) | |
| Ischemic heart disease | ||||
| No | 3143 (93.8%) | 1698 (95.3%) | 1445 (92.2%) | <0.001 |
| Yes | 207 (6.2%) | 84 (4.7%) | 123 (7.8%) | |
| Diabetes Mellitus | ||||
| No | 2557 (76.3%) | 1368 (76.8%) | 1189 (75.8%) | 0.524 |
| Yes | 793 (23.7%) | 414 (23.2%) | 379 (24.2%) | |
| Hypertension | ||||
| No | 1217 (36.3%) | 655 (36.8%) | 562 (35.8%) | 0.583 |
| Yes | 2133 (63.7%) | 1127 (63.2%) | 1006 (64.2%) | |
| Tranexamic acid | ||||
| No | 1885 (56.3%) | 813 (45.6%) | 1072 (68.4%) | <0.001 |
| Yes | 1465 (43.7%) | 969 (54.4%) | 496 (31.6%) | |
| Operation time (h) | ||||
| <2 h | 345 (10.3%) | 194 (10.9%) | 151 (9.6%) | 0.232 |
| >2 h | 3005 (89.7%) | 1588 (89.1%) | 1417 (90.4%) | |
| Anesthesia | ||||
| General | 3002 (89.6%) | 1580 (88.7%) | 1422 (90.7%) | 0.055 |
| Spinal | 348 (10.4%) | 202 (11.3%) | 146 (9.3%) | |
| Preoperative Hb (g/L) | 133 (124–141) | 131 (123–139) | 132 (122–142) | 0.193 |
| Lactate Ringer fluid (ml) | 408.2 (358.8–460.4) | 403.5 (360.2–457.7) | 410.8 (356.2–465.4) | 0.396 |
| Postoperative Hb (g/L) | 111 (102–119) | 115 (107–122) | 106 (97–115) | <0.001 |
| Platelet count (104/μL) | 22.7 (1.92–2.65) | 22.9 (1.95–2.69) | 22.2 (1.88–2.61) | <0.001 |
| PT (s) | 10.1 (9.9–10.4) | 10.1 (9.9–10.4) | 10.1 (9.9–10.4) | 0.765 |
| aPTT (s) | 27.3 (26.0–28.6) | 27.3 (26.0–28.5) | 27.3 (26.0–28.7) | 0.478 |
| Discharge Hb (g/L) | 123 (115–131) | 123 (115–131) | 124 (114–132) | 0.215 |
| Variables (Unit) | n (%)/Unit | Uariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Sex | |||||
| Female | 2601 (77.6%) | 1 | 1 | ||
| Male | 749 (22.4%) | 1.45 (1.23–1.70) | <0.001 | 1.29 (1.08–1.53) | 0.005 |
| Age | 70 (64–75) | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | 0.001 |
| ASA | |||||
| Ⅰ | 63 (1.9%) | 1 | 1 | ||
| Ⅱ | 2042 (61.0%) | 1.17 (0.70–1.95) | 0.547 | 1.07 (0.63–1.82) | 0.793 |
| Ⅲ | 1245 (37.2%) | 1.42 (0.75–2.37) | 0.184 | 1.09 (0.64–1.86) | 0.758 |
| BMI | |||||
| BMI < 18.5 | 8 (0.2%) | 1 | 1 | ||
| BMI = 18.5–24 | 565 (16.9%) | 0.57 (0.14–2.42) | 0.449 | 0.56 (0.12–2.50) | 0.444 |
| BMI > 24 | 2777 (82.9%) | 0.52 (0.12–2.17) | 0.369 | 0.51 (0.11–2.28) | 0.377 |
| Ischemic heart disease | |||||
| No | 3143 (93.8%) | 1 | 1 | ||
| Yes | 207 (6.2%) | 1.72 (1.29–2.29) | <0.001 | 1.19 (0.88–1.62) | 0.262 |
| Diabetes Mellitus | |||||
| No | 2557 (76.3%) | 1 | 1 | ||
| Yes | 793 (23.7%) | 1.05 (0.90–1.24) | 0.524 | 1.04 (0.88–1.24) | 0.625 |
| Hypertension | |||||
| No | 1217 (36.3%) | 1 | 1 | ||
| Yes | 2133 (63.7%) | 1.04 (0.90–1.20) | 0.583 | 1.00 (0.86–1.17) | 0.976 |
| Tranexamic acid | |||||
| No | 1885 (56.3%) | 1 | 1 | ||
| Yes | 1465 (43.7%) | 0.39 (0.34–0.45) | <0.001 | 0.39 (0.34–0.45) | <0.001 |
| Operation time (h) | |||||
| <2 h | 345 (10.3%) | 1 | 1 | ||
| >2 h | 3005 (89.7%) | 1.15 (0.92–1.44) | 0.233 | 0.95 (0.75–1.21) | 0.685 |
| Anesthesia | |||||
| General | 3002 (89.6%) | 1 | 1 | ||
| Spinal | 348 (10.4%) | 0.80 (0.64–1.01) | 0.056 | 0.71 (0.56–0.90) | 0.004 |
| Platelet count # (2 × 104/μL) | 0.45 (0.38–0.53) | 0.95 (0.93–0.97) | <0.001 | 0.96 (0.93–0.98) | 0.001 |
| PT (s) | 10.1 (9.9–10.4) | 1.07 (0.95–1.20) | 0.289 | 0.98 (0.86–1.11) | 0.745 |
| aPTT (s) | 27.3 (26.0–28.6) | 1.01 (0.98–1.04) | 0.441 | 1.01 (0.98–1.04) | 0.550 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.-B.; Hung, K.-C.; Juang, S.-E.; Chin, J.-C.; Lu, H.-F.; Ko, J.-Y. Are Risk Factors for Postoperative Significant Hemorrhage following Total Knee Arthroplasty Potentially Modifiable? A Retrospective Cohort Study. J. Pers. Med. 2022, 12, 434. https://doi.org/10.3390/jpm12030434
Wu E-B, Hung K-C, Juang S-E, Chin J-C, Lu H-F, Ko J-Y. Are Risk Factors for Postoperative Significant Hemorrhage following Total Knee Arthroplasty Potentially Modifiable? A Retrospective Cohort Study. Journal of Personalized Medicine. 2022; 12(3):434. https://doi.org/10.3390/jpm12030434
Chicago/Turabian StyleWu, En-Bo, Kuo-Chuan Hung, Sin-Ei Juang, Jo-Chi Chin, Hsiao-Feng Lu, and Jih-Yang Ko. 2022. "Are Risk Factors for Postoperative Significant Hemorrhage following Total Knee Arthroplasty Potentially Modifiable? A Retrospective Cohort Study" Journal of Personalized Medicine 12, no. 3: 434. https://doi.org/10.3390/jpm12030434
APA StyleWu, E.-B., Hung, K.-C., Juang, S.-E., Chin, J.-C., Lu, H.-F., & Ko, J.-Y. (2022). Are Risk Factors for Postoperative Significant Hemorrhage following Total Knee Arthroplasty Potentially Modifiable? A Retrospective Cohort Study. Journal of Personalized Medicine, 12(3), 434. https://doi.org/10.3390/jpm12030434








