Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Particiants
- -
- Obesity sub-study: the study participants were classified according to their BMI: patients without obesity (BMI < 30 kg/m2; n = 109) or with obesity (BMI ≥ 30 kg/m2; n = 37).
- -
- Metabolic syndrome sub-study: 124 patients of the whole cohort, which had enough clinical information available, were classified depending on the presence of MS (MS, n = 15; non-MS, n = 109) according to Alberti et al. criteria [25].
2.2. Data Collection
2.3. Biochemical Analyses
2.4. Plasma Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Whole Cohort
3.2. Baseline Cytokine Storm Related to COVID-19 Pneumonia Severity
3.3. Biochemical Parameters and Cytokine Levels of the Whole Cohort, at Baseline and 4–6 Weeks after Admission and Clinical Residual Manifestations at 4–6 Weeks
3.4. Correlations between Baseline Biochemical Parameters and Cytokine Levels and the Clinical Residual Manifestations at 4–6 Weeks of the COVID-19 Cohort
3.5. Biochemical Parameters and Cytokine Levels of the COVID-19 Cohort Classified according to the BMI (<30 and ≥30 kg/m2) and according to the Presence of MS
3.6. Correlations between the Baseline Levels of Cytokines with Clinical Characteristics of Patients with COVID-19
3.7. Predictive Value of Cytokine Levels for COVID-19 Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ware, L.B. Physiological and Biological Heterogeneity in COVID-19-Associated Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 1163–1165. [Google Scholar] [CrossRef]
- Hue, S.; Beldi-Ferchiou, A.; Frapard, T.; Rivoal, S.; Razazi, K.; Carteaux, G.; Mekontso-Dessap, A.; Audureau, E.; de Prost, N. Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID-19 Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 11. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Caussy, C.; Wallet, F.; Laville, M.; Disse, E. Obesity Is Associated with Severe Forms of COVID-19. Obesity 2020, 28, 1175. [Google Scholar] [CrossRef]
- Mahase, E. China Coronavirus: WHO Declares International Emergency as Death Toll Exceeds 200. BMJ 2020, 368, m408. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. IJMS 2020, 21, 5793. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-Q.; Peng, H.-J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. JCM 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Perpiñan, C.; Bertran, L.; Terra, X.; Aguilar, C.; Lopez-Dupla, M.; Alibalic, A.; Riesco, D.; Camaron, J.; Perrone, F.; Rull, A.; et al. Predictive Biomarkers of COVID-19 Severity in SARS-CoV-2 Infected Patients with Obesity and Metabolic Syndrome. JPM 2021, 11, 227. [Google Scholar] [CrossRef]
- Luzi, L.; Radaelli, M.G. Influenza and Obesity: Its Odd Relationship and the Lessons for COVID-19 Pandemic. Acta Diabetol. 2020, 57, 759–764. [Google Scholar] [CrossRef]
- Yanai, H. Metabolic Syndrome and COVID-19. Cardiol Res. 2020, 11, 360–365. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An Immune-Based Biomarker Signature Is Associated with Mortality in COVID-19 Patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef] [PubMed]
- Meizlish, M.L.; Pine, A.B.; Bishai, J.D.; Goshua, G.; Nadelmann, E.R.; Simonov, M.; Chang, C.-H.; Zhang, H.; Shallow, M.; Bahel, P.; et al. A Neutrophil Activation Signature Predicts Critical Illness and Mortality in COVID-19. Blood Adv. 2021, 5, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- on behalf of the REACT COVID investigators; Burke, H.; Freeman, A.; Cellura, D.C.; Stuart, B.L.; Brendish, N.J.; Poole, S.; Borca, F.; Phan, H.T.T.; Sheard, N.; et al. Inflammatory Phenotyping Predicts Clinical Outcome in COVID-19. Respir. Res. 2020, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis; World Health Organization: Geneva, Switzerland, 2020; Volume 2020, pp. 1–9. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, E.; Balakrishna, A.; Habboushe, J.; Shawl, A.; Lee, J. Calculated Decisions: COVID-19 Calculators during Extreme Resource-Limited Situations. Emerg. Med. Pract. 2020, 6, 1–5. [Google Scholar]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine Storm in COVID-19: Pathogenesis and Overview of Anti-Inflammatory Agents Used in Treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Clark, I.A.; Virelizier, J.L.; Carswell, E.A.; Wood, P.R. Possible Importance of Macrophage-Derived Mediators in Acute Malaria. Infect. Immun. 1981, 32, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A. Suggested Importance of Monokines in Pathophysiology of Endotoxin Shock and Malaria. Klin. Wochenschr. 1982, 60, 756–758. [Google Scholar] [CrossRef]
- Makhija, R.; Kingsnorth, A.N. Cytokine Storm in Acute Pancreatitis. J. Hep. Bil. Pancr. Surg. 2002, 9, 401–410. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Hensley, L.E.; Martinez, M.J.; LeDuc, J.W.; Rubins, K.H. Exploring the Potential of Variola Virus Infection of Cynomolgus Macaques as a Model for Human Smallpox. Proc. Natl. Acad. Sci. USA 2004, 101, 15196–15200. [Google Scholar] [CrossRef]
- Yuen, K.; Wong, S. Human Infection by Avian Influenza A H5N1. Hong Kong Med. J. 2005, 11, 189–199. [Google Scholar] [PubMed]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Zhan, Y.; Wu, L.; Yu, X.; Zhang, W.; Ye, L.; Xu, S.; Sun, R.; Wang, Y.; et al. Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immun. 2004, 72, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Min, C.-K.; Cheon, S.; Ha, N.-Y.; Sohn, K.M.; Kim, Y.; Aigerim, A.; Shin, H.M.; Choi, J.-Y.; Inn, K.-S.; Kim, J.-H.; et al. Comparative and Kinetic Analysis of Viral Shedding and Immunological Responses in MERS Patients Representing a Broad Spectrum of Disease Severity. Sci. Rep. 2016, 6, 25359. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Ward, S.; Brunner, M.D. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Ceschi, A.; Noseda, R.; Palin, K.; Verhamme, K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine Release Syndrome in Severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Golonka, R.M.; Saha, P.; Yeoh, B.S.; Chattopadhyay, S.; Gewirtz, A.T.; Joe, B.; Vijay-Kumar, M. Harnessing Innate Immunity to Eliminate SARS-CoV-2 and Ameliorate COVID-19 Disease. Physiol. Genom. 2020, 52, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.M.; Himmelfarb, J.; Sezer, M.T.; Chertow, G.M.; Mehta, R.L.; Paganini, E.P.; Soroko, S.; Freedman, S.; Becker, K.; Spratt, D.; et al. Plasma Cytokine Levels Predict Mortality in Patients with Acute Renal Failure. Kidney Int. 2004, 65, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wang, S.-S.; Lu, R.-H.; Chang, F.-Y.; Lee, S.-D. Serum Interleukin 10 and Interleukin 11 in Patients with Acute Pancreatitis. Gut 1999, 45, 895–899. [Google Scholar] [CrossRef][Green Version]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Ramirez, N.; Zou, C.; Popp, S.; Zingg, D.; Brannetti, B.; Wirth, E.; Calzascia, T.; Kovarik, J.; Sommer, L.; Zenke, G.; et al. Improved Cancer Immunotherapy by a CD25-Mimobody Conferring Selectivity to Human Interleukin-2. Sci. Transl. Med. 2016, 8, 367ra166. [Google Scholar] [CrossRef] [PubMed]
- Angioni, R.; Sánchez-Rodríguez, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A.; et al. Age-Severity Matched Cytokine Profiling Reveals Specific Signatures in Covid-19 Patients. Cell Death Dis. 2020, 11, 957. [Google Scholar] [CrossRef]
- Dorgham, K.; Quentric, P.; Gökkaya, M.; Marot, S.; Parizot, C.; Sauce, D.; Guihot, A.; Luyt, C.-E.; Schmidt, M.; Mayaux, J.; et al. Distinct Cytokine Profiles Associated with COVID-19 Severity and Mortality. J. Allergy Clin. Immunol. 2021, 147, 2098–2107. [Google Scholar] [CrossRef]
- Sundén-Cullberg, J.; Nyström, T.; Lee, M.L.; Mullins, G.E.; Tokics, L.; Andersson, J.; Norrby-Teglund, A.; Treutiger, C.J. Pronounced Elevation of Resistin Correlates with Severity of Disease in Severe Sepsis and Septic Shock. Crit. Care Med. 2007, 35, 1536–1542. [Google Scholar] [CrossRef]

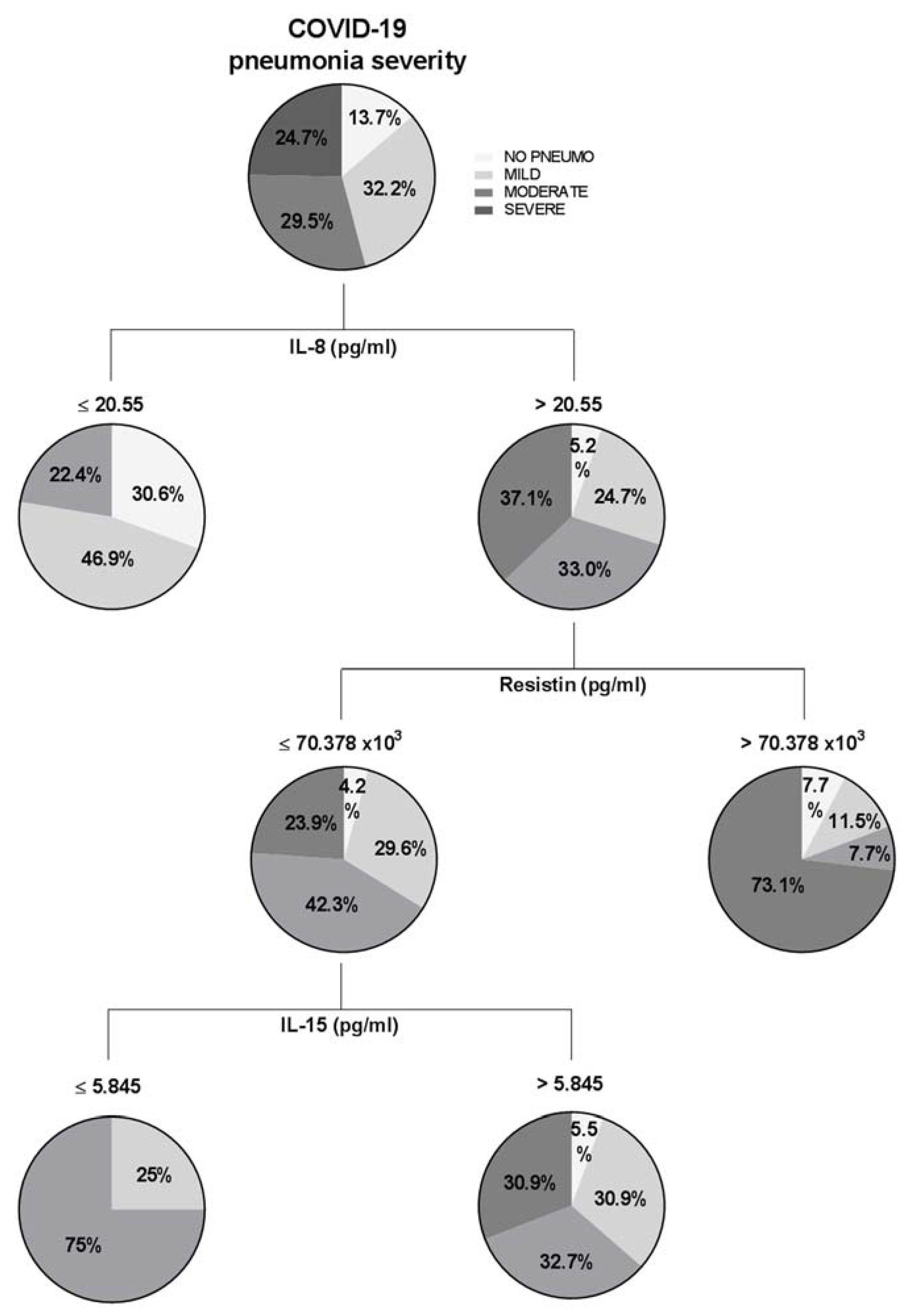
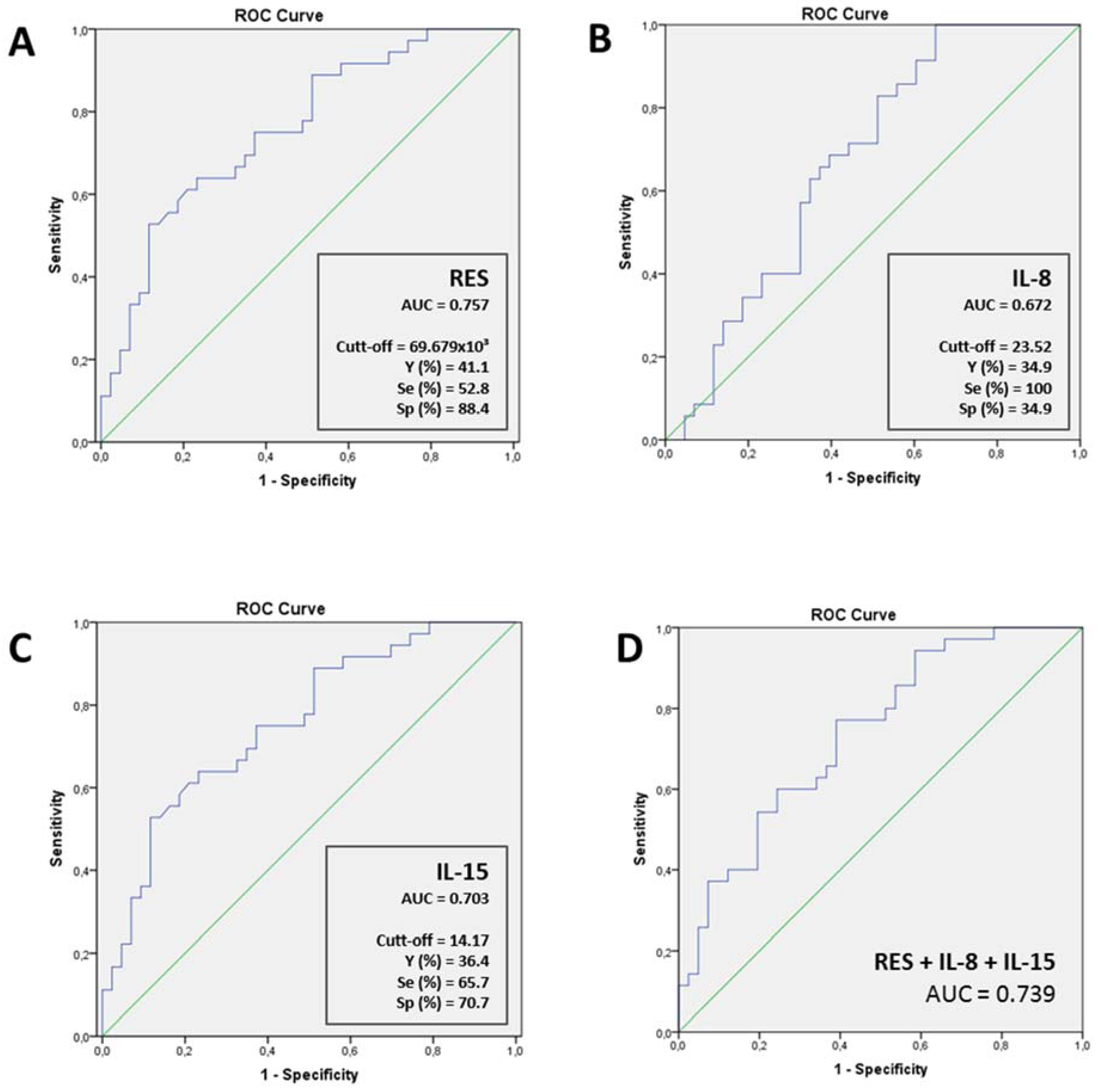
| Pneumonia Severity | ||||
|---|---|---|---|---|
| Variables | Mild (WHO 3) n = 47 | Moderate (WHO 4–5) n = 43 | Severe (WHO 6–7–8) n = 36 | p-Value |
| Gender—n (%): | ||||
| Male | 21 (44.70) | 22 (51.20) | 25 (69.40) | 0.074 |
| Age—mean (SD): | ||||
| 57.70 (18.16) | 62.19 (13.33) | 60.64 (11.49) | 0.478 | |
| Admission stay—mean (SD): | ||||
| 7.83 (9.73) | 8.79 (5.40) | 34.47 (23.83) | <0.001 * | |
| Comorbidities—n (%): | ||||
| Obesity | 10 (23.80) | 13 (31.70) | 9 (26.50) | 0.717 |
| Metabolic syndrome | 7 (15.90) | 2 (8.30) | 4 (11.10) | 0637 |
| Diabetes mellitus | 7 (14.90) | 5 (11.60) | 9 (25.00) | 0.263 |
| Hypertension | 16 (34.00) | 26 (60.50) | 11 (30.60) | 0.011 * |
| Dyslipemia | 15 (31.90) | 12 (27.90) | 12 (33.30) | 0.861 |
| Cardiovascular disease | 5 (10.60) | 4 (9.30) | 3 (8.30) | 0.936 |
| Death—n (%) | ||||
| 0 (0) | 0 (0) | 6 (16.70) | <0.001 * | |
| FOLLOW-UP | |||||
|---|---|---|---|---|---|
| Variables | Baseline Mean (SD) or Median (IQR) n = 146 | 4–6 Weeks Mean (SD) or Median (IQR) n = 146 | p-Value | ||
| Leukocytes (×109/L) | 6.49 | (4.89–8.19) | 6.14 | (4.95–7.49) | 0.734 |
| Lymphocytes (×109/L) | 1.10 | (0.59) | 1.92 | (0.76) | <0.001 * |
| Hemoglobin (g/dL) | 12.95 | (11.62–13.9) | 12.70 | (11.60–13.62) | 0.139 |
| D-Dimer (ng/mL) | 540.00 | (382.75–888.25) | 434.00 | (294.00–683.00) | 0.070 |
| ESR (mm) | 65.15 | (37.22) | 48.83 | (53.89) | 0.655 |
| Ferritin (ng/mL) | 385.00 | (160.00–606.00) | 153.00 | (58.00–248.25) | <0.001 * |
| CRP (mg/dL) | 7.00 | (3.00–14.00) | 0.40 | (0–0.70) | <0.001 * |
| Glucose (mg/dL) | 103.00 | (87.00–128.00) | 93.00 | (85.00–110.25) | 0.054 |
| Total-cholesterol (mg/dL) | 140.61 | (35.39) | 173.51 | (50.64) | 0.005 * |
| Creatinine (mg/dL) | 0.75 | (0.59–0.89) | 0.79 | (0.62–0.89) | 0.160 |
| AST (U/L) | 31.00 | (23.25–44.00) | 23.00 | (18.00–30.00) | <0.001 * |
| ALT (U/L) | 28.00 | (19.25–51.75) | 26.00 | (19.00–46.00) | 0.332 |
| GGT (U/L) | 45.00 | (26.00–79.00) | 31.00 | (20.00–56.00) | 0.003 * |
| AP (U/L) | 66.00 | (51.75–89.25) | 70.00 | (59.00–84.00) | 0.005 * |
| LDH (U/L) | 285.60 | (80.14) | 197.35 | (42.70) | <0.001 * |
| Cytokines—Median (IQR): | |||||
| Resistin (×103 pg/mL) | 38.86 | (25.67–67.69) | 23.69 | (17.73–35.68) | <0.001 * |
| INF-β (pg/mL) | 889.50 | (333.14–2230.71) | 1080.17 | (435.38–2289.21) | 0.779 |
| INF-γ (pg/mL) | 11.83 | (5.50–23.86) | 5.95 | (3.05–14.99) | <0.001 * |
| IL-1β (pg/mL) | 4.91 | (2.22–12.50) | 4.94 | (2.03–15.49) | <0.001 * |
| IL-6 (pg/mL) | 11.70 | (4.90–26.42) | 2.90 | (2.10–5.30) | <0.001 * |
| IL-7 (pg/mL) | 7.38 | (3.93–13.50) | 3.61 | (1.98–6.95) | <0.001 * |
| IL-8 (pg/mL) | 40.68 | (15.88–94.56) | 11.14 | (7.03–23.40) | <0.001 * |
| IL-10 (pg/mL) | 31.12 | (8.97–57.53) | 22.23 | (9.61–92.33) | 0.109 |
| IL-13 (pg/mL) | 14.93 | (9.45–28.17) | 12.86 | (7.56–22.19) | <0.001 * |
| IL-15 (pg/mL) | 10.19 | (6.28–17.20) | 6.42 | (4.17–10.34) | <0.001 * |
| IL-17A (pg/mL) | 11.16 | (2.90–25.53) | 9.73 | (3.48–27.97) | 0.013 * |
| IL-18 (pg/mL) | 58.06 | (33.41–89.35) | 27.52 | (14.36–43.54) | <0.001 * |
| MCP-1 (pg/mL) | 731.42 | (528.77–1047.76) | 775.77 | (605.63–932.75) | 0.826 |
| TNF-α (pg/mL) | 46.42 | (27.22–61.52) | 30.77 | (18.33–47.89) | <0.001 * |
| Variables | WOB Mean (SD); n (%) n = 109 | OB Mean (SD); n (%) n = 37 | p-Value | |
|---|---|---|---|---|
| Age (years) | 58.43 (15.65) | 53.46 (15.17) | 0.091 | |
| Sex N (%) | Male | 57 (55.9) | 14 (40.0) | 0.106 |
| BMI (kg/m2) | 25.27 (2.90) | 34.99 (4.65) | <0.001 * | |
| SBP (mmHg) | 128.75 (19.90) | 131.37 (18.04) | 0.409 | |
| DBP (mmHg) | 78.82 (9.96) | 80 (71–88.25) | 0.104 | |
| Admission stay—mean (SD) | 15.26 (17.16) | 12.70 (16.43) | 0.502 | |
| ICU admission N (%) | 21 (20.60) | 7 (20.00) | 0.941 | |
| Invasive ventilatory support need N (%) | 25 (24.5) | 9 (25.7) | 0.402 | |
| Mortality N (%) | 5 (4.90) | 1 (2.90) | 0.611 | |
| Biochemical parameters—Mean (SD) or Median (IQR): | ||||
| Leukocytes (×109/L) | 6.51 (4.70–8.19) | 6.09 (5.20–7.68) | 0.998 | |
| Lymphocytes (×109/L) | 1.02 (0.56) | 1.31 (0.63) | 0.009 * | |
| Hemoglobin (g/dL) | 13.00 (11.55–13.80) | 12.80 (12.00–14.05) | 0.437 | |
| D-Dimer (ng/mL) | 541 (367.00–1047.00) | 554.5 (411.00–746.00) | 0.751 | |
| ESR (mm) | 68.05 (36.56) | 54.23 (35.88) | 0.083 | |
| Ferritin (ng/mL) | 396 (154.00–701.00) | 351.5 (154.50–457.50) | 0.317 | |
| CRP (mg/dL) | 6.65 (2.82–13.42) | 6.95 (3.10–14.72) | 0.681 | |
| Glucose (mg/dL) | 103.5 (88.00–128.75) | 103 (84.00–130.00) | 0.954 | |
| Total-cholesterol (mg/dL) | 141.44 (38.50) | 140.35 (29.27) | 0.718 | |
| Creatinine (mg/dL) | 0.77 (0.58–0.89) | 0.70 (0.61–0.88) | 0.685 | |
| AST (U/L) | 30 (23.50–43.50) | 31 (23.50–44.50) | 0.583 | |
| ALT (U/L) | 28 (19.00–53.00) | 33 (23.50–51.50) | 0.271 | |
| GGT (U/L) | 44 (24.00–76.00) | 54 (33.00–87.00) | 0.058 | |
| AP (U/L) | 66 (49.00–90.00) | 67 (53.00–90.75) | 0.666 | |
| LDH (U/L) | 280.25 (86.97) | 293.64 (55.43) | 0.138 | |
| Troponin (ng/L) | 6 (2.00–14.25) | 4 (3.00–14.00) | 0.675 | |
| Cytokines—Median (IQR): | ||||
| Resistin (×103 pg/mL) | 36.62 (21.37–54.32) | 35.43 (29.26–69.95) | 0.225 | |
| INF-β (pg/mL) | 1386.98 (568.33–2230.71) | 299.07 (228.25–2497.14) | 0.184 | |
| INF-γ (pg/mL) | 11.69 (5.20–23.83) | 8.78 (5.87–20.56) | 0.846 | |
| IL-1β (pg/mL) | 5.80 (2.63–13.17) | 4.50 (1.88–14.98) | 0.645 | |
| IL-6 (pg/mL) | 8.90 (4.78–23.65) | 11.70 (5.22–16.52) | 0.957 | |
| IL-7 (pg/mL) | 8.12 (3.56–14.13) | 6.61 (3.91–11.96) | 0.828 | |
| IL-8 (pg/mL) | 37.81 (13.25–86.42) | 68.61 (21.73–127.80) | 0.076 | |
| IL-10 (pg/mL) | 50.30 (28.72–90.48) | 35.88 (7.66–55.53) | 0.979 | |
| IL-13 (pg/mL) | 14.79 (8.96–27.36) | 16.44 (11.75–34.77) | 0.462 | |
| IL-15 (pg/mL) | 10.83 (6.30–19.71) | 7.73 (5.49–14.27) | 0.137 | |
| IL-17A (pg/mL) | 11.04 (2.29–30.41) | 11.16 (3.48–20.52) | 0.797 | |
| IL-18 (pg/mL) | 50.30 (28.72–90.48) | 60.94 (40.4–79.69) | 0.302 | |
| MCP-1 (pg/mL) | 725.81 (505.47–1021.62) | 751.19 (514.62–1077.91) | 0.642 | |
| TNF-α (pg/mL) | 43.85 (25.42–58.66) | 53.20 (27.49–70.01) | 0.132 | |
| Variables | Non-MS Mean (SD); n (%) n = 109 | MS Mean (SD); n (%) n = 15 | p-value | |
|---|---|---|---|---|
| Age (years) | 55.60 (16.63) | 68.80 (9.26) | 0.003 * | |
| Sex N (%) | Male | 59.00 (54.10) | 9.00 (60.00) | 0.670 |
| BMI (kg/m2) | 27.20 (5.49) | 30.93 (4.48) | 0.002 * | |
| SBP (mmHg) | 128.64 (20.56) | 134.20 (15.38) | 0.212 | |
| DBP (mmHg) | 79.45 (10.35) | 78.53 (10.22) | 0.615 | |
| Admission stay—mean (SD) | 17.51 (19.43) | 13.71 (20.67) | 0.432 | |
| ICU admission N (%) | 28 (25.70) | 2 (13.30) | 0.297 | |
| Invasive ventilatory support need N (%) | 32 (29.40) | 4 (26.70) | 0.579 | |
| Mortality N (%) | 5 (4.60) | 1 (6.70) | 0.726 | |
| Biochemical parameters—Mean (SD) or Median (IQR): | ||||
| Leukocytes (×109/L) | 6.55 (4.85–8.56) | 6.68 (5.59–7.60) | 0.981 | |
| Lymphocytes (×109/L) | 1.07 (0.60) | 1.14 (0.61) | 0.631 | |
| Hemoglobin (g/dL) | 12.95 (11.62–13.90) | 12.40 (10.75–14.25) | 0.456 | |
| D-Dimer (ng/mL) | 545.50 (389.50–888.25) | 554.50 (313.00–813.75) | 0.634 | |
| ESR (mm) | 66.91 (36.78) | 59.33 (48.37) | 0.402 | |
| Ferritin (ng/mL) | 391.50 (147.25–685.75) | 396.00 (142.00–492.50) | 0.855 | |
| CRP (mg/dL) | 7.10 (3.00–14.00) | 6.45 (2.12–17.75) | 0.748 | |
| Glucose (mg/dL) | 103.00 (86.00–123.00) | 130.00 (102.75–150.75) | <0.001 * | |
| Total-cholesterol (mg/dL) | 135.23 (33.97) | 141.90 (33.83) | 0.462 | |
| Creatinine (mg/dL) | 0.75 (0.58–0.89) | 0.86 (0.73–0.98) | 0.054 | |
| AST (U/L) | 31.50 (25.00–44.75) | 22.50 (19.50–53.75) | 0.272 | |
| ALT (U/L) | 30.50 (20.25–54.00) | 25.50 (16.00–63.75) | 0.484 | |
| GGT (U/L) | 44.00 (27.00–79.00) | 55.00 (23.75–129.50) | 0.406 | |
| AP (U/L) | 67.00 (53.00–89.00) | 64.00 (48.00–115.50) | 0.682 | |
| LDH (U/L) | 192.90 (42.02) | 212.08 (59.68) | 0.835 | |
| Troponin (ng/L) | 6.00 (2–14.25) | 10.50 (4.25–27.75) | 0.094 | |
| Cytokines—Median (IQR): | ||||
| Resistin (×103 pg/mL) | 41.22 (23.52–71.23) | 39.76 (32.58–69.95) | 0.878 | |
| INF-β (pg/mL) | 889.50 (333.15–2466.04) | 996.43 (299.07-) | 0.565 | |
| INF-γ (pg/mL) | 11.40 (5.35–21.77) | 15.35 (5.37–25.69) | 0.825 | |
| IL-1β (pg/mL) | 5.89 (2.43–13.17) | 2.49 (1.09–5.47) | 0.035 * | |
| IL-6 (pg/mL) | 11.8 (5.10–28.15) | 15.60 (3.00–29.10) | 0.905 | |
| IL-7 (pg/mL) | 7.43 (3.36–14.12) | 6.03 (3.95–10.07) | 0.712 | |
| IL-8 (pg/mL) | 40.55 (14.41–98.12) | 68.61 (27.97–94.56) | 0.338 | |
| IL-10 (pg/mL) | 35.03 (8.19–65.49) | 24.42 (4.98–50.54) | 0.474 | |
| IL-13 (pg/mL) | 15.48 (8.96–30.80) | 12.03 (5.15–19.05) | 0.141 | |
| IL-15 (pg/mL) | 10.73 (6.88–17.52) | 9.90 (6.57–15.88) | 0.594 | |
| IL-17A (pg/mL) | 11.39 (2.44–25.53) | 3.48 (2.29–11.40) | 0.103 | |
| IL-18 (pg/mL) | 52.50 (24.62–81.20) | 68.81 (47.18–103.74) | 0.203 | |
| MCP-1 (pg/mL) | 753.29 (559.36–1071.87) | 829.87 (392.41–954.92) | 0.449 | |
| TNF-α (pg/mL) | 44.96 (26.33–65.54) | 44.96 (36.10–57.63) | 0.837 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpiñan, C.; Bertran, L.; Terra, X.; Aguilar, C.; Binetti, J.; Lopez-Dupla, M.; Rull, A.; Reverté, L.; Yeregui, E.; Gómez-Bertomeu, F.; et al. Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome. J. Pers. Med. 2022, 12, 391. https://doi.org/10.3390/jpm12030391
Perpiñan C, Bertran L, Terra X, Aguilar C, Binetti J, Lopez-Dupla M, Rull A, Reverté L, Yeregui E, Gómez-Bertomeu F, et al. Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome. Journal of Personalized Medicine. 2022; 12(3):391. https://doi.org/10.3390/jpm12030391
Chicago/Turabian StylePerpiñan, Carles, Laia Bertran, Ximena Terra, Carmen Aguilar, Jessica Binetti, Miguel Lopez-Dupla, Anna Rull, Laia Reverté, Elena Yeregui, Frederic Gómez-Bertomeu, and et al. 2022. "Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome" Journal of Personalized Medicine 12, no. 3: 391. https://doi.org/10.3390/jpm12030391
APA StylePerpiñan, C., Bertran, L., Terra, X., Aguilar, C., Binetti, J., Lopez-Dupla, M., Rull, A., Reverté, L., Yeregui, E., Gómez-Bertomeu, F., Peraire, J., Auguet, T., & on behalf of COVID-19 Study Group. (2022). Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome. Journal of Personalized Medicine, 12(3), 391. https://doi.org/10.3390/jpm12030391








