The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Sample
2.2. Cognitive Impairment Assessment
2.3. Bodily Pain Assessment
2.4. Covariates
2.5. Statistical Analysis
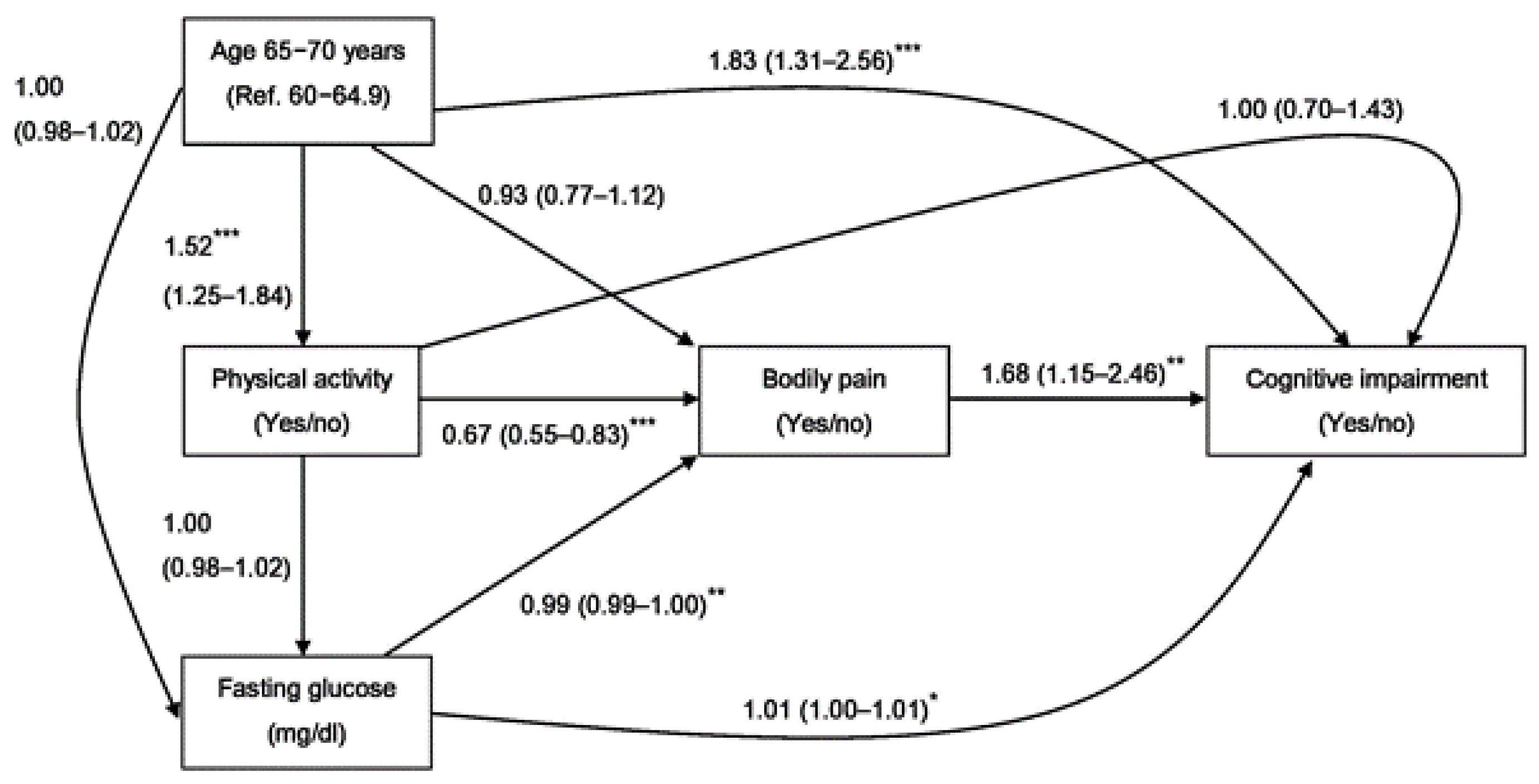
3. Results
3.1. Descriptive Statistics
3.2. Association between Bodily Pain Factors and Cognitive Impairment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachdev, P.S.; Lipnicki, D.M.; Kochan, N.A.; Crawford, J.D.; Thalamuthu, A.; Andrews, G.; Brayne, C.; Matthews, F.E.; Stephan, B.C.M.; Lipton, R.B.; et al. Cohort Studies of Memory in an International, Consortium. The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PLoS ONE 2015, 10, e0142388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lee, H.J.; Yang, S.C.; Chen, T.F.; Lin, K.N.; Lin, C.C.; Wang, P.-N.; Tang, L.-Y.; Chiu, M.-J. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS ONE 2014, 9, e100303. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International: London, UK, 2016. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nieuwenhuis-Mark, R.E. The death knoll for the MMSE: Has it outlived its purpose? J. Geriatr. Psychiatry Neurol. 2010, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Horgas, A.L. Pain Assessment in Older Adults. Nurs. Clin. N. Am. 2017, 52, 375–385. [Google Scholar] [CrossRef]
- Savvas, S.M.; Gibson, S.J. Overview of Pain Management in Older Adults. Clin. Geriatr. Med. 2016, 32, 635–650. [Google Scholar] [CrossRef]
- Abdulla, A.; Adams, N.; Bone, M.; Elliott, A.M.; Gaffin, J.; Jones, D.; Knaggs, R.; Martin, D.; Sampson, L.; Schofield, P.; et al. Guidance on the management of pain in older people. Age Ageing 2013, 42 (Suppl. 1), i1–i57. [Google Scholar] [CrossRef] [Green Version]
- Moriarty, O.; Finn, D.P. Cognition and pain. Curr. Opin. Support. Palliat. Care 2014, 8, 130–136. [Google Scholar] [CrossRef]
- Berryman, C.; Stanton, T.R.; Jane Bowering, K.; Tabor, A.; McFarlane, A.; Lorimer Moseley, G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain 2013, 154, 1181–1196. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepker, C.A.; Leveille, S.G.; Pedersen, M.M.; Ward, R.E.; Kurlinski, L.A.; Grande, L.; Kiely, D.K.; Bean, J.F. Effect of Pain and Mild Cognitive Impairment on Mobility. J. Am. Geriatr. Soc. 2016, 64, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, K.M.; Jordan, K.P.; Croft, P.R. Contributions of prognostic factors for poor outcome in primary care low back pain patients. Eur. J. Pain 2011, 15, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Seminowicz, D.A.; Wideman, T.H.; Naso, L.; Hatami-Khoroushahi, Z.; Fallatah, S.; Ware, M.A.; Jarzem, P.; Bushnell, M.C.; Shir, Y.; Ouellet, J.A.; et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011, 31, 7540–7550. [Google Scholar] [CrossRef] [Green Version]
- van der Leeuw, G.; Eggermont, L.H.; Shi, L.; Milberg, W.P.; Gross, A.L.; Hausdorff, J.M.; Bean, J.F.; Leveille, S.G. Pain and Cognitive Function Among Older Adults Living in the Community. J. Gerontol. Biol. Sci. Med. Sci. 2016, 71, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Weiner, D.K.; Rudy, T.E.; Morrow, L.; Slaboda, J.; Lieber, S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006, 7, 60–70. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Pagonabarraga, J. Cognitive impairment in Parkinson’s disease: Tools for diagnosis and assessment. Mov. Disord. 2009, 24, 1103–1110. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.K.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.-W.; Chang, J.; Song, I.W.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C.; Chen, L.K.; Hsiao, W.W.; Fan, C.T.; Ko, M.L. Next chapter of the Taiwan Biobank: Sustainability and perspectives. Biopreserv. Biobank 2019, 17, 189–197. [Google Scholar] [CrossRef]
- Xiu, S.; Zheng, Z.; Guan, S.; Zhang, J.; Ma, J.; Chan, P. Serum uric acid and impaired cognitive function in community-dwelling elderly in Beijing. Neurosci. Lett. 2017, 637, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.M.; Tiwari, S.C.; Mahdi, A.A.; Kohli, N. Serum Homocysteine and Behavioral and Psychological Symptoms of Dementia: Is There Any Correlation in Alzheimer’s Disease? Ann. Neurosci. 2019, 25, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 13, CD011145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahbour, S.; Hashim, M.; Alhyasat, A.; Salameh, A.; Qtaishat, A.; Braik, R.; Alnimer, T.M.A. Mini-mental state examination (MMSE) scores in elderly Jordanian population. Cereb. Circ. Cogn. Behav. 2021, 2, 100016. [Google Scholar] [CrossRef]
- Hwang, S.J.; Tsai, J.C.; Chen, H.C. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology 2010, 15 (Suppl. 2), 3–9. [Google Scholar] [CrossRef]
- Scemes, E.; Zammit, A.R.; Katz, M.J.; Lipton, R.B.; Derby, C.A. Associations of cognitive function and pain in older adults. Int. J. Geriatr. Psychiatry 2017, 32, 118–120. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.L.; McLachlan, A. The Challenges of Treating Sciatica Pain in Older Adults. Drugs Aging 2016, 33, 779–785. [Google Scholar] [CrossRef]
- Dick, B.; Eccleston, C.; Crombez, G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002, 47, 639–644. [Google Scholar] [CrossRef]
- Povedano, M.; Gascon, J.; Galvez, R.; Ruiz, M.; Rejas, J. Cognitive function impairment in patients with neuropathic pain under standard conditions of care. J. Pain Symptom Manag. 2007, 33, 78–89. [Google Scholar] [CrossRef]
- Eggermont, L.H.; Leveille, S.G.; Shi, L.; Kiely, D.K.; Shmerling, R.H.; Jones, R.N.; Guralnik, J.M.; Bean, J.F. Pain characteristics associated with the onset of disability in older adults: The maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J. Am. Geriatr. Soc. 2014, 62, 1007–1016. [Google Scholar] [CrossRef]
- Thakral, M.; Shi, L.; Foust, J.B.; Patel, K.V.; Shmerling, R.H.; Bean, J.F.; Leveille, S.G. Pain quality descriptors in community-dwelling older adults with nonmalignant pain. Pain 2016, 157, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Valentin, G.H.; Pilegaard, M.S.; Vaegter, H.B.; Rosendal, M.; Ortenblad, L.; Vaeggemose, U.; Christensen, R. Prognostic factors for disability and sick leave in patients with subacute non-malignant pain: A systematic review of cohort studies. BMJ Open 2016, 6, e007616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.D.; Shellman, J.M.; Graham, L.; Harrison, L. The Relationship Between Reminiscence Functions, Optimism, Depressive Symptoms, Physical Activity, and Pain in Older Adults. Res. Gerontol. Nurs. 2016, 9, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; Eccleston, C.; Pillemer, K. Management of chronic pain in older adults. BMJ 2015, 350, h532. [Google Scholar] [CrossRef] [Green Version]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Perlmuter, L.C.; Hakami, M.K.; Hodgson-Harrington, C.; Ginsberg, J.; Katz, J.; Singer, D.E.; Nathan, D.M. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am. J. Med. 1984, 77, 1043–1048. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. 2007, 32, 394–399. [Google Scholar]
- Korb, A.; Bonetti, L.V.; da Silva, S.A.; Marcuzzo, S.; Ilha, J.; Bertagnolli, M.; Partata, W.A.; Faccioni-Heuser, M.C. Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem. Res. 2010, 35, 380–389. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef] [Green Version]
- Kurita, S.; Tsutsumimoto, K.; Doi, T.; Nakakubo, S.; Kim, M.; Ishii, H.; Shimada, H. Association of physical and/or cognitive activity with cognitive impairment in older adults. Geriatr. Gerontol. Int. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Bodily Pain (n = 1332) | No Bodily Pain (n = 690) | p Value | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Age (Years) | 0.090 | ||||
| 60–64.9 | 747 | (56.1) | 359 | (52.0) | |
| 65–70 | 585 | (43.9) | 331 | (48.0) | |
| Gender | <0.001 | ||||
| Male | 577 | (43.3) | 400 | (58.0) | |
| Female | 755 | (56.7) | 290 | (42.0) | |
| Marital status | 0.599 | ||||
| Unmarried | 32 | (2.4) | 12 | (1.7) | |
| Married | 1061 | (79.7) | 557 | (80.7) | |
| Others | 239 | (17.9) | 121 | (17.5) | |
| Alcohol drinking | 0.235 | ||||
| No | 1210 | (90.8) | 615 | (89.1) | |
| Yes | 122 | (9.2) | 75 | (10.9) | |
| Smoking | 0.419 | ||||
| No | 1001 | (75.1) | 507 | (73.5) | |
| Yes | 331 | (24.9) | 183 | (26.5) | |
| Routine physical activity | <0.001 | ||||
| No | 502 | (37.7) | 191 | (27.7) | |
| Yes | 830 | (62.3) | 499 | (72.3) | |
| Body mass index (kg/m2) | 24.5 | (3.1) | 24.4 | (3.1) | 0.327 |
| Waist circumference (cm) | 86.3 | (9.1) | 86.4 | (8.9) | 0.814 |
| Total cholesterol (mg/dL) | 200.8 | (35.6) | 198.4 | (35.7) | 0.151 |
| Triglyceride (mg/dL) | 119.8 | (88.5) | 110.4 | (59.5) | 0.005 |
| HDL cholesterol (mg/dL) | 54.0 | (13.2) | 54.0 | (13.9) | 0.979 |
| LDL cholesterol (mg/dL) | 125.2 | (32.4) | 124.5 | (30.7) | 0.621 |
| Fasting glucose (mg/dL) | 101.2 | (21.7) | 104.5 | (26.7) | 0.006 |
| GFR (mL/min/1.73 m2) | 100.3 | (24.2) | 98.0 | (23.0) | 0.033 |
| Variables | Cognitive Impairment (n = 161) | No Cognitive Impairment (n = 1861) | p Value | Multivariable Model b | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | OR | (95% CI) | p Value | ||
| Bodily Pain | 0.005 | |||||||
| No | 39 | (24.2) | 651 | (35.0) | 1.00 | |||
| Yes | 122 | (75.8) | 1210 | (65.0) | 1.68 | (1.15–1.16) | 0.008 | |
| Number of pain locations | 0.017 | |||||||
| 0 | 39 | (24.2) | 651 | (35.0) | 1.00 | |||
| 1 | 58 | (36.0) | 615 | (33.0) | 1.61 | (1.05–2.48) | 0.029 | |
| ≥2 | 64 | (39.8) | 595 | (32.0) | 1.75 | (1.14–2.68) | 0.010 | |
| Pain location a | ||||||||
| Articular pain | 60 | (37.3) | 581 | (31.2) | 0.133 | 1.30 | (0.92–1.83) | 0.131 |
| Neck pain | 48 | (29.8) | 502 | (27.0) | 0.460 | 1.11 | (0.77–1.59) | 0.576 |
| Low back and waist pain | 63 | (39.1) | 561 | (30.1) | 0.021 | 1.47 | (1.04–2.06) | 0.028 |
| Sciatica | 27 | (16.8) | 199 | (10.7) | 0.026 | 1.66 | (1.06–2.59) | 0.027 |
| Hemicrania continua | 27 | (16.8) | 259 | (13.9) | 0.345 | 1.24 | (0.80–1.94) | 0.337 |
| Other pain | 7 | (4.4) | 71 | (3.8) | 0.670 | 1.20 | (0.54–2.69) | 0.655 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Lee, L.-H.; Lin, W.-S.; Hsiao, T.-H.; Chen, I.-C.; Lin, C.-H. The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults. J. Pers. Med. 2022, 12, 350. https://doi.org/10.3390/jpm12030350
Huang C-C, Lee L-H, Lin W-S, Hsiao T-H, Chen I-C, Lin C-H. The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults. Journal of Personalized Medicine. 2022; 12(3):350. https://doi.org/10.3390/jpm12030350
Chicago/Turabian StyleHuang, Chun-Che, Li-Hui Lee, Wei-Szu Lin, Tzu-Hung Hsiao, I-Chieh Chen, and Ching-Heng Lin. 2022. "The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults" Journal of Personalized Medicine 12, no. 3: 350. https://doi.org/10.3390/jpm12030350
APA StyleHuang, C.-C., Lee, L.-H., Lin, W.-S., Hsiao, T.-H., Chen, I.-C., & Lin, C.-H. (2022). The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults. Journal of Personalized Medicine, 12(3), 350. https://doi.org/10.3390/jpm12030350






