MicroRNAs as Predictors of Future Uterine Malignancy in Endometrial Hyperplasia without Atypia
Abstract
:1. Introduction
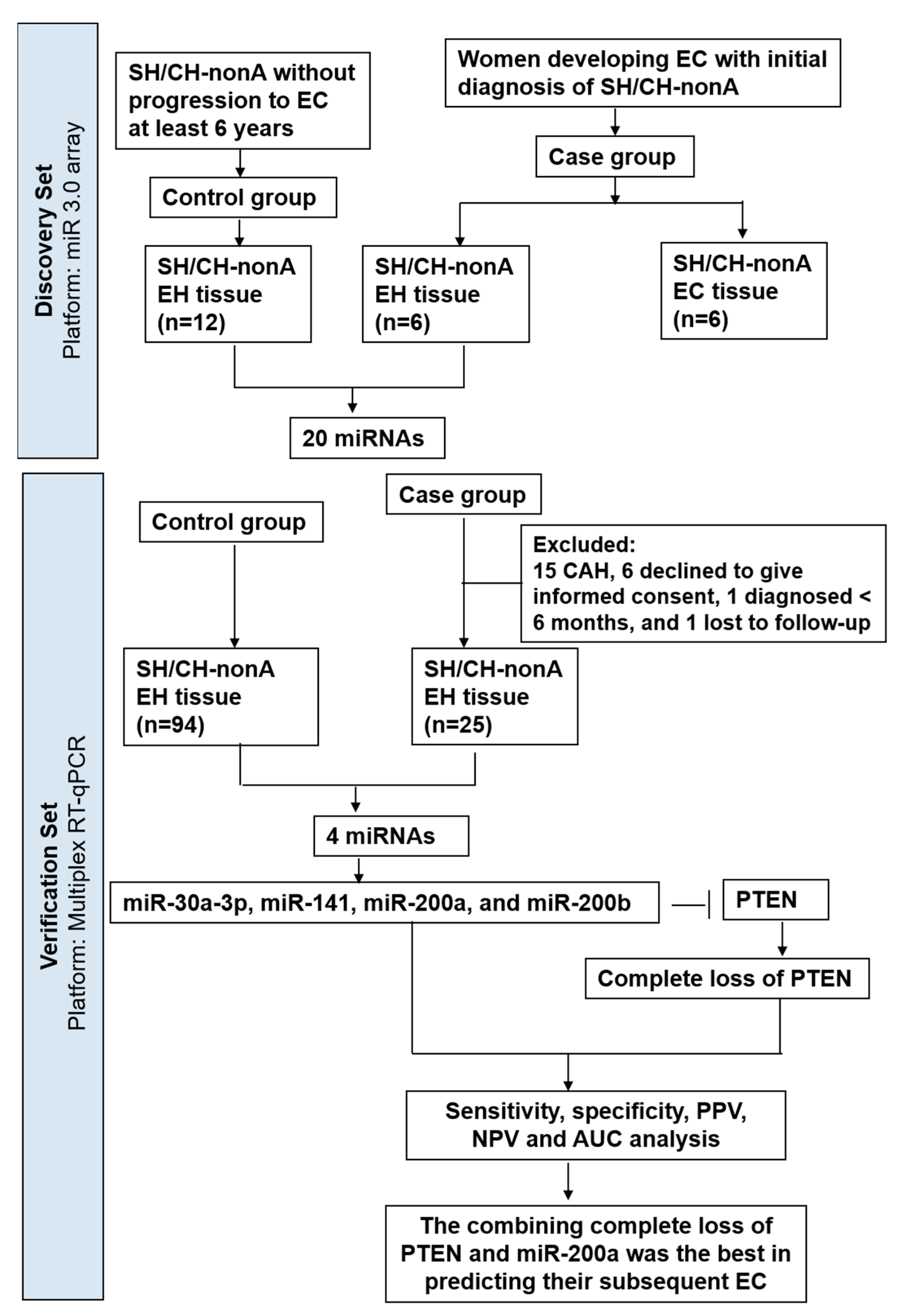
2. Materials and Methods
2.1. Tissue Samples
2.2. RNA Extraction from Formalin-Fixed Paraffin-Embedded Tissues
2.3. MiRNA 3.0 Array Analyses and Target Prediction of miRNA
2.4. Reverse Transcription
2.5. Quantitative PCR
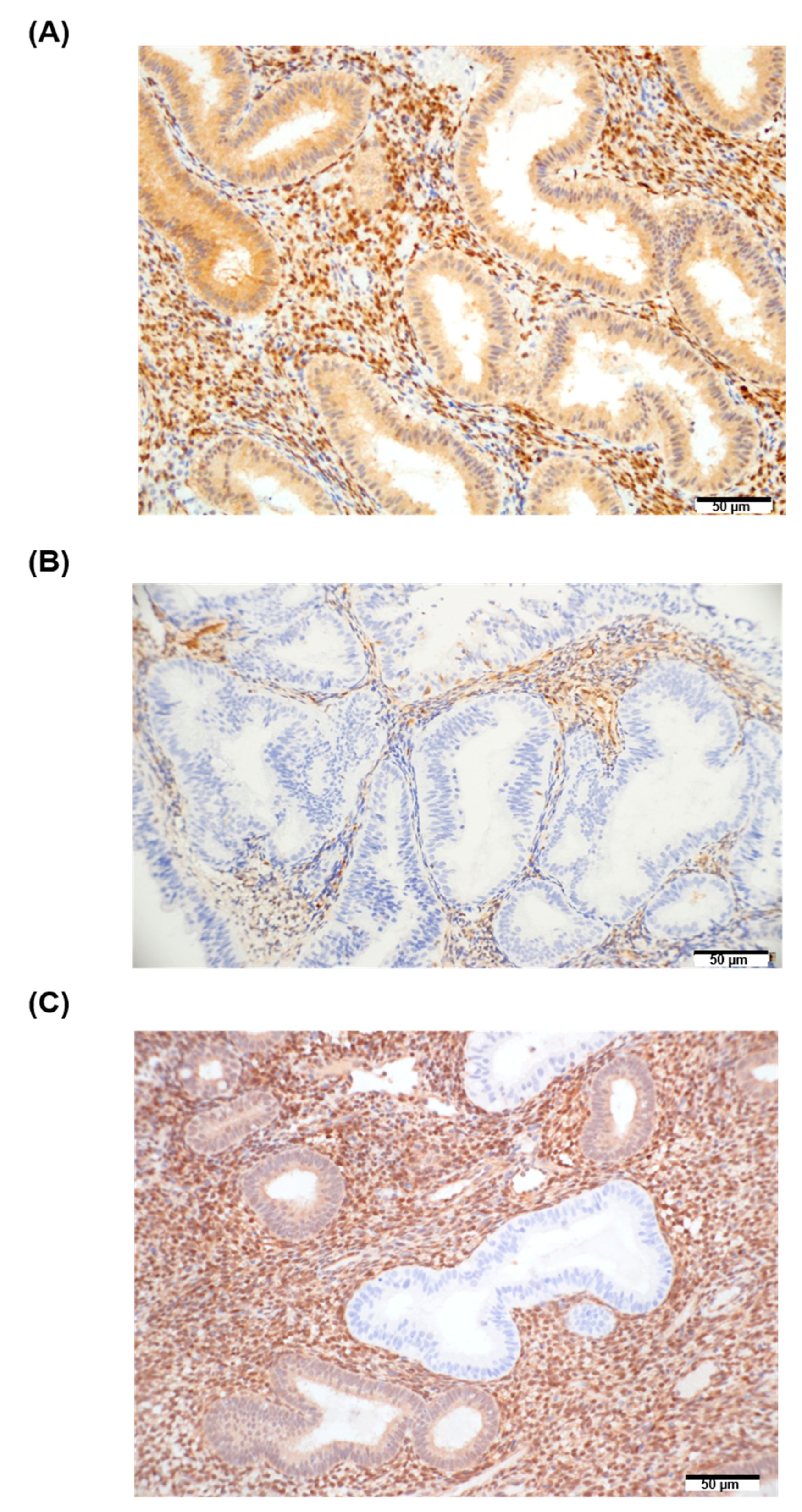
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
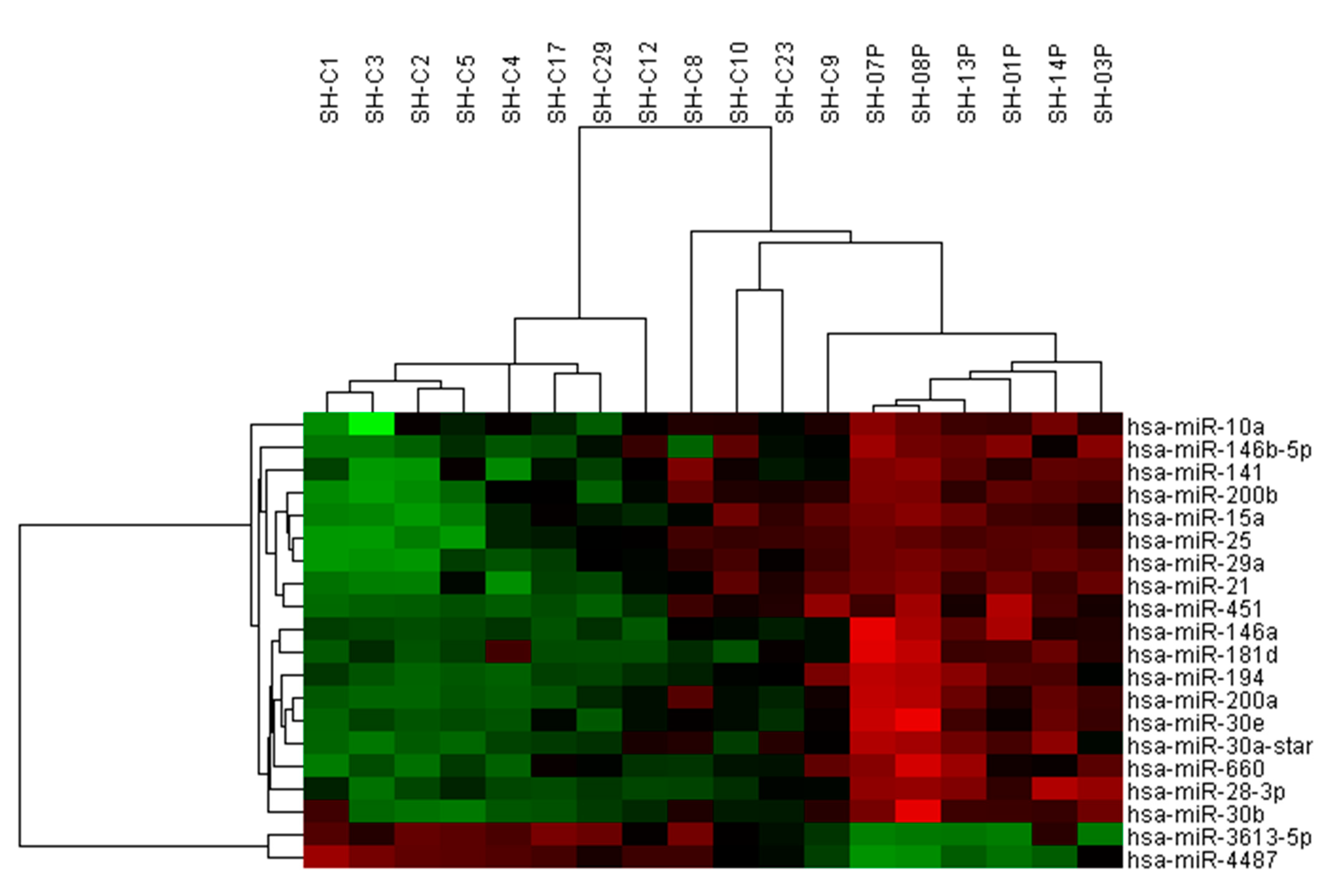
3.1. Use of miRNA Arrays to Measure miRNA Levels in Clinical Samples
3.2. TaqMan RT-qPCR to Measure miRNA Expression in Expanded Cohort
3.3. BECN1 and PTEN in EH Tissue Samples and Their Association with miRNA Expression
3.4. The miRNA Expression in EC and EH Tissue of Case Group
3.5. Combination of the miRNAs and PTEN Panel to Predict SH/CH-nonA Progression to EC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration Ministry of Health and Welfare Taiwan. 2018 Cancer Registry Annual Report, Taiwan. Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/6069/File_5962.pdf (accessed on 25 July 2021).
- Sobczuk, K.; Sobczuk, A. New classification system of endometrial hyperplasia WHO 2014 and its clinical implications. Prz. Menopauzalny 2017, 16, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef]
- Zaino, R.J.; Kauderer, J.; Trimble, C.L.; Silverberg, S.G.; Curtin, J.P.; Lim, P.C.; Gallup, D.G. Reproducibility of the diagnosis of atypical endometrial hyperplasia: A Gynecologic Oncology Group study. Cancer 2006, 106, 804–811. [Google Scholar] [CrossRef]
- Alexovic, M.; Sabo, J.; Longuespee, R. Automation of single-cell proteomic sample preparation. Proteomics 2021, 21, e2100198. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Lin, C.Y.; Lee, Y.S.; Tsai, C.L.; Wei, P.C.; Hsueh, S.; Wu, T.I.; Tsai, C.N.; Wang, C.J.; Chao, A.S.; et al. Regulation of ovarian cancer progression by microRNA-187 through targeting Disabled homolog-2. Oncogene 2012, 31, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sianou, A.; Galyfos, G.; Moragianni, D.; Andromidas, P.; Kaparos, G.; Baka, S.; Kouskouni, E. The role of microRNAs in the pathogenesis of endometrial cancer: A systematic review. Arch. Gynecol. Obstet. 2015, 292, 271–282. [Google Scholar] [CrossRef]
- Snowdon, J.; Zhang, X.; Childs, T.; Tron, V.A.; Feilotter, H. The microRNA-200 family is upregulated in endometrial carcinoma. PLoS ONE 2011, 6, e22828. [Google Scholar] [CrossRef]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int. J. Cancer 2013, 132, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Donkers, H.; Bekkers, R.; Galaal, K. Diagnostic value of microRNA panel in endometrial cancer: A systematic review. Oncotarget 2020, 11, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, H.J.; Kang, C.S.; Lee, H.J.; Lee, W.S.; Park, C.S. Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma. Mod. Pathol. 2012, 25, 1508–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tantbirojn, P.; Triratanachat, S.; Trivijitsilp, P.; Niruthisard, S. Detection of PTEN immunoreactivity in endometrial hyperplasia and adenocarcinoma. J. Med. Assoc. Thai. 2008, 91, 1161–1165. [Google Scholar] [PubMed]
- Sangwan, K.; Garg, M.; Pathak, N.; Bharti, L. Expression of Cyclin D1 in Hyperplasia and Carcinoma of Endometrium and Its Correlation with Histologic Grade, Tumor Type, and Clinicopathological Features. J. Lab. Physicians 2020, 12, 165–170. [Google Scholar] [CrossRef]
- Russo, M.; Newell, J.M.; Budurlean, L.; Houser, K.R.; Sheldon, K.; Kesterson, J.; Phaeton, R.; Hossler, C.; Rosenberg, J.; DeGraff, D.; et al. Mutational profile of endometrial hyperplasia and risk of progression to endometrioid adenocarcinoma. Cancer 2020, 126, 2775–2783. [Google Scholar] [CrossRef]
- von Ahlfen, S.; Missel, A.; Bendrat, K.; Schlumpberger, M. Determinants of RNA quality from FFPE samples. PLoS ONE 2007, 2, e1261. [Google Scholar] [CrossRef]
- Zhong, S.; Ma, T.; Zhang, X.; Lv, M.; Chen, L.; Tang, J.; Zhao, J. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in vinorelbine-resistant breast cancer cells. Gene 2015, 556, 113–118. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Q.; Gong, Y.; Huang, L.; Lin, H.; Zhou, X.; Liang, X.; Guo, W. MiR-486 promotes proliferation and suppresses apoptosis in myeloid cells by targeting Cebpa in vitro. Cancer Med. 2018, 7, 4627–4638. [Google Scholar] [CrossRef]
- Kaddis, J.S.; Wai, D.H.; Bowers, J.; Hartmann, N.; Baeriswyl, L.; Bajaj, S.; Anderson, M.J.; Getts, R.C.; Triche, T.J. Influence of RNA labeling on expression profiling of microRNAs. J. Mol. Diagn. 2012, 14, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Chao, A.; Wu, R.C.; Lee, L.Y.; Ueng, S.H.; Tsai, C.L.; Lee, Y.S.; Peng, M.T.; Yang, L.Y.; Huang, H.J.; et al. Synergistic effects of pazopanib and hyperthermia against uterine leiomyosarcoma growth mediated by downregulation of histone acetyltransferase 1. J. Mol. Med. 2020, 98, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, R.K.; Shaha, C.; Madhubala, R. MicroRNA expression profiling of Leishmania donovani-infected host cells uncovers the regulatory role of MIR30A-3p in host autophagy. Autophagy 2016, 12, 1817–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Park, Y.A.; Choi, J.J.; Lee, Y.Y.; Kim, C.J.; Choi, C.; Kim, T.J.; Lee, N.W.; Kim, B.G.; Bae, D.S. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol. Oncol. 2011, 120, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.J.W. miRinGO: Prediction of biological processes indirectly targeted by human microRNAs. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, T.S.; Jeon, H.W.; Kim, Y.B.; Kim, Y.A.; Kim, M.A.; Kang, S.B. Aberrant microRNA expression in endometrial carcinoma using formalin-fixed paraffin-embedded (FFPE) tissues. PLoS ONE 2013, 8, e81421. [Google Scholar] [CrossRef]
- Huang, G.L.; Sun, J.; Lu, Y.; Liu, Y.; Cao, H.; Zhang, H.; Calin, G.A. MiR-200 family and cancer: From a meta-analysis view. Mol. Asp. Med. 2019, 70, 57–71. [Google Scholar] [CrossRef]
- Yoneyama, K.; Ishibashi, O.; Kawase, R.; Kurose, K.; Takeshita, T. miR-200a, miR-200b and miR-429 are onco-miRs that target the PTEN gene in endometrioid endometrial carcinoma. Anticancer. Res. 2015, 35, 1401–1410. [Google Scholar]
- Mutter, G.L.; Lin, M.C.; Fitzgerald, J.T.; Kum, J.B.; Baak, J.P.; Lees, J.A.; Weng, L.P.; Eng, C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl. Cancer Inst. 2000, 92, 924–930. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.S.; Deftereos, G.; Morihara, J.; Stern, J.E.; Hawes, S.E.; Swisher, E.; Kiviat, N.B.; Feng, Q. MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features. World J. Surg. Oncol. 2012, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Kim, H.; Kim, Y.; Jeoung, D. miR-30a Regulates the Expression of CAGE and p53 and Regulates the Response to Anti-Cancer Drugs. Mol. Cells 2016, 39, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, O.; Miura, K.; Mishima, H.; Abe, S.; Kaneuchi, M.; Higashijima, A.; Miura, S.; Kinoshita, A.; Yoshiura, K.; Masuzaki, H. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. Gynecol. Oncol. 2014, 132, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, H.; Liu, X.; Li, B.; Chen, Y.; Ren, X.; Liu, C.G.; Yang, J.M. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 2009, 5, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giatromanolaki, A.; Koukourakis, M.I.; Koutsopoulos, A.; Chloropoulou, P.; Liberis, V.; Sivridis, E. High Beclin 1 expression defines a poor prognosis in endometrial adenocarcinomas. Gynecol. Oncol. 2011, 123, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Klionsky, D.J. Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res. 2007, 17, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Cai, Y.; Xu, K.; Ren, X.; Sun, J.; Lu, S.; Chen, J.; Xu, P. Beclin1 overexpression suppresses tumor cell proliferation and survival via an autophagydependent pathway in human synovial sarcoma cells. Oncol. Rep. 2018, 40, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Senfter, D.; Madlener, S.; Krupitza, G.; Mader, R.M. The microRNA-200 family: Still much to discover. Biomol. Concepts 2016, 7, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Li, Y.Y.; Fu, S.; Wang, X.P.; Huang, M.Y.; Zhang, X.; Shao, Q.; Deng, L.; Zeng, M.S.; Zeng, Y.X.; et al. MicroRNA-30a promotes invasiveness and metastasis in vitro and in vivo through epithelial-mesenchymal transition and results in poor survival of nasopharyngeal carcinoma patients. Exp. Biol. Med. 2014, 239, 891–898. [Google Scholar] [CrossRef]
- Iwata, T.; Fujita, T.; Hirao, N.; Matsuzaki, Y.; Okada, T.; Mochimaru, H.; Susumu, N.; Matsumoto, E.; Sugano, K.; Yamashita, N.; et al. Frequent immune responses to a cancer/testis antigen, CAGE, in patients with microsatellite instability-positive endometrial cancer. Clin. Cancer Res. 2005, 11, 3949–3957. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Li, J.; Chen, L.; Huang, B.; Li, S.; Yang, G.; Ding, H.; Wang, F.; Liu, N.; Zhao, Q.; et al. MicroRNA-200b regulates cyclin D1 expression and promotes S-phase entry by targeting RND3 in HeLa cells. Mol. Cell. Biochem. 2010, 344, 261–266. [Google Scholar] [CrossRef]
- Shawana, S.; Kehar, S.I.; Masood, S.; Aamir, I. Immunoexpression of Cyclin D1 and PTEN in Various Endometrial Pathologies. J. Coll. Physicians Surg. Pak. 2016, 26, 277–282. [Google Scholar]

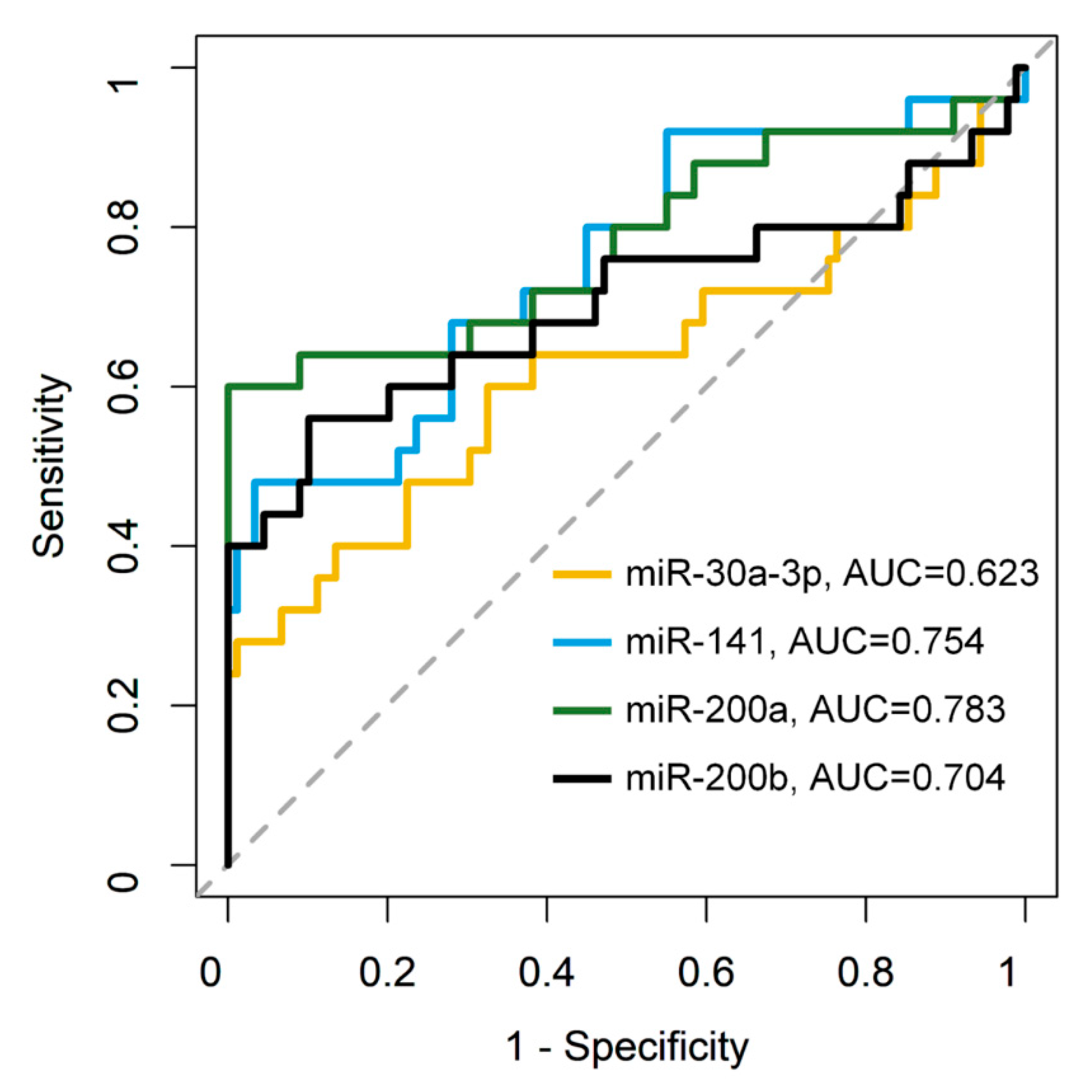
| Variables | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| miR-30a-3p | 0.600 | 0.674 | 0.658 |
| miR-141 | 0.480 | 0.966 | 0.860 |
| miR-200a | 0.600 | 1.000 | 0.912 |
| miR-200b | 0.560 | 0.899 | 0.825 |
| PTEN | 0.520 | 1.000 | 0.895 |
| miR-30a-3p + PTEN | 0.500 | 0.989 | 0.878 |
| miR-141 + PTEN | 0.520 | 1.000 | 0.895 |
| miR-200a + PTEN | 0.760 | 1.000 | 0.947 |
| miR-200b + PTEN | 0.600 | 1.000 | 0.912 |
| Count (%) | PTEN Complete Loss | PTEN Heterogeneous | PTEN Positive | Total |
|---|---|---|---|---|
| Control-SH/CH-nonA | 0 * (0%) | 53 (56.4%) | 41 (43.6%) | 94 (100%) |
| Case-SH/CH-nonA | 6 *,# (24.0%) | 10 (40.0%) | 9 + (36.0%) | 25 (100%) |
| Subsequent endometrial cancer of Case-SH/CH-nonA | 14 # (56.0%) | 11 (44.0%) | 0 + (0%) | 25 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Wu, R.-C.; Yang, L.-Y.; Jung, S.-M.; Ueng, S.-H.; Tang, Y.-H.; Huang, H.-J.; Tung, H.-J.; Lin, C.-T.; Chen, H.-Y.; et al. MicroRNAs as Predictors of Future Uterine Malignancy in Endometrial Hyperplasia without Atypia. J. Pers. Med. 2022, 12, 311. https://doi.org/10.3390/jpm12020311
Lin C-Y, Wu R-C, Yang L-Y, Jung S-M, Ueng S-H, Tang Y-H, Huang H-J, Tung H-J, Lin C-T, Chen H-Y, et al. MicroRNAs as Predictors of Future Uterine Malignancy in Endometrial Hyperplasia without Atypia. Journal of Personalized Medicine. 2022; 12(2):311. https://doi.org/10.3390/jpm12020311
Chicago/Turabian StyleLin, Chiao-Yun, Ren-Chin Wu, Lan-Yan Yang, Shih-Ming Jung, Shir-Hwa Ueng, Yun-Hsin Tang, Huei-Jean Huang, Hsiu-Jung Tung, Cheng-Tao Lin, Hsuan-Yu Chen, and et al. 2022. "MicroRNAs as Predictors of Future Uterine Malignancy in Endometrial Hyperplasia without Atypia" Journal of Personalized Medicine 12, no. 2: 311. https://doi.org/10.3390/jpm12020311
APA StyleLin, C.-Y., Wu, R.-C., Yang, L.-Y., Jung, S.-M., Ueng, S.-H., Tang, Y.-H., Huang, H.-J., Tung, H.-J., Lin, C.-T., Chen, H.-Y., Chao, A., & Lai, C.-H. (2022). MicroRNAs as Predictors of Future Uterine Malignancy in Endometrial Hyperplasia without Atypia. Journal of Personalized Medicine, 12(2), 311. https://doi.org/10.3390/jpm12020311







