The Sociodemographic and Risk Factors for Fuchs’ Endothelial Dystrophy: A Nationwide, Matched Case–Control Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
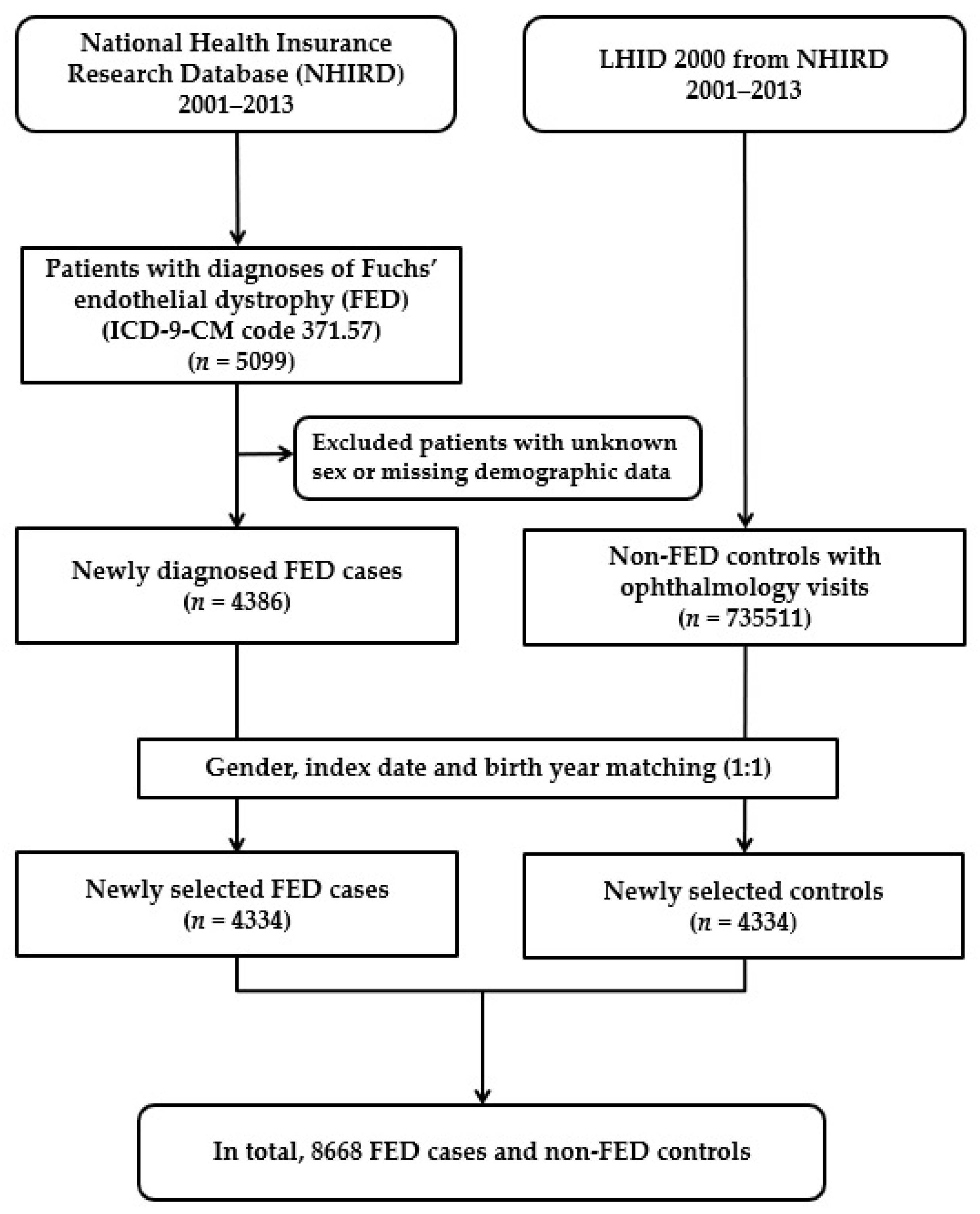
2.2. Selection of Patients and Variables
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Associated Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Patel, D.V. The pathophysiology of Fuchs’ endothelial dystrophy—A review of molecular and cellular insights. Exp. Eye Res. 2015, 130, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef] [PubMed]
- Hamill, C.E.; Schmedt, T.; Jurkunas, U. Fuchs endothelial cornea dystrophy: A review of the genetics behind disease development. Semin. Ophthalmol. 2013, 28, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Somani, A.N.; Vaidyanathan, U.; Patel, B.C. Fuchs Endothelial Dystrophy (FED); StatPearls: Treasure Island, FL, USA, 2020.
- Zhang, X.; Igo, R.P., Jr.; Fondran, J.; Mootha, V.V.; Oliva, M.; Hammersmith, K.; Sugar, A.; Lass, J.H.; Iyengar, S.K. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J.; Tripathi, R.C. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Gottsch, J.D. Fuchs’ corneal dystrophy. Expert Rev. Ophthalmol. 2010, 5, 147–159. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Purcell, J.J., Jr.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef]
- Afshari, N.A.; Pittard, A.B.; Siddiqui, A.; Klintworth, G.K. Clinical study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: A 30-year experience. Arch. Ophthalmol. 2006, 124, 777–780. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Janson, B.J.; Skeie, J.M.; Ling, J.J.; Greiner, M.A. The effects of diabetes mellitus on the corneal endothelium: A review. Surv. Ophthalmol. 2020, 65, 438–450. [Google Scholar] [CrossRef]
- Leonardi, A.; Brun, P.; Abatangelo, G.; Plebani, M.; Secchi, A.G. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3052–3058. [Google Scholar] [CrossRef][Green Version]
- Maatta, M.; Kari, O.; Tervahartiala, T.; Wahlgren, J.; Peltonen, S.; Kari, M.; Rytila, P.; Saari, M.; Sorsa, T. Elevated expression and activation of matrix metalloproteinase 8 in tear fluid in atopic blepharoconjunctivitis. Cornea 2008, 27, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: Relevance in keratoconus. Clin. Exp. Optom. 2013, 96, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eli, H.; Erdinest, N.; Solomon, A. Pathogenesis and complications of chronic eye rubbing in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Kojima, M.; Sasaki, H.; Shui, Y.B.; Chew, S.J.; Cheng, H.M.; Ono, M.; Morikawa, Y.; Sasaki, K. Prevalence of primary cornea guttata and morphology of corneal endothelium in aging Japanese and Singaporean subjects. Ophthalmic Res. 2002, 34, 135–138. [Google Scholar] [CrossRef]
- Zoega, G.M.; Fujisawa, A.; Sasaki, H.; Kubota, A.; Sasaki, K.; Kitagawa, K.; Jonasson, F. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology 2006, 113, 565–569. [Google Scholar] [CrossRef]
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef]
- Li, Q.J.; Ashraf, M.F.; Shen, D.F.; Green, W.R.; Stark, W.J.; Chan, C.C.; O’Brien, T.P. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch. Ophthalmol. 2001, 119, 1597–1604. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Ceriello, A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 2003, 26, 1589–1596. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
| Fuchs’ Endothelial Dystrophy n = 4334 | Comparison n = 4334 | p Value | |
|---|---|---|---|
| Sociodemographic factors | n (%) | n (%) | |
| Age (year; Mean ± SD) | 49.80 ± 17.83 | 49.78 ± 17.81 | 0.9988 a |
| Age (year) | |||
| <25 | 366 (8.44) | 366 (8.44) | 1.000 b |
| 25–34 | 625 (14.42) | 625 (14.42) | |
| 35–44 | 780 (18.00) | 780 (18.00) | |
| 45–54 | 875 (20.19) | 875 (20.19) | |
| 55–64 | 736 (16.98) | 736 (16.98) | |
| ≥65 | 952 (21.97) | 952 (21.97) | |
| Gender | |||
| Male | 2105 (48.57) | 2105 (48.57) | 1.000 b |
| Female | 2229 (51.43) | 2229 (51.43) | |
| Income | 0.0124 b | ||
| <TWD 30,000 | 2523 (58.21) | 2620 (60.45) | |
| TWD 30,000–60,000 | 1507 (34.77) | 1378 (31.80) | |
| TWD 60,000–90,000 | 242 (5.58) | 253 (5.84) | |
| >TWD 90,000 | 62 (1.37) | 83 (1.91) | |
| Geographical region of Taiwan | <0.0001 b | ||
| Northern | 3547 (81.84) | 2225 (51.34) | |
| Central | 305 (7.04) | 809 (18.67) | |
| Southern | 433 (9.99) | 1165 (26.88) | |
| Eastern | 49 (1.13) | 135 (3.11) | |
| Residential city status | <0.0001 b | ||
| Metropolis | 3703 (85.44) | 3055 (70.49) | |
| Satellite | 148 (3.41) | 291 (6.71) | |
| Rural | 483 (11.14) | 988 (22.80) | |
| Occupation | <0.0001 b | ||
| Public servant | 2460 (56.76) | 2255 (52.03) | |
| Farmer | 298 (6.88) | 588 (13.57) | |
| Fisherman | 42 (0.97) | 86 (1.98) | |
| Other | 1534 (35.39) | 1405 (32.42) | |
| Ophthalmology visit times (times; Mean ± SD) | 17.00 ± 28.70 | 6.53 ± 12.86 | <0.0001 a |
| Comorbid conditions | |||
| Ocular allergic condition | 204 (4.71) | 8 (0.18) | <0.0001 b |
| Asthma | 312 (7.20) | 313 (7.22) | 0.9669 b |
| Allergic rhinitis | 152 (3.51) | 140 (3.23) | 0.4750 b |
| Atopic dermatitis | 62 (1.43) | 79 (1.82) | 0.1489 b |
| Diabetes mellitus | 310 (7.15) | 428 (9.88) | <0.0001 b |
| Chronic renal disease | 75 (1.73) | 89 (2.05) | 0.2697 b |
| Mitral valve prolapses | 34 (0.78) | 39 (0.90) | 0.5567 b |
| Odds Ratio a (95% CI) | p Value | Adjusted Odds Ratio b (95% CI) | p Value | |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Income | ||||
| <TWD 30,000 | 1.27 (0.90–1.78) | 0.1704 | 1.92 (1.28–2.87) | 0.0016 |
| TWD 30,000–60,000 | 1.45 (1.04–2.04) | 0.0302 | 1.72 (1.16–2.56) | 0.0074 |
| TWD 60,000–90,000 | 1.28 (0.88–1.87) | 0.1934 | 1.40 (0.90–2.18) | 0.1351 |
| >TWD 90,000 | 1.00 | 1.00 | ||
| Geographical region of Taiwan | ||||
| Northern | 4.80 (3.37–6.83) | <0.0001 | 5.33 (3.42–8.30) | <0.0001 |
| Central | 1.06 (0.73–1.53) | 0.7635 | 1.13 (0.73–1.73) | 0.5904 |
| Southern | 1.06 (0.74–1.54) | 0.7418 | 1.06 (0.68–1.65) | 0.7893 |
| Eastern | 1.00 | 1.00 | ||
| Residential city status | ||||
| Metropolis | 2.53 (2.23–2.88) | <0.0001 | 0.97 (0.80–1.18) | 0.7552 |
| Satellite | 0.96 (0.77–1.19) | 0.7010 | 0.37 (0.28–0.50) | <0.0001 |
| Rural | 1.00 | 1.00 | ||
| Occupation | ||||
| Public servant | 1.04 (0.94–1.15) | 0.4298 | 1.02 (0.89–1.17) | 0.7675 |
| Farmer | 0.43 (0.37–0.51) | <0.0001 | 0.92 (0.74–1.15) | 0.4866 |
| Fisherman | 0.47 (0.32–0.68) | <0.0001 | 0.92 (0.58–1.46) | 0.7197 |
| Other | 1.00 | 1.00 | ||
| Ophthalmology visit times | 1.05 (1.05–1.06) | <0.0001 | 1.05 (1.05–1.06) | <0.0001 |
| Comorbid conditions | ||||
| Ocular allergic condition | 25.50 (12.58–51.68) | <0.0001 | 25.26 (11.24–56.77) | <0.0001 |
| Asthma | 1.00 (0.85–1.18) | 0.9665 | 0.90 (0.73–1.12) | 0.3406 |
| Allergic rhinitis | 1.09 (0.86–1.38) | 0.4718 | 0.97 (0.71–1.32) | 0.8477 |
| Atopic dermatitis | 0.78 (0.56–1.09) | 0.1504 | 0.76 (0.49–1.18) | 0.2267 |
| Diabetes mellitus | 0.68 (0.58–0.80) | <0.0001 | 0.52 (0.42–0.64) | <0.0001 |
| Chronic renal disease | 0.84 (0.62–1.15) | 0.269 | 0.78 (0.52–1.18) | 0.2372 |
| Mitral valve prolapses | 0.87 (0.55–1.38) | 0.5532 | 0.63 (0.35–1.13) | 0.1191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-S.; Ho, C.-H.; Wang, J.-J.; Tseng, S.-H.; Jan, R.-L. The Sociodemographic and Risk Factors for Fuchs’ Endothelial Dystrophy: A Nationwide, Matched Case–Control Study in Taiwan. J. Pers. Med. 2022, 12, 305. https://doi.org/10.3390/jpm12020305
Chang Y-S, Ho C-H, Wang J-J, Tseng S-H, Jan R-L. The Sociodemographic and Risk Factors for Fuchs’ Endothelial Dystrophy: A Nationwide, Matched Case–Control Study in Taiwan. Journal of Personalized Medicine. 2022; 12(2):305. https://doi.org/10.3390/jpm12020305
Chicago/Turabian StyleChang, Yuh-Shin, Chung-Han Ho, Jhi-Joung Wang, Sung-Huei Tseng, and Ren-Long Jan. 2022. "The Sociodemographic and Risk Factors for Fuchs’ Endothelial Dystrophy: A Nationwide, Matched Case–Control Study in Taiwan" Journal of Personalized Medicine 12, no. 2: 305. https://doi.org/10.3390/jpm12020305
APA StyleChang, Y.-S., Ho, C.-H., Wang, J.-J., Tseng, S.-H., & Jan, R.-L. (2022). The Sociodemographic and Risk Factors for Fuchs’ Endothelial Dystrophy: A Nationwide, Matched Case–Control Study in Taiwan. Journal of Personalized Medicine, 12(2), 305. https://doi.org/10.3390/jpm12020305







