Adjuvant Radiotherapy Is Associated with an Increase in the Survival of Old (Aged over 80 Years) and Very Old (Aged over 90 Years) Women with Breast Cancer Receiving Breast-Conserving Surgery
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Study Covariates and Propensity Score Matching
2.4. Statistics
3. Results
3.1. Study Cohort
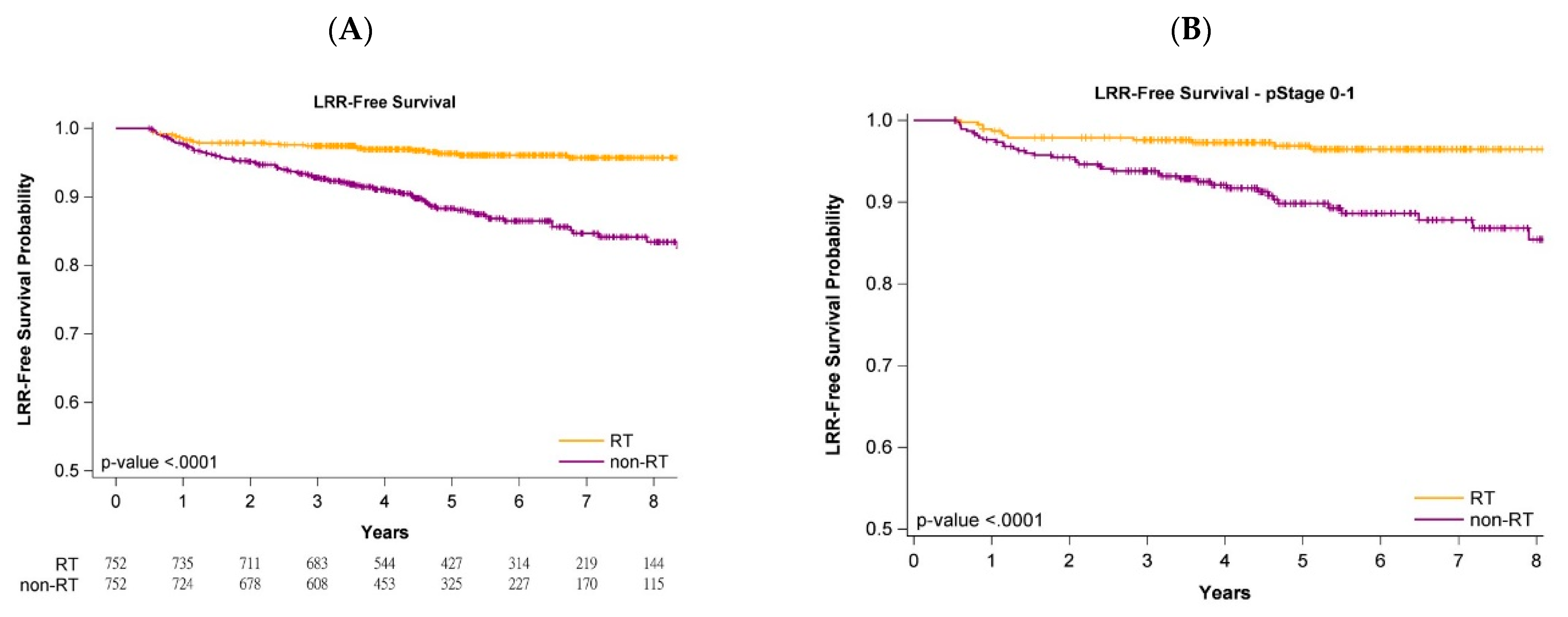
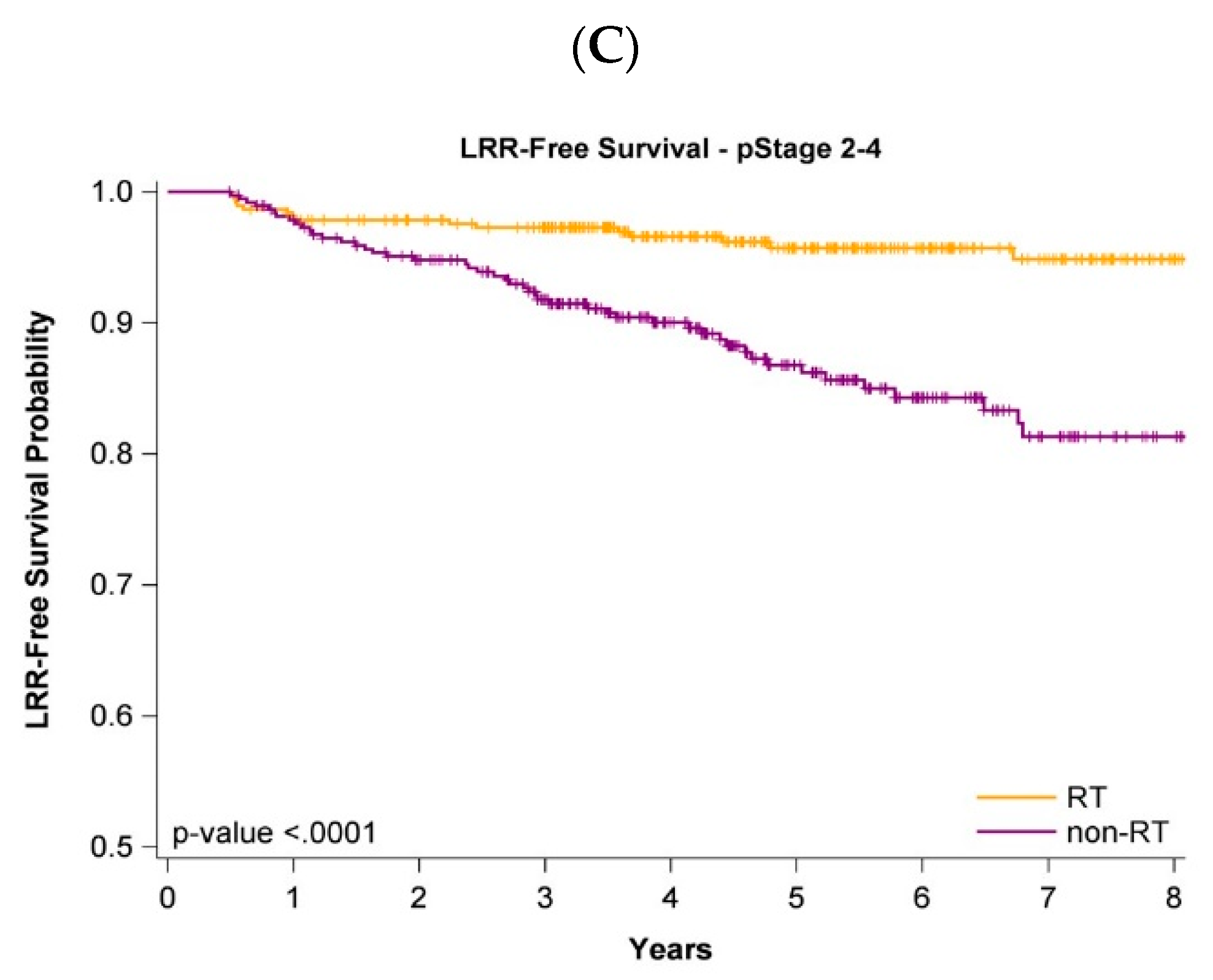
3.2. Impact of Adjuvant WBRT on Oncologic Outcomes of Old and Very Old Women
3.3. Age Stratification in Multivariable Cox Regression Analysis
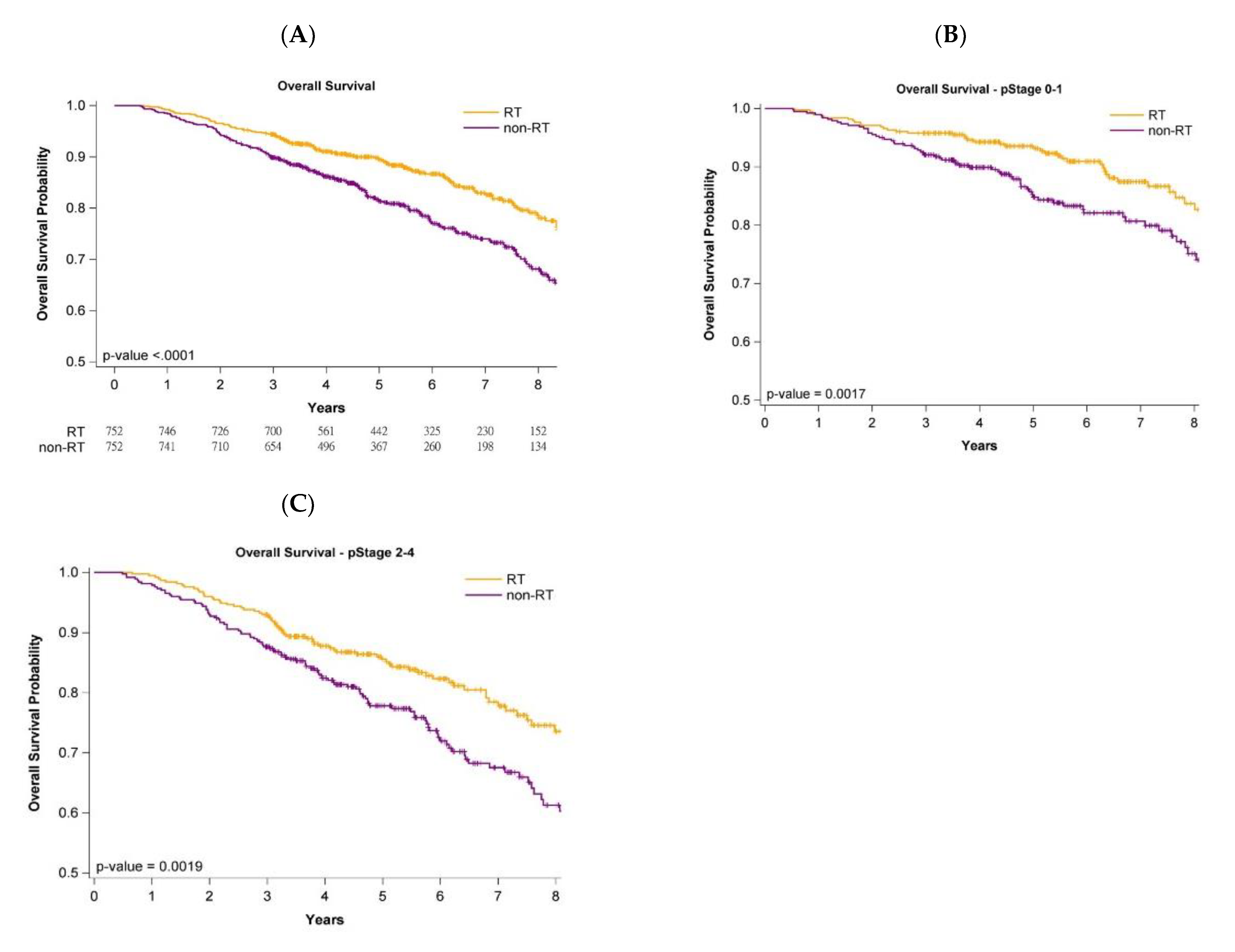
3.4. Survival Curves with or without Adjuvant WBRT
3.5. Survival Curves of Cancer Stages and Age Stratification
4. Discussion
4.1. No Solution Regarding Adjuvant WBRT for Older Women with Breast Cancer
4.2. Value of PSM in This Population
4.3. Conditions Different from Previous Studies
4.4. Cancer Stage and Age Stratification
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mohler, J.; Bahnson, R.R.; Boston, B.; Busby, J.E.; D’Amico, A.; Eastham, J.A.; Enke, C.A.; George, D.; Horwitz, E.M.; Huben, R.P. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 17 February 2021).
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M.; on behalf of the PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- van de Water, W.; Bastiaannet, E.; Scholten, A.N.; Kiderlen, M.; de Craen, A.J.; Westendorp, R.G.; van de Velde, C.J.; Liefers, G.J. Breast-conserving surgery with or without radiotherapy in older breast patients with early stage breast cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2014, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [Green Version]
- Bertolo, A.; Rosso, C.; Voutsadakis, I.A. Breast Cancer in Patients 80 Years-Old and Older. Eur. J. Breast Health 2020, 16, 208–212. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative, G.; Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.; Bryant, J.; Dignam, J.J.; Wickerham, D.L.; Mamounas, E.P.; Fisher, E.R.; Margolese, R.G.; Nesbitt, L.; Paik, S.; Pisansky, T.M.; et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Gnant, M.; Kwasny, W.; Tausch, C.; Handl-Zeller, L.; Pakisch, B.; Taucher, S.; Hammer, J.; Luschin-Ebengreuth, G.; Schmid, M.; et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int. J. Radiat Oncol. Biol. Phys. 2007, 68, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinterri, C.; Gatzemeier, W.; Zanini, V.; Regolo, L.; Pedrazzoli, C.; Rondini, E.; Amanti, C.; Gentile, G.; Taffurelli, M.; Fenaroli, P.; et al. Conservative surgery with and without radiotherapy in elderly patients with early-stage breast cancer: A prospective randomised multicentre trial. Breast 2009, 18, 373–377. [Google Scholar] [CrossRef]
- Ford, H.T.; Coombes, R.C.; Gazet, J.C.; Gray, R.; McConkey, C.C.; Sutcliffe, R.; Quilliam, J.; Lowndes, S. Long-term follow-up of a randomised trial designed to determine the need for irradiation following conservative surgery for the treatment of invasive breast cancer. Ann. Oncol. 2006, 17, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.Y.; Chen, C.H.; Chen, H.M.; Wu, S.Y. Effect of pathologic stages on postmastectomy radiation therapy in breast cancer receiving neoadjuvant chemotherapy and total mastectomy: A Cancer Database Analysis. Breast 2020, 54, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.Y.; Qin, L.; Chen, H.M.; Wu, S.Y. Breast-conserving surgery with or without irradiation in women with invasive ductal carcinoma of the breast receiving preoperative systemic therapy: A cohort study. Breast 2020, 54, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast with High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw. Open 2021, 4, e211785. [Google Scholar] [CrossRef]
- Liu, W.C.; Liu, H.E.; Kao, Y.W.; Qin, L.; Lin, K.C.; Fang, C.Y.; Tsai, L.L.; Shia, B.C.; Wu, S.Y. Definitive radiotherapy or surgery for early oral squamous cell carcinoma in old and very old patients: A propensity-score-matched, nationwide, population-based cohort study. Radiother Oncol. 2020, 151, 214–221. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 20 December 2021).
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, J.H.; Yen, Y.C.; Yang, H.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine 2016, 95, e3268. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Gorina, Y.; Hoyert, D.; Lentzner, H.; Goulding, M. Trends in Causes of Death among Older Persons in the United States. Available online: https://www.cdc.gov/nchs/data/ahcd/agingtrends/06olderpersons.pdf (accessed on 30 October 2005).
- Berry, S.D.; Ngo, L.; Samelson, E.J.; Kiel, D.P. Competing risk of death: An important consideration in studies of older adults. J. Am. Geriatr. Soc. 2010, 58, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Lau, B.; Cole, S.R.; Gange, S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009, 170, 244–256. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Collins, G.S.; Spence, J.; Daures, J.P.; Devereaux, P.J.; Landais, P.; Le Manach, Y. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 2017, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, T.Y.; Koca, E.; Dizdar, O.; Arslan, C.; Eren, T.; Yalcin, S.; Kucukoztas, N.; Aksoy, S.; Rahatli, S.; Dede, D.S.; et al. Breast cancer in octogenarian women: Clinical characteristics and outcome. J. BUON 2013, 18, 328–334. [Google Scholar] [PubMed]
- Zhang, J.; Chang, C.L.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with breast cancer receiving breast conservative surgery compared with volatile inhalational without propofol in general anesthesia. Biomed. Pharmacother. 2021, 142, 111991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Pathologic response rates for breast cancer stages as a predictor of outcomes in patients receiving neoadjuvant chemotherapy followed by breast-conserving surgery. Surg. Oncol. 2020, 36, 91–98. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Chang, E.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Pathologic response as predictor of recurrence, metastasis, and survival in breast cancer patients receiving neoadjuvant chemotherapy and total mastectomy. Am. J. Cancer Res. 2020, 10, 3415–3427. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Lu, C.Y.; Qin, L.; Chen, H.M.; Wu, S.Y. Outcome of post-mastectomy radiotherapy after primary systemic treatment in patients with different clinical tumor and nodal stages of breast cancer: A cohort study. Am. J. Cancer Res. 2020, 10, 2185–2198. [Google Scholar]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lai, M.S.; Syu, C.Y.; Chang, S.C.; Tseng, F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 2005, 104, 157–163. [Google Scholar] [PubMed]

| Raw Population | Propensity Score-Matched Population | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N = 3703 | Adjuvant WBRT N = 2776 | Non-WBRT N = 927 | Adjuvant WBRT N = 752 | Non-WBRT N = 752 | |||||||||
| Variables | n | (%) | n | (%) | n | (%) | p Value | n | (%) | n | (%) | p Value | |
| Age | Mean (SD) | 84.8 | (6.1) | 84.4 | (4.9) | 85.9 | (7.2) | <0.0001 | 85.3 | (6.0) | 85.9 | (6.3) | 0.9674 |
| Median (IQR, Q1–Q3) | 84 | (81–88) | 84 | (81–88) | 84 | (82–89) | 84 | (82–89) | 84 | (82–90) | |||
| 80–84 | 1815 | (49.0) | 1598 | (57.6) | 217 | (23.4) | <0.0001 | 215 | (28.6) | 215 | (28.6) | 1.0000 | |
| 85–89 | 1285 | (34.7) | 879 | (31.7) | 406 | (43.8) | 238 | (31.6) | 238 | (31.6) | |||
| 90+ | 603 | (16.3) | 299 | (10.8) | 304 | (32.8) | 299 | (39.8) | 299 | (39.8) | |||
| Differentiation | I | 851 | (23.0) | 631 | (22.7) | 220 | (23.7) | 0.3441 | 171 | (22.7) | 182 | (24.2) | 0.3075 |
| II | 2071 | (55.9) | 1544 | (55.6) | 527 | (56.9) | 406 | (54.0) | 418 | (55.6) | |||
| III | 781 | (21.1) | 601 | (21.6) | 180 | (19.4) | 175 | (23.3) | 152 | (20.2) | |||
| AJCC Clinical stage | I | 2033 | (54.9) | 1568 | (56.5) | 465 | (50.2) | 0.0012 | 402 | (53.5) | 398 | (52.9) | 0.9532 |
| II | 1547 | (41.8) | 1112 | (40.1) | 435 | (46.9) | 329 | (43.8) | 332 | (44.1) | |||
| III | 123 | (3.3) | 96 | (3.5) | 27 | (2.9) | 21 | (2.8) | 22 | (2.9) | |||
| AJCC Pathologic stage | 0 | 41 | (1.1) | 33 | (1.2) | 8 | (0.9) | 0.0029 | 4 | (0.5) | 4 | (0.5) | 1.0000 |
| I | 1894 | (51.1) | 1449 | (52.2) | 445 | (48.0) | 375 | (49.9) | 375 | (49.9) | |||
| II | 1531 | (41.3) | 1103 | (39.7) | 428 | (46.2) | 331 | (44.0) | 331 | (44.0) | |||
| III | 237 | (6.4) | 191 | (6.9) | 46 | (5.0) | 42 | (5.6) | 42 | (5.6) | |||
| pT | 0 | 58 | (1.6) | 47 | (1.7) | 11 | (1.2) | <0.0001 | 5 | (0.7) | 6 | (0.8) | 0.5977 |
| 1 | 2214 | (59.8) | 1710 | (61.6) | 504 | (54.4) | 429 | (57.0) | 424 | (56.4) | |||
| 2 | 1356 | (36.6) | 975 | (35.1) | 381 | (41.1) | 301 | (40.0) | 301 | (40.0) | |||
| 3 | 45 | (1.2) | 24 | (0.9) | 21 | (2.3) | 8 | (1.1) | 14 | (1.9) | |||
| 4 | 30 | (0.8) | 20 | (0.7) | 10 | (1.1) | 9 | (1.2) | 7 | (0.9) | |||
| pT | 0–1 | 2272 | (61.4) | 1757 | (63.3) | 515 | (55.6) | <0.0001 | 434 | (57.7) | 430 | (57.2) | 0.6625 |
| 2–4 | 1431 | (38.6) | 1019 | (36.7) | 412 | (44.4) | 318 | (42.3) | 322 | (42.8) | |||
| pN | 0 | 2890 | (78.0) | 2122 | (76.4) | 768 | (82.8) | 0.0004 | 618 | (82.2) | 608 | (80.9) | 0.8552 |
| 1 | 613 | (16.6) | 488 | (17.6) | 125 | (13.5) | 103 | (13.7) | 113 | (15.0) | |||
| 2 | 140 | (3.8) | 114 | (4.1) | 26 | (2.8) | 22 | (2.9) | 23 | (3.1) | |||
| 3 | 60 | (1.6) | 52 | (1.9) | 8 | (0.9) | 9 | (1.2) | 8 | (1.1) | |||
| pN | 0 | 2890 | (78.0) | 2122 | (76.4) | 768 | (82.8) | <0.0001 | 618 | (82.2) | 608 | (80.9) | 0.4111 |
| 1+ | 813 | (22.0) | 654 | (23.6) | 159 | (17.2) | 134 | (17.8) | 144 | (19.1) | |||
| Neoadjuvant Chemotherapy | 115 | (3.1) | 107 | (3.9) | 8 | (0.9) | <0.0001 | 8 | (1.1) | 7 | (0.9) | 0.7389 | |
| Adjuvant chemotherapy | 1270 | (34.3) | 1126 | (40.6) | 144 | (15.5) | <0.0001 | 162 | (21.5) | 142 | (18.9) | 0.0588 | |
| Hormone receptor positive | 1871 | (50.5) | 1394 | (50.2) | 477 | (51.5) | 0.5132 | 368 | (48.9) | 372 | (49.5) | 0.8168 | |
| HER2 positive | 231 | (6.2) | 189 | (6.8) | 42 | (4.5) | 0.0130 | 41 | (5.5) | 40 | (5.3) | 0.9081 | |
| Nodal surgery | ALND | 2259 | (61.0) | 1688 | (60.8) | 571 | (61.6) | 0.6301 | 432 | (57.4) | 424 | (56.4) | 0.3608 |
| SLNB | 1444 | (39.0) | 1088 | (39.2) | 356 | (38.4) | 320 | (42.6) | 328 | (43.6) | |||
| CCI Scores | 0 | 1513 | (40.9) | 1178 | (42.4) | 335 | (36.1) | <0.0001 | 279 | (37.1) | 283 | (37.6) | 0.9752 |
| 1 | 1133 | (30.6) | 863 | (31.1) | 270 | (29.1) | 226 | (30.1) | 224 | (29.8) | |||
| 2+ | 1057 | (28.5) | 735 | (26.5) | 322 | (34.7) | 247 | (32.8) | 245 | (32.6) | |||
| Hypertension | 2430 | (65.6) | 1765 | (63.6) | 665 | (71.7) | <0.0001 | 543 | (72.2) | 530 | (70.5) | 0.4460 | |
| Ischemic heart diseases | 925 | (25.0) | 582 | (21.0) | 343 | (37.0) | <0.0001 | 260 | (34.6) | 258 | (34.3) | 0.9811 | |
| Cerebrovascular diseases | 377 | (10.2) | 229 | (8.2) | 148 | (17.0) | <0.0001 | 125 | (16.6) | 117 | (15.6) | 0.2624 | |
| COPD | 552 | (14.9) | 301 | (10.8) | 251 | (27.1) | <0.0001 | 211 | (28.1) | 211 | (128.1) | 1.0000 | |
| Diabetes | 1180 | (31.9) | 862 | (31.1) | 318 | (34.3) | 0.0658 | 268 | (35.6) | 254 | (33.8) | 0.4423 | |
| Hospital level | Medical center | 1973 | (53.3) | 1394 | (50.2) | 579 | (62.5) | <0.0001 | 446 | (59.3) | 461 | (61.3) | 0.3258 |
| Non-Medical centers | 1730 | (46.7) | 1382 | (49.8) | 348 | (37.5) | 306 | (40.7) | 291 | (38.7) | |||
| Hospital area | North | 2017 | (54.5) | 1563 | (56.3) | 454 | (49.0) | <0.0001 | 384 | (51.1) | 374 | (49.7) | 0.5139 |
| Center | 761 | (20.6) | 489 | (17.6) | 272 | (29.3) | 196 | (26.1) | 211 | (28.1) | |||
| South/East | 925 | (25.0) | 724 | (26.1) | 201 | (21.7) | 172 | (22.9) | 167 | (22.2) | |||
| Income | <NTD 18,000 | 1331 | (35.9) | 987 | (35.6) | 344 | (37.1) | 0.0599 | 279 | (37.1) | 281 | (37.4) | 0.9108 |
| NTD 18,000–24,000 | 1240 | (33.5) | 928 | (33.4) | 312 | (33.7) | 240 | (31.9) | 248 | (33.0) | |||
| NTD 24,000–36,000 | 350 | (9.5) | 283 | (10.2) | 67 | (7.2) | 55 | (7.3) | 56 | (7.4) | |||
| NTD 36,000+ | 782 | (21.1) | 578 | (20.8) | 204 | (22.0) | 178 | (23.7) | 167 | (22.2) | |||
| Follow-up time, months | Mean (SD) | 68.8 | (29.1) | 70.7 | (28.7) | 63.1 | (29.3) | 70.3 | (29.2) | 64.4 | (28.8) | ||
| Death | 606 | (16.4) | 336 | (12.1) | 270 | (29.1) | <0.0001 | 123 | (16.4) | 182 | (24.2) | <0.0001 | |
| Locoregional recurrence | 245 | (6.6) | 144 | (5.2) | 101 | (10.9) | <0.0001 | 28 | (3.7) | 88 | (11.7) | <0.0001 | |
| Distant metastasis | 331 | (8.9) | 214 | (7.7) | 117 | (12.6) | <0.0001 | 54 | (7.2) | 108 | (14.4) | <0.0001 | |
| All-Cause Death | Locoregional Recurrence | Distant Metastasis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aHR * | (95% CI) | p Value | aHR * | (95% CI) | p Value | aHR * | (95% CI) | p Value | ||
| Adjuvant WBRT | No | 1 | <0.0001 | 1 | <0.0001 | 1 | <0.0001 | |||
| Yes | 0.56 | (0.44–0.70) | 0.29 | (0.19–0.45) | 0.45 | (0.32–0.62) | ||||
| Age | 80–84 | 1 | <0.0001 | 1 | 0.6874 | 1 | 0.1827 | |||
| 85–89 | 1.85 | (1.28–2.69) | 0.94 | (0.61–1.45) | 1.05 | (0.71–1.56) | ||||
| 90+ | 1.67 | (1.47–3.46) | 0.77 | (0.42–1.41) | 0.66 | (0.36–1.18) | ||||
| Differentiation | I | 1 | 0.6671 | 1 | 0.3917 | 1 | 0.3724 | |||
| II | 1.17 | (0.90–1.88) | 1.09 | (0.76–1.71) | 1.28 | (0.79–1.40) | ||||
| III | 1.94 | (0.97–2.19) | 1.64 | (0.77–2.75) | 1.59 | (0.69–2.46) | ||||
| AJCC clinical stage | I | 1 | 0.4779 | 1 | 0.5677 | 1 | 0.3347 | |||
| II | 1.08 | (0.91–1.76) | 1.18 | (0.73–1.91) | 1.14 | (0.78–1.67) | ||||
| III | 1.12 | (0.87–1.81) | 1.75 | (0.61–5.03) | 1.59 | (0.75–5.39) | ||||
| pT | pT0–1 | 1 | 0.7845 | 1 | 0.8537 | 1 | 0.7764 | |||
| pT2–4 | 1.06 | (0.73–1.27) | 1.05 | (0.65–1.67) | 1.06 | (0.73–1.52) | ||||
| pN | pN0 | 1 | 0.0676 | 1 | 0.3442 | 1 | 0.3685 | |||
| pN1+ | 1.30 | (0.98–1.73) | 1.25 | (0.79–2.00) | 1.20 | (0.81–1.77) | ||||
| Adjuvant chemotherapy | Yes | 0.83 | (0.43–1.12) | 0.1168 | 0.93 | (0.57–1.51) | 0.7727 | 1.18 | (0.78–1.80) | 04319 |
| Hormone receptor positive | Yes | 0.88 | (0.61–1.09) | 0.2451 | 0.80 | (0.55–1.18) | 0.2617 | 0.84 | (0.60–1.18) | 0.3169 |
| HER2 positive | Yes | 1.06 | (0.72–1.31) | 0.2206 | 1.04 | (0.75–1.18) | 0.3494 | 1.14 | (0.76–1.21) | 0.4070 |
| Nodal surgery | ALND | 1 | 0.2361 | 1 | 0.2561 | 1 | 0.4612 | |||
| SLNB | 0.77 | (0.49–1.22) | 1.16 | (0.74–1.84) | 1.05 | (0.71–1.55) | ||||
| CCI Scores | 0 | 1 | 0.4551 | 1 | 0.2721 | 1 | 0.0318 | |||
| 1 | 1.09 | (0.82–1.34) | 0.90 | (0.57–1.42) | 0.94 | (0.65–1.35) | ||||
| 2+ | 1.23 | (0.81–1.79) | 0.69 | (0.43–1.09) | 0.58 | (0.38–0.89) | ||||
| Hospital level | Medical center | 1 | 0.3925 | 1 | 0.1240 | 1 | 0.9823 | |||
| Non-Medical centers | 1.11 | (0.88–1.41) | 0.73 | (0.49–1.09) | 1.00 | (0.71–1.42) | ||||
| Age 80–89 | Age ≥90 | ||||||
|---|---|---|---|---|---|---|---|
| aHR * | (95% CI) | p Value | aHR * | (95% CI) | p Value | ||
| Adjuvant RT | No | 1 | 0.0156 | 1 | 0.0040 | ||
| Yes | 0.60 | (0.40–0.91) | 0.64 | (0.48–0.87) | |||
| Age | 80–84 | 1 | 0.0382 | – | |||
| 85–89 | 1.48 | (1.07–2.27) | – | ||||
| 90–94 | – | 1 | 0.0095 | ||||
| 95+ | – | 1.50 | (1.10–2.04) | ||||
| Differentiation | I | 1 | 0.2286 | 1 | 0.3581 | ||
| II | 1.01 | (0.58–1.76) | 1.08 | (0.84–1.94) | |||
| III | 1.90 | (0.93–2.50) | 1.88 | (0.90–2.32) | |||
| AJCC clinical stage | I | 1 | 0.4135 | 1 | 0.3453 | ||
| II | 1.13 | (0.88–1.54) | 1.19 | (0.83–1.70) | |||
| III | 1.75 | (0.78–2.02) | 1.66 | (0.70–2.48) | |||
| pT | pT0–1 | 1 | 0.7816 | 1 | 0.8476 | ||
| pT2–4 | 1.07 | (0.66–1.75) | 1.04 | (0.73–1.47) | |||
| pN | pN0 | 1 | 0.1494 | 1 | 0.5985 | ||
| pN1+ | 1.36 | (0.86–1.96) | 1.11 | (0.75–1.66) | |||
| Adjuvant chemotherapy | 0.64 | (0.52–1.22) | 0.2338 | 0.65 | (0.52–1.91) | 0.2533 | |
| HR positive | 0.92 | (0.60–1.39) | 0.6880 | 0.92 | (0.67–1.26) | 0.6025 | |
| HER2 positive | 1.12 | (0.77–1.41) | 0.5702 | 1.31 | (0.87–1.96) | 0.4925 | |
| Nodal surgery | ALND | 1 | 0.8517 | 1 | 0.0102 | ||
| SLNB/+ALND | 0.94 | (0.57–1.57) | 0.77 | (0.52–1.15) | |||
| CCI Scores | 0 | 1 | 0.7365 | 1 | 0.8771 | ||
| 1 | 1.11 | (0.83–1.41) | 1.07 | (0.84–1.90) | |||
| 2+ | 1.53 | (0.86–2.10) | 1.31 | (0.85–2.42) | |||
| Hospital level | Medical centers | 1 | 0.8969 | 1 | 0.4276 | ||
| Non-medical centers | 1.03 | (0.68–1.57) | 1.13 | (0.84–1.52) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Chang, C.-L.; Sun, M.; Chiang, M.-F.; Sum, S.-Y.; Zhang, J.; Wu, S.-Y. Adjuvant Radiotherapy Is Associated with an Increase in the Survival of Old (Aged over 80 Years) and Very Old (Aged over 90 Years) Women with Breast Cancer Receiving Breast-Conserving Surgery. J. Pers. Med. 2022, 12, 287. https://doi.org/10.3390/jpm12020287
Huang C-C, Chang C-L, Sun M, Chiang M-F, Sum S-Y, Zhang J, Wu S-Y. Adjuvant Radiotherapy Is Associated with an Increase in the Survival of Old (Aged over 80 Years) and Very Old (Aged over 90 Years) Women with Breast Cancer Receiving Breast-Conserving Surgery. Journal of Personalized Medicine. 2022; 12(2):287. https://doi.org/10.3390/jpm12020287
Chicago/Turabian StyleHuang, Chung-Chien, Chia-Lun Chang, Mingyang Sun, Ming-Feng Chiang, Shao-Yin Sum, Jiaqiang Zhang, and Szu-Yuan Wu. 2022. "Adjuvant Radiotherapy Is Associated with an Increase in the Survival of Old (Aged over 80 Years) and Very Old (Aged over 90 Years) Women with Breast Cancer Receiving Breast-Conserving Surgery" Journal of Personalized Medicine 12, no. 2: 287. https://doi.org/10.3390/jpm12020287
APA StyleHuang, C.-C., Chang, C.-L., Sun, M., Chiang, M.-F., Sum, S.-Y., Zhang, J., & Wu, S.-Y. (2022). Adjuvant Radiotherapy Is Associated with an Increase in the Survival of Old (Aged over 80 Years) and Very Old (Aged over 90 Years) Women with Breast Cancer Receiving Breast-Conserving Surgery. Journal of Personalized Medicine, 12(2), 287. https://doi.org/10.3390/jpm12020287






