Abstract
(1) Background: Neoadjuvant therapy is the main therapeutic strategy for human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients, and the combination of trastuzumab and pertuzumab (HP) has become a routine treatment. How to predict and screen patients who are less likely to respond to neoadjuvant therapy is the focus of research. The androgen receptor (AR) is a biomarker that is widely expressed in all breast cancer subtypes and is probably related to treatment response and prognosis. In this study, we investigated the relationship between AR expression and treatment response in HER2-positive breast cancer patients treated with HP neoadjuvant therapy. (2) Methods: We evaluated early breast cancer patients treated with HP neoadjuvant therapy from Jan. 2019 to Oct. 2020 at Peking University First Hospital Breast Cancer Center. The inclusion criteria were as follows: early HER2-positive breast cancer patients diagnosed by core needle biopsy who underwent both HP neoadjuvant therapy and surgery. We compared the clinical and pathological features between pathological complete response (pCR) and non-pCR patients. (3) Results: We included 44 patients. A total of 90.9% of patients received neoadjuvant therapy of taxanes, carboplatin, trastuzumab and pertuzumab (TCHP), and the total pCR rate was 50%. pCR was negatively related to estrogen receptor (ER) positivity (OR 0.075 [95% confidence interval (CI) 0.008–0.678], p = 0.021) and positively related to high expression levels of AR (OR 33.145 [95% CI 2.803–391.900], p = 0.005). We drew a receiver operating characteristic (ROC) curve to assess the predictive value of AR expression for pCR, and the area under the curve was 0.737 (95% CI 0.585–0.889, p = 0.007). The optimal cutoff of AR for predicting pCR was 85%. (4) Conclusion: AR is a potential marker for the prediction of pCR in HER2-positive breast cancer patients treated with HP neoadjuvant therapy.
1. Background
Neoadjuvant therapy is the main therapeutic strategy for human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients; it can downsize the lesion for surgery, provide information on treatment response and prognosis and help to adjust follow-up treatment strategies to promote survival in non-pathological complete response (non-pCR) patients [1]. At present, neoadjuvant therapy using trastuzumab and pertuzumab (HP) is commonly adopted for HER2-positive patients. Although most patients respond to this therapy, with a promising pathological complete response (pCR) rate of over 50% [2,3,4,5,6], there are some patients who do not benefit as much from this therapy. How to predict a patient’s response to neoadjuvant treatment and how to identify patients who do not respond very well to the treatment are the focus of current research.
The androgen receptor (AR) can be detected in most breast cancers, but both its expression level and prognostic effect vary. In triple-negative breast cancers (TNBCs), the luminal AR subtype expresses high levels of AR and shows decreased relapse-free survival [7,8,9,10]. In hormone receptor (HR)-positive breast cancers, AR expression is associated with better outcomes [10,11,12,13]. However, in HER2-positive breast cancers, the relationship between AR and survival is unclear. In HER2-enriched metastatic breast cancer, AR-positivity with a cut-off value of 10% was associated with both longer progression-free survival (PFS) and an increased overall survival (OS) rate and could also predict the efficacy of first-line trastuzumab treatment [14]. AR positivity was also associated with significantly better OS in AR+ non-luminal HER2 positive breast cancer treated with neoadjuvant chemotherapy [15]. However, a higher AR mRNA level was associated with worse disease outcomes in HER2-positive tumors [11]. Another analysis showed that although AR positivity was associated with favorable clinical outcome in the entire early-stage breast cancer, AR positivity was not significantly associated with disease-free survival (DFS) and was significantly associated with a worse OS rate in the HER2 positive breast cancer subgroup [16]. Molecular apocrine tumors (estrogen receptor [ER] negative and AR positive) with HER2 positive status also showed a poor outcome [12]. There are also studies that show no relationship between AR and outcome. However, a study of 4147 patients, which followed them for a median 16.5 years, showed that AR was not associated with survival among ER+/HER2+ tumors or ER−/HER2+ tumors [10]. Studies show that AR can probably predict neoadjuvant treatment effects. AR expression seemed to be related to a lower pCR rate and worse outcome in TNBC [3,17,18,19,20], but there was also controversy related to these results [21]. In HER2 positive breast cancer, AR+ positivity was associated with pCR, but in this study, only 57.3% of patients received trastuzumab [15]. Currently, no study has focused on the relationship between AR expression and the response to neoadjuvant therapy including HP in HER2-positive breast cancer patients.
There is no acknowledged cutoff for the AR expression level. Most researchers suggest adopting 1% or 10% for both ER and the progesterone receptor (PR), but a cutoff of 35% is also advised because of its prognostic value for recurrence-free survival (RFS) after surgery [22]. In our study, we explored the potential cutoff for the purpose of predicting the pCR rate of neoadjuvant therapy in HER2-positive breast cancer patients.
2. Materials and Methods
2.1. Patients
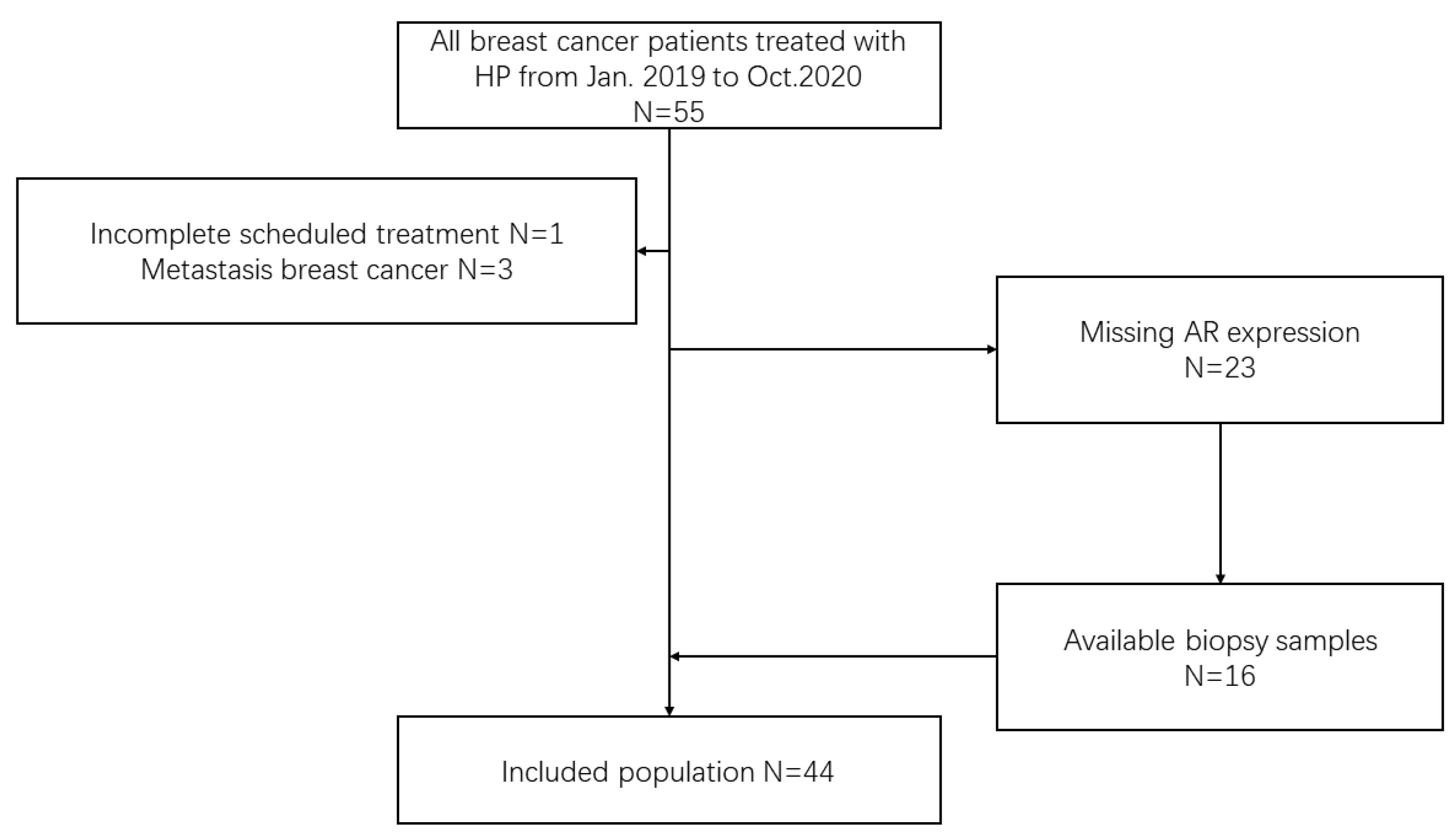
We reviewed early breast cancer patients treated with HP neoadjuvant therapy from January 2019 to October 2020 at Peking University First Hospital Breast Cancer Center. The inclusion criteria were as follows: early HER2-positive breast cancer patients diagnosed by core needle biopsy who underwent both HP neoadjuvant therapy and surgery. The exclusion criteria were as follows: (1) metastatic breast cancer; (2) failure to complete the scheduled treatment; and (3) missing AR expression and unable to be tested (Figure 1). This study is reported according to the reporting recommendations for tumor MARKer prognostic studies (REMARK) criteria [23].
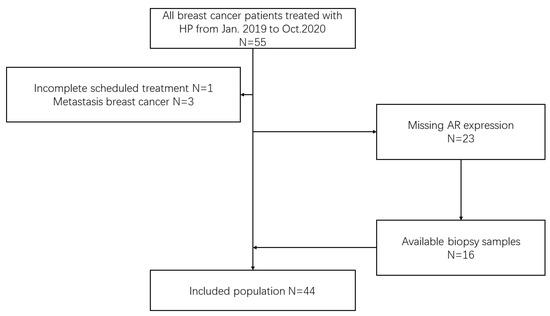
Figure 1.
Flow diagram of the patient population. HP neoadjuvant therapy containing trastuzumab and pertuzumab, AR androgen receptor.
2.2. Specimen Preparation and Hematoxylin and Eosin (HE) Staining
The protocol of tissue handling was standardized according to the recommendations of the 2007 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines [24]. The breast tissue samples were fixed in 10% neutral buffered formalin, dehydrated through a serial alcohol gradient, and embedded in paraffin wax blocks. The sections were then stained with HE. Two experienced pathologists reviewed and distinguished the histological subtype and grading based on the World Health Organization (WHO) classification of breast cancer and Nottingham grading [25].
2.3. Immunohistochemistry
Biomarkers of ER, PR, HER2 and AR were detected in all the samples using the immunohistochemical stainer Ventana Benchmark XT for HER2 and Dako Autostainer Link 48 for other markers. The antibodies were ER (1D5, Dako, at 1:50 dilution), PR (636, Dako, at 1:200 dilution), HER2 (4B5, Ventana), Ki67 (MIB1, Dako, at 1:100 dilution) or AR (EP120, GBI). According to the 2010 ASCO/CAP guidelines [26], ER or PR was positive if ≥1% of tumor cells were immunoreactive and negative if <1% of tumor cells were immunoreactive. HER2 expression was determined according to the 2013 ASCO/CAP guideline [27]. A score of 3+ was regarded positive, and a score of 0–1 was negative. A score of 2+ was interpreted as equivocal and was tested with fluorescence in situ hybridization (FISH). According to the 2018 ASCO/CAP guidelines [28], a HER2/CEP17 ratio ≥ 2.0 with a HER2 signals/cell ratio ≥ 4.0 or a HER2/CEP17 ratio < 2.0, with a HER2 signals/cell ≥ 6.0, were positive. The molecular subtypes were differentiated according to the 2011 St. Gallen International Expert Consensus: Luminal B HER2 positive (ER+, PR+ or −, HER2+ and Ki-67 ≥ 15%) and HER2-overexpressed (ER-, PR- and HER2+) [29]. Then, the expression of AR was described by the proportion and intensity of positive staining of tumor cell nuclei. Ki-67 was evaluated by the percentage of tumor cell nuclei with positive immunostaining. Based on the Nottingham combined histologic grade [25], histological scores and grades were made according to adeno-tube formation, nuclei size and shape, chromosome heterogeneity and nuclear division. Anatomical staging was performed according to the 8th edition of the American Joint Commission on Cancer (AJCC) breast cancer staging system [30].
2.4. Neoadjuvant Therapy and Evaluation of Efficacy
In our study, the scheduled neoadjuvant therapy was six cycles of taxanes, carboplatin, trastuzumab and pertuzumab (TCHP) as follows: (1) T: docetaxel 75 mg/m2 administered by intravenous infusion every 3 weeks or albumin-bound paclitaxel 200–260 mg/m2 administered by intravenous infusion every 3 weeks; (2) C: carboplatin, total dose (mg) = Area Under Curve(AUC) × (glomerular filtration rate + 25) (AUC is 4–5), administered by intravenous infusion every 3 weeks; (3) H: trastuzumab 8 mg/kg administered by intravenous infusion for the first time and 6 mg/kg administered by intravenous infusion afterward every 3 weeks and (4) P: pertuzumab 840 mg administered by intravenous infusion for the first time and 420 mg administered by intravenous infusion afterward every 3 weeks.
The clinical response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [31]. Patients underwent surgery after scheduled neoadjuvant therapy. The Miller–Payne system was used to assess the effect of neoadjuvant treatment [32], and the pathological response was evaluated according to Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC). pCR was defined as the absence of invasive cancer in the breast and axillary nodes, irrespective of ductal carcinoma in situ [33].
2.5. Statistical Analysis
The clinicopathological characteristics of the groups were compared by using the independent-sample t test for continuous variables, Pearson χ2 test or Fisher’s exact probability test for categorical variables and the Mann–Whitney U test for grade variables. Relevant factors were analyzed by logistic regression. The diagnostic test was analyzed by receiver operating characteristic (ROC) curve analysis. p < 0.05 indicated that the difference was statistically significant. All tests were two-sided tests. All analyses were performed with IBM SPSS 26.
3. Results
3.1. Patients
A total of 44 patients were enrolled in our study. All the patients were women. The median age was 46.5 years, and the Eastern Cooperative Oncology Group (ECOG) performance status was 0–1. Of the patients, 23 (52.3%) had the HER2-overexpressed subtype and 21 (47.7%) had the luminal B HER2-positive subtype. Of the 21 Luminal B HER2 positive patients, 6 patients were ER+ PR- and 15 were ER+ PR+. One patient was in stage T1, 36 patients were in stage T2 and 7 patients were in stage T3. A total of 22 patients (50%) were in stage N1. Five patients had Ki-67 <30% and 38 had Ki-67 ≥ 30%. Six patients did not express AR. Thirteen patients had an AR expression level of 50–89% and eighteen had an AR expression level ≥90%. Forty patients received TCHP neoadjuvant therapy and four patients received other therapies (Table 1).

Table 1.
Baseline characteristics of the patients. BMI Body Mass Index, ECOG the Eastern Cooperative Oncology Group, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, FISH fluorescence in situ hybridization, AR androgen receptor, pCR pathological complete response, TCHP taxanes, carboplatin, trastuzumab and pertuzumab, NPHP N: vinorelbine 25–30 mg/m2 administered by intravenous infusion on day 1 and day 8 every 3 weeks; P: cisplatin 75 mg/m2 administered by intravenous infusion, completed in 2–3 days, every 3 weeks; H: trastuzumab; P: pertuzumab.
3.2. Factors Influencing Neoadjuvant Therapy Response
We performed multiple logistic regression including factors such as age, Body Mass Index (BMI), tumor stage and molecular biomarkers. AR expression over 90% was the only statistically significant factor for pCR (OR 729.322, 95% Confidence Interval, CI 1.778–299,130.165, p = 0.032). We then used the backward LR method to select the factors. It showed that AR expression over 90% was positively correlated with pCR (OR 33.145, 95% CI 2.803–391.900, p = 0.005) and that ER positivity was negatively correlated with pCR (OR 0.075, 95% CI 0.008–0.678, p = 0.021). Age, BMI, tumor stage, histological grade, PR, HER2 and Ki-67 were not significantly related to pathological response (Table 2 and Table 3).

Table 2.
Factors influencing neoadjuvant therapy response. BMI Body Mass Index, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, FISH fluorescence in situ hybridization, AR androgen receptor, pCR pathological complete response, TCHP taxanes, carboplatin, trastuzumab and pertuzumab, NPHP vinorelbine, cisplatin, trastuzumab and pertuzumab, OR odds ratio, CI confidence interval.

Table 3.
Factors influencing neoadjuvant therapy response after selection. ER estrogen receptor, AR androgen receptor, OR odds ratio, CI confidence interval.
3.3. ROC Curve Analysis of AR for the Prediction of pCR
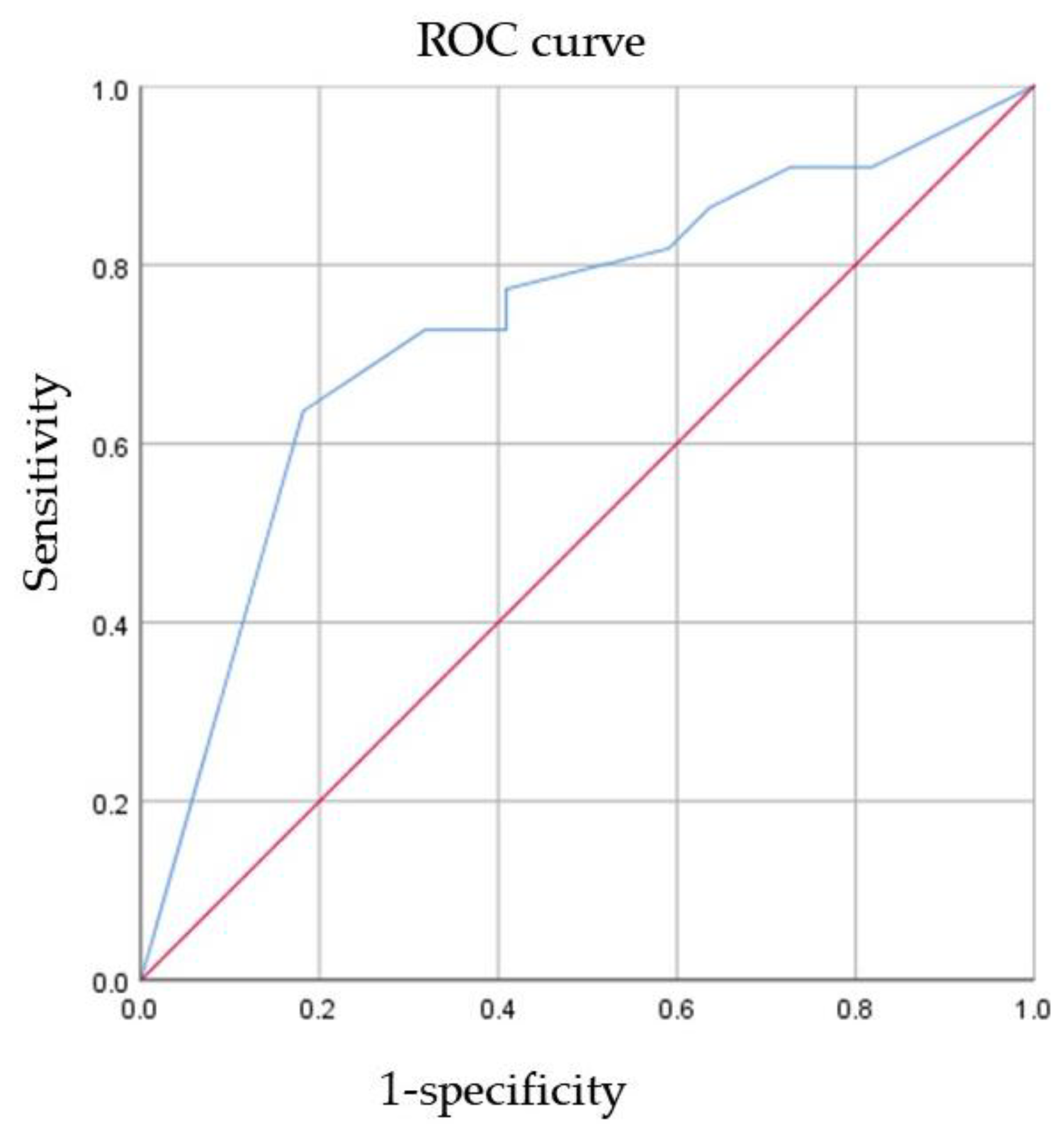
ROC curve analysis of AR expression for the prediction of pCR was performed (Figure 2). The AUC was 0.737 (95% CI 0.585–0.889, p = 0.007). This result indicated that as the AR expression level increased, the patient was more likely to have pCR. To maximize sensitivity and specificity, 85% was the best cutoff of AR expression level to predict pCR. The sensitivity was 0.636, and the specificity was 0.818.
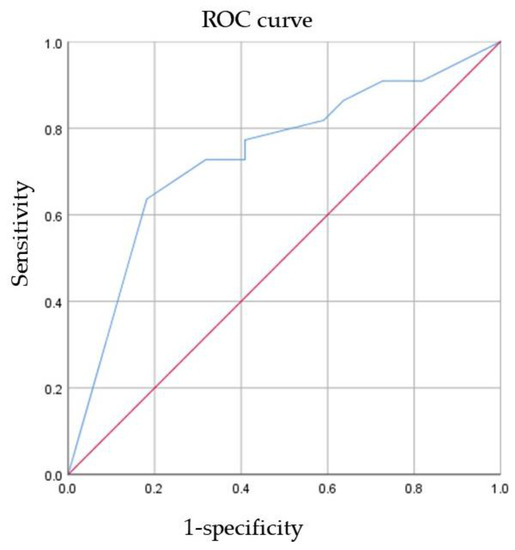
Figure 2.
The ability of AR to predict pCR. ROC receiver operating characteristic, AR androgen receptor, pCR pathological complete response.
4. Discussion
According to the St. Gallen Conference of 2013, breast cancer can be classified into subtypes according to molecular expression [29]. The two subtypes of HER2 positivity are the HER2-overexpressed and luminal B HER2-positive subtypes, accounting for 15–20% of all breast cancer cases. HER2 modulates cell differentiation, apoptosis and other activities through pathways such as PI3K/AKT and RAS/RAB/MEK/MAPK [34]. Anti-HER2 targeted therapy is growing quickly and is widely used in neoadjuvant treatments, adjuvant treatments and salvage treatments. Clinical trials have shown the promising effect of HP in neoadjuvant treatments, but pertuzumab was inaccessible in China until 2019, when it was included in healthcare insurance. To date, there have been few studies on Chinese breast cancer patients treated with HP.
AR widely exists in all breast cancer subtypes. Studies of AR have mostly focused on the TNBC subtype, but few have focused on the HER2-positive subtypes. In HER2-positive breast cancer cells, AR promotes the expression of the HER3 gene, and the HER2/HER3 heterodimer activates the PI3K/AKT pathway and the MYC gene and promotes cell proliferation. HER2 can also activate AR transcription and ERK, which in turn modulates HER2 and AR [35,36]. An additional subtype of molecular apocrine breast cancer was identified in recent microarray studies. This subtype features ER negativity, AR positivity and apocrine differentiation, and is also associated with HER2 amplification [37,38,39]. AR is highly expressed in HER2 enriched (HER2E) breast cancer, and HER2E breast cancers are hormonally driven, either by ER in ER+ HER2E tumors or by AR in ER- HER2E tumors [40]. AR pathway activity and AR expression are also found to be positively correlated in HER2-positive breast cancer and inversely correlated in HER2-negative breast cancer [41]. These studies show a crosstalk between AR and HER2.
In our study, the median age of the enrolled patients was 46.5 years, which was consistent with the characteristics of early age of onset in China. Approximately 70–90% of luminal breast cancers and 60–70% of HER2-positive breast cancers express AR [42,43,44,45,46,47,48,49,50]. In our study, the proportion of breast cancers with AR expression was 86%, which matched the literature. All patients received full-course neoadjuvant therapy, including HP, and the pCR rate was 50%. However, in the literature, the pCR rate of neoadjuvant therapy, including trastuzumab and pertuzumab, was 54–67% [2,3,4,5,6], which was higher. We inferred that this was due to the difference in age and regions and that the small population could also cause bias. A larger study is warranted to determine the true pCR rate of HER2-targeted neoadjuvant therapy in China. In the previous studies, pCR was related to histological grade, ER, PR, Ki-67 and lymph node status [51,52,53]. However, we found that only high AR expression and ER negativity were significantly related to pCR. In our study, the population was rather small, and only five patients had Ki-67 expression < 30%. This could probably explain why Ki-67 was not significantly associated with pCR in our analysis.
AR expression has been suggested to predict the response to neoadjuvant therapy in the literature. AR-negative TNBC patients had higher pCR rates than AR-positive TNBC patients [3,18,19,20]. In these studies, researchers often adopted 1% or 10% as the cutoff of AR. In a study of 82 HER2-positive breast cancer patients treated with neoadjuvant therapy [15], AR scores ≥ 4 were regarded as AR positive. Although only 57% of patients received HER2-targeted treatment, the pCR rate was as high as 49%. AR-positive and non-luminal types were both associated with pCR. For survival analysis, AR positivity was associated with better OS and DFS in several subgroups. Our result agreed with the literature. There are other studies focusing on the cutoff of AR. AR > 78% has been shown to predict the survival of ERα-positive patients [54], and AR > 35% has been shown to predict RFS in patients with surgically resected breast cancer [22]. In our study, we found that a cutoff value of 85% could predict the response to neoadjuvant therapy including HP. However, the raw data of AR expression level in our study were all integers of 10%, which meant that the cutoff of 85% was the dividing line between AR ≥ 90% and AR ≤ 80%. Therefore, we suggest a cutoff value of 90% as a more practical boundary line. The higher the AR expression level, the more likely it is that the patient has pCR. This cutoff could probably be used to predict and select non-pCR patients and help to adjust treatment strategies to achieve better outcomes.
The highlight of our study is that this is the earliest study on the predictive value of AR in neoadjuvant treatment with HP. Only a short amount of time has passed since pertuzumab was approved for use in China, and there are few studies on the effect of HP in the Chinese population. Our study is relatively early and significant in China at the moment. However, there are still limitations. First, the population was small and from a single center. A larger, multicenter study is needed to show the effect of HER2-targeted neoadjuvant treatment in Chinese people and the predictive value of AR. Second, pertuzumab has only been accessible in China since 2019 and some patients are still receiving adjuvant HER2-targeted therapy. The follow-up time was too short to assess long-term survival. We will continue to follow up with the patients for further study on survival.
5. Conclusions
Neoadjuvant therapy including HP is a major strategy for treating HER2-positive breast cancer patients. AR expression has the potential to predict the effects of treatment, but a larger population and longer follow-up are needed to show its value. AR is also a probable drug target in breast cancer. We look forward to the promising performance of AR in the treatment of breast cancer.
Author Contributions
Conceptualization, J.Y. and Y.L.; methodology, C.Y.; software, J.L. and C.Y.; validation, L.X. (Ling Xu); formal analysis, C.Y.; investigation, J.L.; resources, S.Z. and H.Z.; data curation, J.Y.; writing—original draft preparation, J.L.; writing—review and editing, L.X. (Ling Xu); visualization, J.L.; supervision, Q.L. and Y.C.; project administration, L.X. (Ling Xin) and X.D.; funding acquisition, L.X. (Ling Xu). All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key R&D Program of China, grant number 2016YFC0901302, and the Interdisciplinary Clinical Research Project of Peking University First Hospital, grant number 2019CR38.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Peking University First Hospital (No 2020-784).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to patients’ individual privacy but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.-S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Schneeweiss, A.; Schmatloch, S.; Aktas, B.; Denkert, C.; Wiebringhaus, H.; Kümmel, S.; Warm, M.; Paepke, S.; et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: A subanalysis of data from the randomized phase III GeparSepto trial. Ann. Oncol. 2017, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Tan, A.R.; Im, S.A.; Mattar, A.; Colomer, R.; Stroyakovskii, D.; Nowecki, Z.; De Laurentiis, M.; Pierga, J.Y.; Jung, K.H.; Schem, C.; et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet. Oncol. 2021, 22, 85–97. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [Green Version]
- Govindan, S.; Siraganahalli Eswaraiah, M.; Basavaraj, C.; Adinarayan, M.; Sankaran, S.; Bakre, M. Androgen Receptor mRNA levels determine the prognosis in triple-negative breast cancer patients. BMC Cancer 2020, 20, 745. [Google Scholar] [CrossRef]
- Choi, J.E.; Kang, S.H.; Lee, S.J.; Bae, Y.K. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann. Surg. Oncol. 2015, 22, 82–89. [Google Scholar] [CrossRef]
- Kensler, K.H.; Poole, E.M.; Heng, Y.J.; Collins, L.C.; Glass, B.; Beck, A.H.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. J. Natl. Cancer Inst. 2019, 111, 700–708. [Google Scholar] [CrossRef]
- Venema, C.M.; Bense, R.D.; Steenbruggen, T.G.; Nienhuis, H.H.; Qiu, S.Q.; van Kruchten, M.; Brown, M.; Tamimi, R.M.; Hospers, G.A.P.; Schröder, C.P.; et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol. Ther. 2019, 200, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W.; Lee, K.S. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann. Oncol. 2011, 22, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, B.; Quaquarini, E.; Palumbo, R.; Balletti, E.; Presti, D.; Malovini, A.; Agozzino, M.; Teragni, C.M.; Terzoni, A.; Bernardo, A.; et al. Role of androgen receptor expression in early stage ER+/PgR-/HER2- breast cancer. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bi, X.; Huang, Z.; Huang, J.; Xia, W.; Shi, W.; Yuan, Z. The prognostic value of androgen receptor (AR) in HER2-enriched metastatic breast cancer. Endocr.-Relat. Cancer 2020, 27, 199–208. [Google Scholar] [CrossRef]
- Akashi, M.; Yamaguchi, R.; Kusano, H.; Ogasawara, S.; Abe, E.; Obara, H.; Yamaguchi, M.; Akiba, J.; Kakuma, T.; Tanaka, M.; et al. Androgen receptor expression is useful to predict the therapeutic effect in HER2-positive breast carcinoma. Breast Cancer Res. Treat. 2020, 184, 277–285. [Google Scholar] [CrossRef]
- Bozovic-Spasojevic, I.; Zardavas, D.; Brohée, S.; Ameye, L.; Fumagalli, D.; Ades, F.; de Azambuja, E.; Bareche, Y.; Piccart, M.; Paesmans, M.; et al. The Prognostic Role of Androgen Receptor in Patients with Early-Stage Breast Cancer: A Meta-analysis of Clinical and Gene Expression Data. Clin. Cancer Res. 2017, 23, 2702–2712. [Google Scholar] [CrossRef] [Green Version]
- Arici, S.; Sengiz Erhan, S.; Geredeli, C.; Cekin, R.; Sakin, A.; Cihan, S. The Clinical Importance of Androgen Receptor Status in Response to Neoadjuvant Chemotherapy in Turkish Patients with Local and Locally Advanced Breast Cancer. Oncol. Res. Treat. 2020, 43, 435–440. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Elsayed, F.M.; Algazar, M.; Rashed, H.E.; Anter, A.H. Neoadjuvant Chemotherapy in Triple Negative Breast Cancer: Correlation between Androgen Receptor Expression and Pathological Response. Asian Pac. J. Cancer Prev. 2020, 21, 563–568. [Google Scholar] [CrossRef]
- Di Leone, A.; Fragomeni, S.M.; Scardina, L.; Ionta, L.; Mulè, A.; Magno, S.; Terribile, D.; Masetti, R.; Franceschini, G. Androgen receptor expression and outcome of neoadjuvant chemotherapy in triple-negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1910–1915. [Google Scholar] [CrossRef]
- Wetzel, C.L.; Sutton, T.L.; Gardiner, S.; Farinola, M.; Johnson, N.; Garreau, J.R. Loss of HER2-positivity following neoadjuvant targeted therapy for breast cancer is not associated with inferior oncologic outcomes. J. Surg. Oncol. 2021, 124, 1224–1234. [Google Scholar] [CrossRef]
- Jongen, L.; Floris, G.; Wildiers, H.; Claessens, F.; Richard, F.; Laenen, A.; Desmedt, C.; Ardui, J.; Punie, K.; Smeets, A.; et al. Tumor characteristics and outcome by androgen receptor expression in triple-negative breast cancer patients treated with neo-adjuvant chemotherapy. Breast Cancer Res. Treat. 2019, 176, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Min, A.; Lee, K.H.; Ryu, H.S.; Kim, T.Y.; Woo, G.U.; Suh, K.J.; Lee, D.W.; Lee, H.B.; Moon, H.G.; et al. Prognostic Role of Androgen Receptor Expression in Surgically Resected Early Breast Cancer Patients. J. Breast Cancer 2020, 23, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res. Treat. 2006, 100, 229–235. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhani, S.R.; Ellis., I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumours of the Breast, 4th ed.; IARC Press: Lyon, France, 2012. [Google Scholar]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [Green Version]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ogston, K.N.; Miller, I.D.; Payne, S.; Hutcheon, A.W.; Sarkar, T.K.; Smith, I.; Schofield, A.; Heys, S.D. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 2003, 12, 320–327. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Chia, K.M.; Liu, J.; Francis, G.D.; Naderi, A. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia 2011, 13, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, K.; Milioli, H.; Portman, N.; Laven-Law, G.; Coulson, R.; Yong, A.; Segara, D.; Parker, A.; Caldon, C.E.; Deng, N.; et al. Non-canonical AR activity facilitates endocrine resistance in breast cancer. Endocr. Relat. Cancer 2019, 26, 251–264. [Google Scholar] [CrossRef]
- Farmer, P.; Bonnefoi, H.; Becette, V.; Tubiana-Hulin, M.; Fumoleau, P.; Larsimont, D.; Macgrogan, G.; Bergh, J.; Cameron, D.; Goldstein, D.; et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 2005, 24, 4660–4671. [Google Scholar] [CrossRef] [Green Version]
- Guedj, M.; Marisa, L.; de Reynies, A.; Orsetti, B.; Schiappa, R.; Bibeau, F.; MacGrogan, G.; Lerebours, F.; Finetti, P.; Longy, M.; et al. A refined molecular taxonomy of breast cancer. Oncogene 2012, 31, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Lehmann-Che, J.; Hamy, A.S.; Porcher, R.; Barritault, M.; Bouhidel, F.; Habuellelah, H.; Leman-Detours, S.; de Roquancourt, A.; Cahen-Doidy, L.; Bourstyn, E.; et al. Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res. BCR 2013, 15, R37. [Google Scholar] [CrossRef] [Green Version]
- Daemen, A.; Manning, G. HER2 is not a cancer subtype but rather a pan-cancer event and is highly enriched in AR-driven breast tumors. Breast Cancer Res. BCR 2018, 20, 8. [Google Scholar] [CrossRef]
- Liu, D. AR pathway activity correlates with AR expression in a HER2-dependent manner and serves as a better prognostic factor in breast cancer. Cell Oncol. 2020, 43, 321–333. [Google Scholar] [CrossRef]
- Anand, A.; Singh, K.R.; Kumar, S.; Husain, N.; Kushwaha, J.K.; Sonkar, A.A. Androgen Receptor Expression in an Indian Breast Cancer Cohort with Relation to Molecular Subtypes and Response to Neoadjuvant Chemotherapy—A Prospective Clinical Study. Breast Care 2017, 12, 160–164. [Google Scholar] [CrossRef]
- Bravaccini, S.; Ravaioli, S.; Amadori, D.; Scarpi, E.; Puccetti, M.; Rocca, A.; Tumedei, M.M.; Masalu, N.; Kahima, J.; Pangan, A.; et al. Are There Differences in Androgen Receptor Expression in Invasive Breast Cancer in African (Tanzanian) Population in Comparison With the Caucasian (Italian) Population? Front. Endocrinol. 2018, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Bravaccini, S.; Ravaioli, S.; Puccetti, M.; Scarpi, E.; Andreis, D.; Tumedei, M.M.; Sarti, S.; Cecconetto, L.; Pietri, E.; et al. Androgen Receptor Expression in Breast Cancer: What Differences Between Primary Tumor and Metastases? Transl. Oncol. 2018, 11, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Allia, E.; Accortanzo, V.; Vandone, A.M.; Chiusa, L.; Arisio, R.; Durando, A.; Donadio, M.; Bussolati, G.; Coates, A.S.; et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res. Treat. 2010, 124, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.F.; Li, J.J.; Kang, S.H.; Song, C.G. The Expression, Clinicopathologic Characteristics, and Prognostic Value of Androgen Receptor in Breast Cancer: A Bioinformatics Analysis Using Public Databases. DNA Cell Biol. 2020, 39, 864–874. [Google Scholar] [CrossRef]
- García, X.; Elía, A.; Galizzi, L.; May, M.; Spengler, E.; Martínez Vázquez, P.; Burruchaga, J.; Gass, H.; Lanari, C.; Lamb, C.A. Increased androgen receptor expression in estrogen receptor-positive/progesterone receptor-negative breast cancer. Breast Cancer Res. Treat. 2020, 180, 257–263. [Google Scholar] [CrossRef]
- Ismael, N.; Khairy, R.A.; Talaat, S.M.; El-Fattah, F.A.A. Immunohistochemical Expression of Androgen Receptors (AR) in Various Breast Cancer Subtypes. Open Access Maced. J. Med. Sci. 2019, 7, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrøm, M.J.; Bofin, A.M. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar] [CrossRef]
- Vidula, N.; Yau, C.; Wolf, D.; Rugo, H.S. Androgen receptor gene expression in primary breast cancer. NPJ Breast Cancer 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Kurozumi, S.; Inoue, K.; Takei, H.; Matsumoto, H.; Kurosumi, M.; Horiguchi, J.; Takeyoshi, I.; Oyama, T. ER, PgR, Ki67, p27Kip1, and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer 2015, 15, 622. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wu, S.; Xing, H.; Han, M.; Li, J.; Liu, Y. Development and Validation of a Novel Model for Predicting Prognosis of Non-PCR Patients After Neoadjuvant Therapy for Breast Cancer. Front. Oncol. 2021, 11, 675533. [Google Scholar] [CrossRef]
- Boér, K.; Kahán, Z.; Landherr, L.; Csőszi, T.; Máhr, K.; Ruzsa, Á.; Horváth, Z.; Budai, B.; Rubovszky, G. Pathologic Complete Response Rates After Neoadjuvant Pertuzumab and Trastuzumab with Chemotherapy in Early Stage HER2-Positive Breast Cancer—Increasing Rates of Breast Conserving Surgery: A Real-World Experience. Pathol. Oncol. Res. POR 2021, 27, 1609785. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.A.; Sakko, A.J.; et al. The Magnitude of Androgen Receptor Positivity in Breast Cancer Is Critical for Reliable Prediction of Disease Outcome. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).