High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Methods
2.2. Oncological Outcomes
2.3. Functional Outcomes
2.4. Statistical Analysis
3. Results
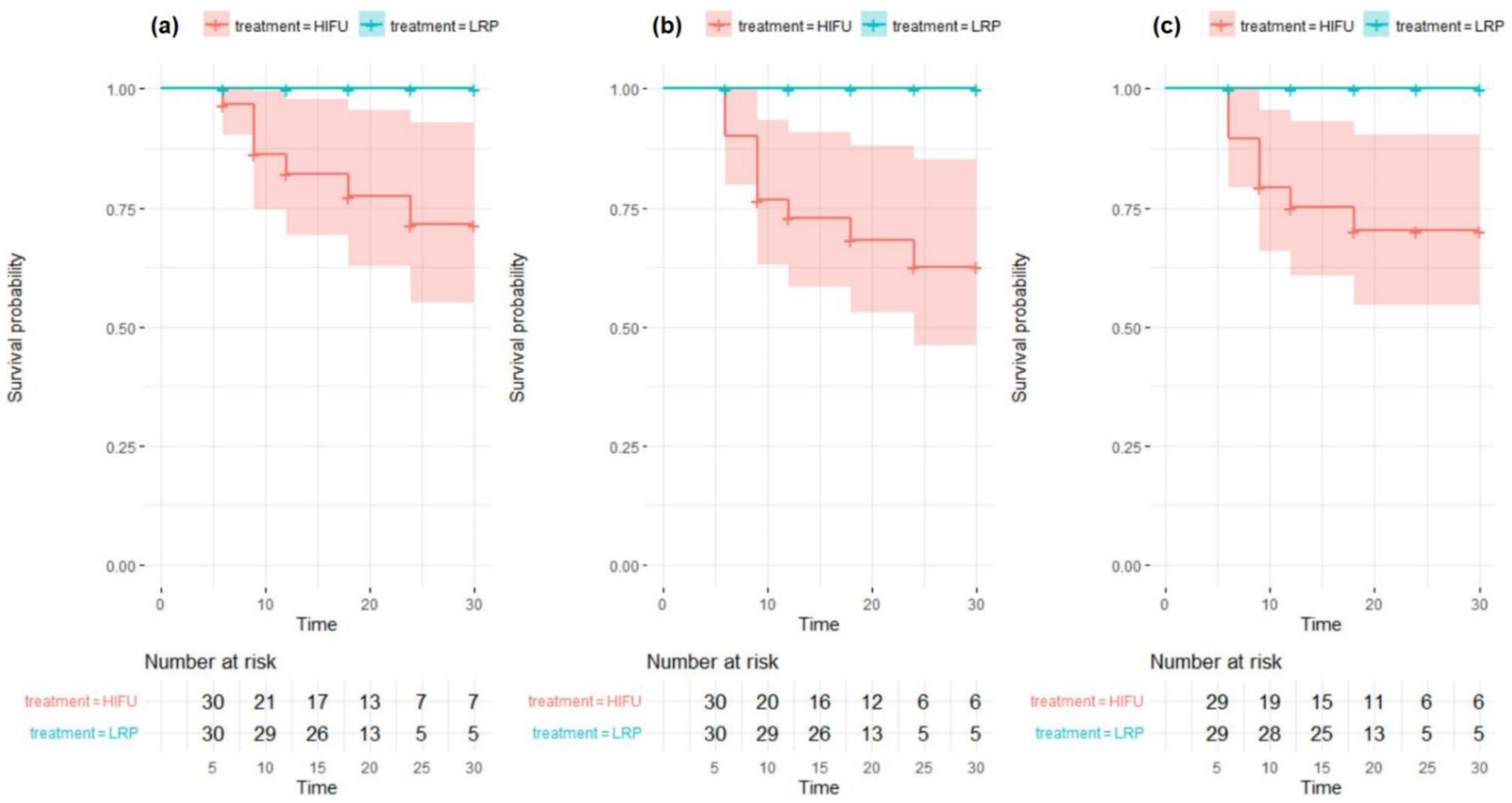
3.1. Oncological Outcomes
3.1.1. High-Intensity Focused Ultrasound-Focal Therapy (HIFU-FT) Treatment Failure Defined as a Prostate-Specific Antigen (PSA) Rise >1.2 ng/mL above the Nadir Only
3.1.2. HIFU-FT Treatment Failure Defined as Either PSA Rise > 1.2 ng/mL or a Positive Biopsy during Follow-Up
3.1.3. HIFU-FT Treatment Failure Defined as a Positive Biopsy Only
3.2. Urinary Continence
3.3. Erectile Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 24 May 2021).
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan 2021; EAU: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Blana, A.; Brown, S.C.; Chaussy, C.; Conti, G.N.; Eastham, J.A.; Ganzer, R.; Murat, F.J.; Pasticier, G.; Rebillard, X.; Rewcastle, J.C.; et al. High-intensity focused ultrasound for prostate cancer: Comparative definitions of biochemical failure. Br. J. Urol. 2009, 104, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.; Gorin, M.A.; Ahmed, H.U.; Nigam, R. Focal therapy for localized prostate cancer in the era of routine multi-parametric MRI. Prostate Cancer Prostatic Dis. 2020, 23, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.T.; Reddy, D.; Peters, M.; Ball, D.; Kim, N.H.; Gomez, E.G.; Miah, S.; Evans, D.E.; Guillaumier, S.; van Rossum, P.S.N.; et al. Focal therapy compared to radical prostatectomy for non-metastatic prostate cancer: A propensity score-matched study. Prostate Cancer Prostatic Dis. 2021, 24, 567–574. [Google Scholar] [CrossRef]
- Guillaumier, S.; Peters, M.; Arya, M.; Afzal, N.; Charman, S.; Dudderidge, T.; Hosking-Jervis, F.; Hindley, R.G.; Lewi, H.; McCartan, N.; et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur. Urol. 2018, 74, 422–429. [Google Scholar] [CrossRef]
- Albisinni, S.; Aoun, F.; Bellucci, S.S.; Biaou, I.; Limani, K.; Hawaux, E.; Peltier, A.; Van Velthoven, R. Comparing High-Intensity Focal Ultrasound Hemiablation to Robotic Radical Prostatectomy in the Management of Unilateral Prostate Cancer: A Matched-Pair Analysis. J. Endourol. 2017, 31, 14–19. [Google Scholar] [CrossRef]
- Arnouil, N.; Gelet, A.; Matillon, X.; Rouviere, O.; Colombel, M.; Ruffion, A.; Mège-Lechevallier, F.; Subtil, F.; Badet, L.; Crouzet, S. Traitement focal par HIFU versus prostatectomie radicale robot-assistée pour cancer de la prostate localisé: Résultats carcinologiques et fonctionnels à 1 an [Focal HIFU vs robot-assisted total prostatectomy: Functionnal and oncologic outcomes at one year]. Prog. Urol. 2018, 28, 603–610. [Google Scholar] [CrossRef]
- Valerio, M.; Ahmed, H.U.; Emberton, M.; Lawrentschuk, N.; Lazzeri, M.; Montironi, R.; Nguyen, P.L.; Trachtenberg, J.; Polascik, T.J. The Role of Focal Therapy in the Management of Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2014, 66, 732–751. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.U.; Hindley, R.G.; Dickinson, L.; Freeman, A.; Kirkham, A.P.; Sahu, M.; Scott, R.; Allen, C.; Van der Meulen, J.; Emberton, M. Focal therapy for localised unifocal and multifocal prostate cancer: A prospective development study. Lancet Oncol. 2012, 13, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Feijoo, E.R.C.; Sivaraman, A.; Barret, E.; Sanchez-Salas, R.; Galiano, M.; Rozet, F.; Prapotnich, D.; Cathala, N.; Mombet, A.; Cathelineau, X. Focal High-intensity Focused Ultrasound Targeted Hemiablation for Unilateral Prostate Cancer: A Prospective Evaluation of Oncologic and Functional Outcomes. Eur. Urol. 2016, 69, 214–220. [Google Scholar] [CrossRef]
- Barqawi, A.B.; Huebner, E.; Krughoff, K.; O’Donnell, C.I. Prospective Outcome Analysis of the Safety and Efficacy of Partial and Complete Cryoablation in Organ-confined Prostate Cancer. Urology 2018, 112, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bahn, D.; Abreu, A.L.D.C.; Gill, I.S.; Hung, A.J.; Silverman, P.; Gross, M.E.; Lieskovsky, G.; Ukimura, O. Focal Cryotherapy for Clinically Unilateral, Low-Intermediate Risk Prostate Cancer in 73 Men with a Median Follow-Up of 3.7 Years. Eur. Urol. 2012, 62, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bos, W.V.D.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.; Thompson, J.E.; Ting, F.; Böhm, M.; Haynes, A.-M.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. Br. J. Urol. 2018, 121, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Azzouzi, A.-R.; Barret, E.; Villers, A.; Muir, G.H.; Barber, N.J.; Bott, S.; Trachtenberg, J.; Arumainayagam, N.; Gaillac, B.; et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. Br. J. Urol. 2014, 116, 888–896. [Google Scholar] [CrossRef]
- Valerio, M.; Cerantola, Y.; Eggener, S.E.; Lepor, H.; Polascik, T.J.; Villers, A.; Emberton, M. New and Established Technology in Focal Ablation of the Prostate: A Systematic Review. Eur. Urol. 2017, 71, 17–34. [Google Scholar] [CrossRef]
- Kongnyuy, M.; Lipsky, M.J.; Islam, S.; Robins, D.J.; Hager, S.; Halpern, D.M.; Kosinski, K.E.; Schiff, J.T.; Corcoran, A.T.; Wenske, S.; et al. Predictors of biochemical recurrence after primary focal cryosurgery (hemiablation) for localized prostate cancer: A multi-institutional analytic comparison of Phoenix and Stuttgart criteria. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 530.e15–530.e19. [Google Scholar] [CrossRef]
- Donaldson, I.A.; Alonzi, R.; Barratt, D.; Barret, E.; Berge, V.; Bott, S.; Bottomley, D.; Eggener, S.; Ehdaie, B.; Emberton, M.; et al. Focal Therapy: Patients, Interventions, and Outcomes—A Report from a Consensus Meeting. Eur. Urol. 2015, 67, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Rosenhammer, B.; Niessen, C.; Rotzinger, L.; Reiss, J.; Schnabel, M.J.; Burger, M.; Bründl, J. Oncological Outcome and Value of Postoperative Magnetic Resonance Imaging after Focal High-Intensity Focused Ultrasound Therapy for Prostate Cancer. Urol. Int. 2019, 103, 270–278. [Google Scholar] [CrossRef]
- Garcia-Barreras, S.; Sanchez-Salas, R.; Sivaraman, A.; Barret, E.; Secin, F.; Nunes-Silva, I.; Linares-Espinós, E.; Rozet, F.; Galiano, M.; Cathelineau, X. Comparative Analysis of Partial Gland Ablation and Radical Prostatectomy to Treat Low and Intermediate Risk Prostate Cancer: Oncologic and Functional Outcomes. J. Urol. 2018, 199, 140–146. [Google Scholar] [CrossRef]
- Asimakopoulos, A.D.; Fraga, C.T.P.; Annino, F.; Pasqualetti, P.; Calado, A.A.; Mugnier, C. Randomized Comparison between Laparoscopic and Robot-Assisted Nerve-Sparing Radical Prostatectomy. J. Sex. Med. 2011, 8, 1503–1512. [Google Scholar] [CrossRef]
- Willis, D.L.; Gonzalgo, M.L.; Brotzman, M.; Feng, Z.; Trock, B.; Su, L.-M. Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: A study of comparative effectiveness based upon validated quality of life outcomes. Br. J. Urol. 2011, 109, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kim, W.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Seo, S.I. Comparison of oncological and functional outcomes of pure versus robotic-assisted laparoscopic radical prostatectomy performed by a single surgeon. Scand. J. Urol. 2012, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Valerio, M.; Emberton, M.; Heidenreich, A.; Crook, J.M.; Bossi, A.; Pisters, L.L. Salvage Local Treatments After Focal Therapy for Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Marconi, L.; Stonier, T.; Tourinho-Barbosa, R.; Moore, C.; Ahmed, H.U.; Cathelineau, X.; Emberton, M.; Sanchez-Salas, R.; Cathcart, P. Robot-assisted Radical Prostatectomy After Focal Therapy: Oncological, Functional Outcomes and Predictors of Recurrence. Eur. Urol. 2019, 76, 27–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitznagel, T.; Hardenberg, J.; Schmid, F.A.; Rupp, N.J.; Westhoff, N.; Worst, T.S.; Weis, C.A.; Mortezavi, A.; Eberli, D. Salvage Robotic-assisted Laparoscopic Radical Prostatectomy Following Focal High-Intensity Focused Ultrasound for ISUP 2/3 Cancer. Urology 2021, 156, 147–153. [Google Scholar] [CrossRef]
| Parameter | HIFU-FT (n = 30) | LRP, Prior to PSM (n = 96) | LRP, After PSM (n = 30) |
|---|---|---|---|
| Median age (years) | 64.5 | 65.7 | 64.0 |
| Mean PSA (ng/mL) | 6.6 | 6.6 | 6.5 |
| Mean PV (mL) | 38.0 | 42.6 | 39.4 |
| Mean baseline IIEF-5 score | 19.2 | 14.7 | 19.9 |
| Median follow-up (months) | 19.1 | 10.4 | 12.5 |
| cT1c a | 17 (57%) | 63 (66%) | 16 (53%) |
| cT2a a | 12 (40%) | 14 (15%) | 6 (20%) |
| cT2b a | 1 (3%) | 19 (20%) | 8 (27%) |
| Grade group 1 | 20 (67%) | 63 (66%) | 18 (60%) |
| Grade group 2 | 9 (30%) | 33 (34%) | 12 (40%) |
| Grade group 3 | 1 (3%) | 0 (0%) | 0 (0%) |
| Nerve-sparing | |||
| any | N/A | 79 (82%) | 27 (90%) |
| bilateral | N/A | 59 (61%) | 25 (83%) |
| Intrafascial b | N/A | 47 (49%) | 20 (67%) |
| Median hospital stay (days) | 2.0 | 3.6 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyk, Ł.; Michalak, W.; Szempliński, S.; Woźniak, R.; Zagożdżon, B.; Krajewski, W.; Kryst, P.; Kamecki, H.; Poletajew, S. High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients. J. Pers. Med. 2022, 12, 251. https://doi.org/10.3390/jpm12020251
Nyk Ł, Michalak W, Szempliński S, Woźniak R, Zagożdżon B, Krajewski W, Kryst P, Kamecki H, Poletajew S. High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients. Journal of Personalized Medicine. 2022; 12(2):251. https://doi.org/10.3390/jpm12020251
Chicago/Turabian StyleNyk, Łukasz, Wojciech Michalak, Stanisław Szempliński, Rafał Woźniak, Bartłomiej Zagożdżon, Wojciech Krajewski, Piotr Kryst, Hubert Kamecki, and Sławomir Poletajew. 2022. "High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients" Journal of Personalized Medicine 12, no. 2: 251. https://doi.org/10.3390/jpm12020251
APA StyleNyk, Ł., Michalak, W., Szempliński, S., Woźniak, R., Zagożdżon, B., Krajewski, W., Kryst, P., Kamecki, H., & Poletajew, S. (2022). High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients. Journal of Personalized Medicine, 12(2), 251. https://doi.org/10.3390/jpm12020251







