The Impact of Total Knee Replacement with a Customized Cruciate-Retaining Implant Design on Patient-Reported and Functional Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Custom Cruciate-Retaining TKA Implant and Planning
2.3. Surgical Technique
2.4. Outcome Parameters
2.5. Statistical Analysis
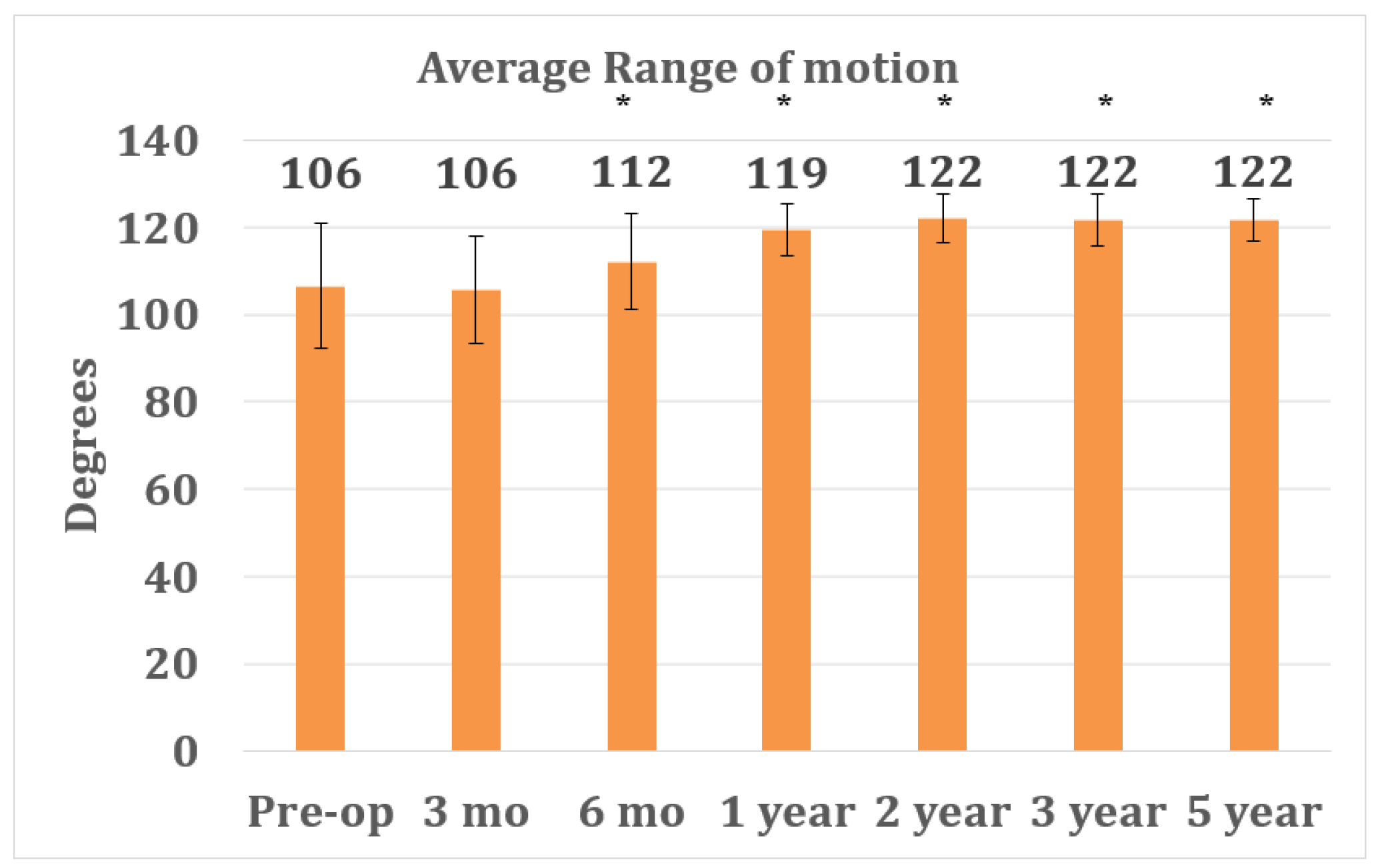
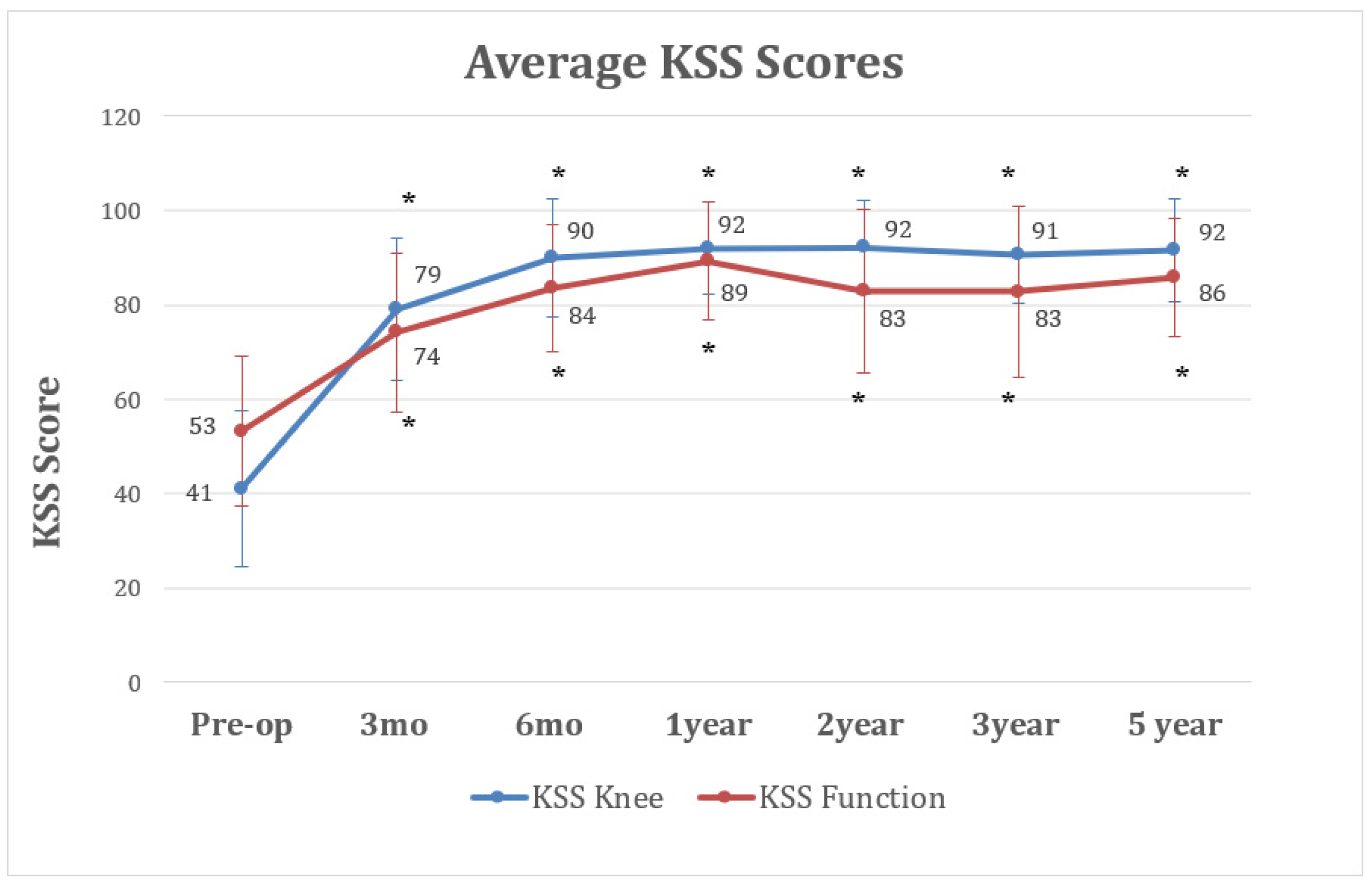
3. Results
3.1. Study Population and Intraoperative Parameters
3.2. Complications and Survial
3.3. Outcome Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, P.C.; Conditt, M.A.; Cook, K.F.; Mathis, K.B. The John Insall Award: Patient expectations affect satisfaction with total knee arthroplasty. Clin. Orthop. Relat. Res. 2006, 452, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvizi, J.; Nunley, R.M.; Berend, K.R.; Lombardi, A.V., Jr.; Ruh, E.L.; Clohisy, J.C.; Hamilton, W.G.; Della Valle, C.J.; Barrack, R.L. High level of residual symptoms in young patients after total knee arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, D.; Nunley, R.M.; Barrack, R.L. Patient dissatisfaction following total knee replacement: A growing concern? Bone Joint J. 2014, 96, 96–100. [Google Scholar] [CrossRef]
- Batailler, C.; Swan, J.; Sappey Marinier, E.; Servien, E.; Lustig, S. New Technologies in Knee Arthroplasty: Current Concepts. J. Clin. Med. 2020, 10, 47. [Google Scholar] [CrossRef]
- Steinert, A.F.; Sefrin, L.; Hoberg, M.; Arnholdt, J.; Rudert, M. Individualized total knee arthroplasty. Orthopade 2015, 44, 294–301. [Google Scholar] [CrossRef]
- Steinert, A.F.; Holzapfel, B.M.; Sefrin, L.; Arnholdt, J.; Hoberg, M.; Rudert, M. Total knee arthroplasty. Patient-specific instruments and implants. Orthopade 2016, 45, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Sefrin, L.; Jansen, B.; Schroder, L.; Holzapfel, B.M.; Arnholdt, J.; Rudert, M. Patient-specific cruciate-retaining total knee replacement with individualized implants and instruments (iTotal CR G2). Oper. Orthop. Traumatol. 2021, 33, 170–180. [Google Scholar] [CrossRef]
- Arnholdt, J.; Kamawal, Y.; Horas, K.; Holzapfel, B.M.; Gilbert, F.; Ripp, A.; Rudert, M.; Steinert, A.F. Accurate implant fit and leg alignment after cruciate-retaining patient-specific total knee arthroplasty. BMC Musculoskelet Disord 2020, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- Arnholdt, J.; Holzapfel, B.M.; Sefrin, L.; Rudert, M.; Beckmann, J.; Steinert, A.F. Individualized unicondylar knee replacement: Use of patient-specific implants and instruments. Oper. Orthop. Traumatol. 2017, 29, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Koeck, F.X.; Beckmann, J.; Luring, C.; Rath, B.; Grifka, J.; Basad, E. Evaluation of implant position and knee alignment after patient-specific unicompartmental knee arthroplasty. Knee 2011, 18, 294–299. [Google Scholar] [CrossRef]
- Arnholdt, J.; Kamawal, Y.; Holzapfel, B.M.; Ripp, A.; Rudert, M.; Steinert, A.F. Evaluation of implant fit and frontal plane alignment after bi-compartmental knee arthroplasty using patient-specific instruments and implants. Arch. Med. Sci. 2018, 14, 1424–1431. [Google Scholar] [CrossRef]
- Beckmann, J.; Steinert, A.F.; Huber, B.; Rudert, M.; Kock, F.X.; Buhs, M.; Rolston, L. Customised bi-compartmental knee arthroplasty shows encouraging 3-year results: Findings of a prospective, multicenter study. Knee Surg. Sports Traumatol. Arthrosc 2020, 28, 1742–1749. [Google Scholar] [CrossRef]
- Steinert, A.F.; Beckmann, J.; Holzapfel, B.M.; Rudert, M.; Arnholdt, J. Bicompartmental individualized knee replacement: Use of patient-specific implants and instruments (iDuo). Oper. Orthop. Traumatol. 2017, 29, 51–58. [Google Scholar] [CrossRef]
- Insall, J.N.; Dorr, L.D.; Scott, R.D.; Scott, W.N. Rationale of the Knee Society clinical rating system. Clin. Orthop. Relat. Res. 1989, 248, 13–14. [Google Scholar] [CrossRef]
- Noble, P.C.; Scuderi, G.R.; Brekke, A.C.; Sikorskii, A.; Benjamin, J.B.; Lonner, J.H.; Chadha, P.; Daylamani, D.A.; Scott, W.N.; Bourne, R.B. Development of a new Knee Society scoring system. Clin. Orthop. Relat. Res. 2012, 470, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Stucki, G.; Meier, D.; Stucki, S.; Michel, B.A.; Tyndall, A.G.; Dick, W.; Theiler, R. Evaluation of a German version of WOMAC (Western Ontario and McMaster Universities) Arthrosis Index. Z Rheumatol. 1996, 55, 40–49. [Google Scholar]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Hepinstall, M.S.; Rutledge, J.R.; Bornstein, L.J.; Mazumdar, M.; Westrich, G.H. Factors that impact expectations before total knee arthroplasty. J. Arthroplast. 2011, 26, 870–876. [Google Scholar] [CrossRef]
- Gandhi, R.; Davey, J.R.; Mahomed, N. Patient expectations predict greater pain relief with joint arthroplasty. J. Arthroplast. 2009, 24, 716–721. [Google Scholar] [CrossRef]
- Meier, M.; Zingde, S.; Best, R.; Schroeder, L.; Beckmann, J.; Steinert, A.F. High variability of proximal tibial asymmetry and slope: A CT data analysis of 15,807 osteoarthritic knees before TKA. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1105–1112. [Google Scholar] [CrossRef]
- Meier, M.; Zingde, S.; Steinert, A.; Kurtz, W.; Koeck, F.; Beckmann, J. What Is the Possible Impact of High Variability of Distal Femoral Geometry on TKA? A CT Data Analysis of 24,042 Knees. Clin. Orthop. Relat. Res. 2019, 477, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Culler, S.D.; Martin, G.M.; Swearingen, A. Comparison of adverse events rates and hospital cost between customized individually made implants and standard off-the-shelf implants for total knee arthroplasty. Arthroplast. Today 2017, 3, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.; Bunn, A.; Bugbee, W.D.; Colwell, C.W., Jr.; D’Lima, D.D. Patient-specific implants with custom cutting blocks better approximate natural knee kinematics than standard TKA without custom cutting blocks. Knee 2015, 22, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Zeller, I.M.; Sharma, A.; Kurtz, W.B.; Anderle, M.R.; Komistek, R.D. Customized versus Patient-Sized Cruciate-Retaining Total Knee Arthroplasty: An In Vivo Kinematics Study Using Mobile Fluoroscopy. J. Arthroplast. 2017, 32, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Ivie, C.B.; Probst, P.J.; Bal, A.K.; Stannard, J.T.; Crist, B.D.; Sonny Bal, B. Improved radiographic outcomes with patient-specific total knee arthroplasty. J. Arthroplast. 2014, 29, 2100–2103. [Google Scholar] [CrossRef]
- Schroeder, L.; Martin, G. In Vivo Tibial Fit and Rotational Analysis of a Customized, Patient-Specific TKA versus Off-the-Shelf TKA. J. Knee Surg. 2019, 32, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Klasan, A.; Twiggs, J.G.; Fritsch, B.A.; Miles, B.P.; Heyse, T.J.; Solomon, M.; Parker, D.A. Correlation of tibial component size and rotation with outcomes after total knee arthroplasty. Arch. Orthop. Trauma Surg. 2020, 140, 1819–1824. [Google Scholar] [CrossRef]
- Schwarzkopf, R.; Brodsky, M.; Garcia, G.A.; Gomoll, A.H. Surgical and Functional Outcomes in Patients Undergoing Total Knee Replacement with Patient-Specific Implants Compared with “Off-the-Shelf“ Implants. Orthop. J. Sports Med. 2015, 3, 2325967115590379. [Google Scholar] [CrossRef]
- Hirschmann, M.T.; Moser, L.B.; Amsler, F.; Behrend, H.; Leclerq, V.; Hess, S. Functional knee phenotypes: A novel classification for phenotyping the coronal lower limb alignment based on the native alignment in young non-osteoarthritic patients. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, C.S.; White, P.B.; West, S.; Ranawat, A.S. Clinical and Radiographic Results of Attune and PFC Sigma Knee Designs at 2-Year Follow-Up: A Prospective Matched-Pair Analysis. J. Arthroplast. 2017, 32, 431–436. [Google Scholar] [CrossRef]
- Chaudhary, R.; Beaupre, L.A.; Johnston, D.W. Knee range of motion during the first two years after use of posterior cruciate-stabilizing or posterior cruciate-retaining total knee prostheses. A randomized clinical trial. J. Bone Joint Surg. Am. 2008, 90, 2579–2586. [Google Scholar] [CrossRef]
- Harato, K.; Bourne, R.B.; Victor, J.; Snyder, M.; Hart, J.; Ries, M.D. Midterm comparison of posterior cruciate-retaining versus -substituting total knee arthroplasty using the Genesis II prosthesis. A multicenter prospective randomized clinical trial. Knee 2008, 15, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.J.; Crua, E.; Chong, B.C.; Gordon, R.; McAuslan, A.; Pitto, R.P.; Clatworthy, M.G. A randomized prospective study comparing mobile-bearing against fixed-bearing PFC Sigma cruciate-retaining total knee arthroplasties with ten-year minimum follow-up. Bone Joint J. 2018, 100-B, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.; Sloan, K.; Clark, G. Functional outcomes comparing Triathlon versus Duracon total knee arthroplasty: Does the Triathlon outperform its predecessor? Int. Orthop. 2014, 38, 1375–1378. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.E.; Clement, N.D.; MacDonald, D.J.; Hamilton, D.F.; Gaston, P.; Howie, C.R.; Burnett, R. Five-year survivorship and patient-reported outcome of the Triathlon single-radius total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1676–1683. [Google Scholar] [CrossRef]
- Behrend, H.; Giesinger, K.; Giesinger, J.M.; Kuster, M.S. The "forgotten joint" as the ultimate goal in joint arthroplasty: Validation of a new patient-reported outcome measure. J. Arthroplast. 2012, 27, 430–436.e431. [Google Scholar] [CrossRef]




| Metric | Min | Max | |
|---|---|---|---|
| Knees (N) | 73 | - | - |
| Patient Gender (% Female) | 38% | - | - |
| Mean Age at Surgery (years) | 64 | 40 | 81 |
| Mean Body Mass Index (BMI) | 31 | 23 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinert, A.F.; Schröder, L.; Sefrin, L.; Janßen, B.; Arnholdt, J.; Rudert, M. The Impact of Total Knee Replacement with a Customized Cruciate-Retaining Implant Design on Patient-Reported and Functional Outcomes. J. Pers. Med. 2022, 12, 194. https://doi.org/10.3390/jpm12020194
Steinert AF, Schröder L, Sefrin L, Janßen B, Arnholdt J, Rudert M. The Impact of Total Knee Replacement with a Customized Cruciate-Retaining Implant Design on Patient-Reported and Functional Outcomes. Journal of Personalized Medicine. 2022; 12(2):194. https://doi.org/10.3390/jpm12020194
Chicago/Turabian StyleSteinert, Andre F., Lennart Schröder, Lukas Sefrin, Björn Janßen, Jörg Arnholdt, and Maximilian Rudert. 2022. "The Impact of Total Knee Replacement with a Customized Cruciate-Retaining Implant Design on Patient-Reported and Functional Outcomes" Journal of Personalized Medicine 12, no. 2: 194. https://doi.org/10.3390/jpm12020194
APA StyleSteinert, A. F., Schröder, L., Sefrin, L., Janßen, B., Arnholdt, J., & Rudert, M. (2022). The Impact of Total Knee Replacement with a Customized Cruciate-Retaining Implant Design on Patient-Reported and Functional Outcomes. Journal of Personalized Medicine, 12(2), 194. https://doi.org/10.3390/jpm12020194







