Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Subjects, Comorbidities, and Medications
2.3. Statistical Analysis
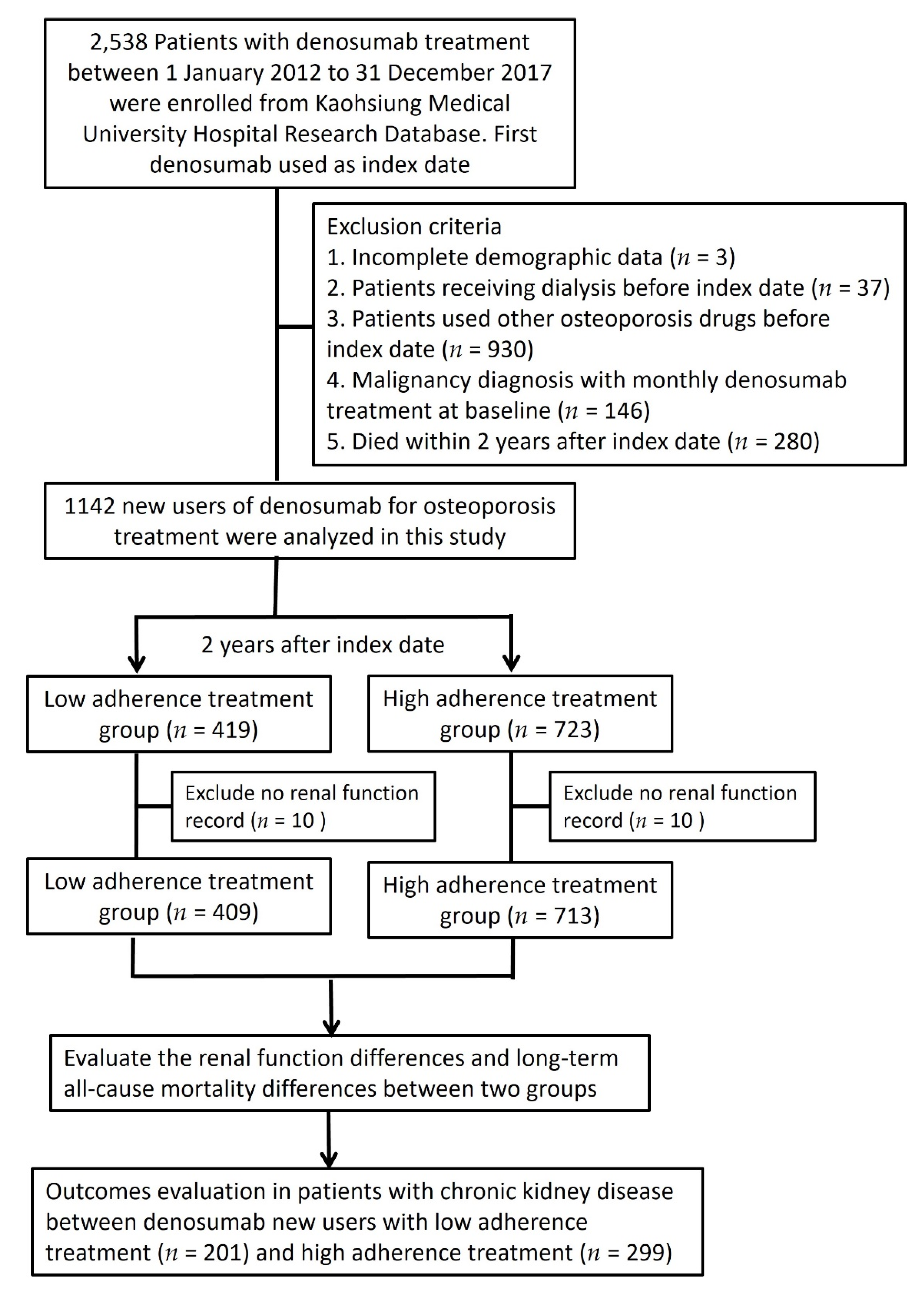
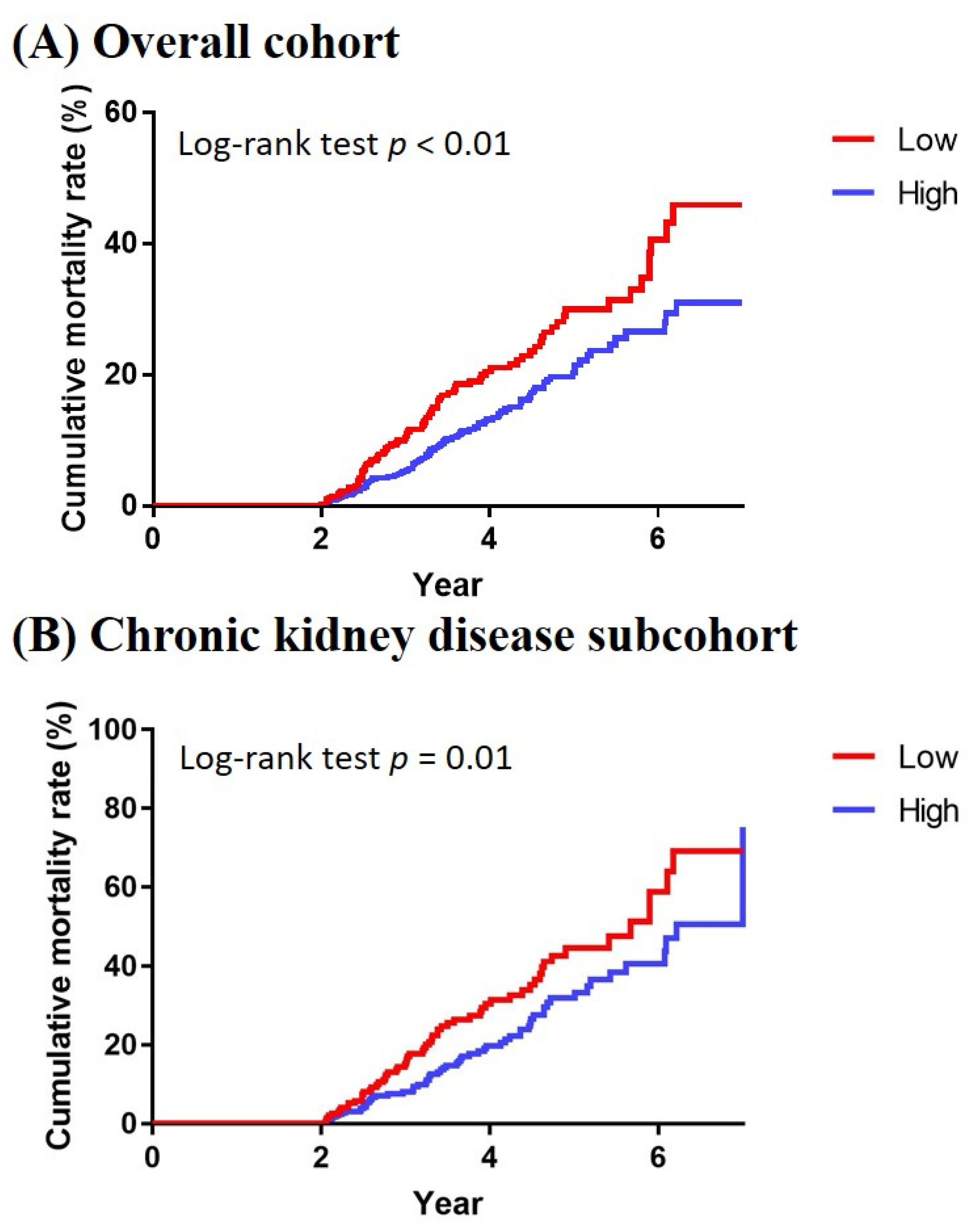
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazama, J.J. Chronic kidney disease and fragility fracture. Clin. Exp. Nephrol. 2017, 21, 46–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pimentel, A.; Ureña-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD—diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Sidibé, A.; Auguste, D.; Desbiens, L.-C.; Fortier, C.; Wang, Y.P.; Jean, S.; Moore, L.; Mac-Way, F. Fracture Risk in Dialysis and Kidney Transplanted Patients: A Systematic Review. JBMR Plus 2019, 3, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Chen, L.-R.; Chen, K.-H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef] [PubMed]
- Broadwell, A.; Chines, A.; Ebeling, P.R.; Franek, E.; Huang, S.; Smith, S.; Kendler, D.; Messina, O.; Miller, P.D. Denosumab Safety and Efficacy Among Participants in the FREEDOM Extension Study With Mild to Moderate Chronic Kidney Disease. J. Clin. Endocrinol. Metab. 2021, 106, 397–409. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [CrossRef]
- Nickolas, T.L.; McMahon, D.J.; Shane, E. Relationship between Moderate to Severe Kidney Disease and Hip Fracture in the United States. J. Am. Soc. Nephrol. 2006, 17, 3223–3232. [Google Scholar] [CrossRef]
- Alem, A.M.; Sherrard, D.J.; Gillen, D.L.; Weiss, N.S.; Beresford, S.A.; Heckbert, S.R.; Wong, C.; Stehman-Breen, C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000, 58, 396–399. [Google Scholar] [CrossRef]
- Kim, S.M.; Long, J.; Montez-Rath, M.; Leonard, M.; Chertow, G.M. Hip Fracture in Patients with Non-Dialysis-Requiring Chronic Kidney Disease. J. Bone Miner. Res. 2016, 31, 1803–1809. [Google Scholar] [CrossRef]
- Klawansky, S.; Komaroff, E.; Cavanaugh, P.; Mitchell, D.Y.; Gordon, M.J.; Connelly, J.E.; Ross, S.D. Relationship between age, renal function and bone mineral density in the US population. Osteoporos. Int. 2003, 14, 570–576. [Google Scholar] [CrossRef]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Ljunggren, Ö.; Stehman-Breen, C.; Cummings, S.R.; McClung, M.; Goemaere, S.; Ebeling, P.R.; Franek, E.; Yang, Y.-C.; Egbuna, O.I.; et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J. Bone Miner. Res. 2011, 26, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.L.; Siris, E.; Kendler, D.L.; Belazi, D.; Brown, J.P.; Gold, D.T.; Lewiecki, E.M.; Papaioannou, A.; Simonelli, C.; Ferreira, I.; et al. Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: Interim results from a prospective observational study. Osteoporos. Int. 2015, 26, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Bolognese, M.A.; Lewiecki, E.M.; McClung, M.; Ding, B.; Austin, M.; Liu, Y.; Martin, J.S. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: A randomized blinded phase 2 clinical trial. Bone 2008, 43, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.; Miller, P.D.; Yang, Y.-C.; Grazette, L.; Martin, J.S.; Gallagher, J.C. Effects of Denosumab Treatment and Discontinuation on Bone Mineral Density and Bone Turnover Markers in Postmenopausal Women with Low Bone Mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef]
- Hadji, P.; Papaioannou, N.; Gielen, E.; Tepie, M.F.; Zhang, E.; Frieling, I.; Geusens, P.; Makras, P.; Resch, H.; Moller, G.L.; et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos. Int. 2015, 26, 2479–2489. [Google Scholar] [CrossRef]
- Migliaccio, S.; Francomano, D.; Romagnoli, E.; Marocco, C.; Fornari, R.; Resmini, G.; Buffa, A.; Di Pietro, G.; Corvaglia, S.; Gimigliano, F.; et al. Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: A multicenter observational real practice study in Italy. J. Endocrinol. Investig. 2017, 40, 1321–1326. [Google Scholar] [CrossRef]
- Durden, E.; Pinto, L.; Lopez-Gonzalez, L.; Juneau, P.; Barron, R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch. Osteoporos. 2017, 12, 22. [Google Scholar] [CrossRef]
- Fahrleitner-Pammer, A.; Papaioannou, N.; Gielen, E.; Tepie, M.F.; Toffis, C.; Frieling, I.; Geusens, P.; Makras, P.; Boschitsch, E.; Callens, J.; et al. Factors associated with high 24-month persistence with denosumab: Results of a real-world, non-interventional study of women with postmenopausal osteoporosis in Germany, Austria, Greece, and Belgium. Arch. Osteoporos. 2017, 12, 58. [Google Scholar] [CrossRef]
- Silverman, S.L.; Siris, E.; Belazi, D.; Recknor, C.; Papaioannou, A.; Brown, J.P.; Gold, T.D.; Lewiecki, E.M.; Quinn, G.; Balasubramanian, A.; et al. Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: Results of a prospective cohort study. Arch. Osteoporos. 2018, 13, 85. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ferrari, S.; Eastell, R.; Gilchrist, N.; Jensen, J.-E.B.; McClung, M.; Roux, C.; Törring, O.; Valter, I.; Wang, A.T.; et al. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J. Bone Miner. Res. 2018, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ando, K.; Machino, M.; Morozumi, M.; Kanbara, S.; Ito, S.; Inoue, T.; Yamaguchi, H.; Ishiguro, N.; Imagama, S. Persistence of Denosumab Therapy among Patients with Osteoporosis. Asian Spine J. 2020, 14, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Goldenstein, P.T.; Jamal, S.A.; Moyses, R. Fractures in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.M.; Najar, M.S.; Muzamil, M. Prevalence of osteoporosis in patients with chronic kidney disease (stages 3–5) in comparison with age- and sex-matched controls: A study from Kashmir Valley Tertiary Care Center. Saudi J. Kidney Dis. Transplant. 2017, 28, 538. [Google Scholar] [CrossRef]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Miller, P.D. The Role of Bone Biopsy in Patients with Chronic Renal Failure. Clin. J. Am. Soc. Nephrol. 2008, 3, S140–S150. [Google Scholar] [CrossRef]
- Miller, P.D. Fragility fractures in chronic kidney disease: An opinion-based approach. Clevel. Clin. J. Med. 2009, 76, 715–723. [Google Scholar] [CrossRef][Green Version]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Papapoulos, S.E.; Lippuner, K.; Roux, C.; Lin, C.J.F.; Kendler, D.L.; Lewiecki, E.M.; Brandi, M.L.; Czerwiński, E.; Franek, E.; Lakatos, P.L.; et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the FREEDOM Extension study. Osteoporos. Int. 2015, 26, 2773–2783. [Google Scholar] [CrossRef]
- Kunizawa, K.; Hiramatsu, R.; Hoshino, J.; Mizuno, H.; Ozawa, Y.; Sekine, A.; Kawada, M.; Sumida, K.; Hasegawa, E.; Yamanouchi, M.; et al. Denosumab for dialysis patients with osteoporosis: A cohort study. Sci. Rep. 2020, 10, 2496. [Google Scholar] [CrossRef] [PubMed]
- Morizio, P.; Burkhart, J.I.; Ozawa, S. Denosumab: A Unique Perspective on Adherence and Cost-effectiveness Compared With Oral Bisphosphonates in Osteoporosis Patients. Ann. Pharmacother. 2018, 52, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guañabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-F.; Cheng, J.-S.; Chen, Y.-C.; Chen, J.-F.; Hsu, C.-Y.; Lai, H.-M.; Ko, C.-H.; Chiu, W.-C.; Su, Y.-J.; Cheng, T.-T. Adherence to anti-osteoporosis medication associated with lower mortality following hip fracture in older adults: A nationwide propensity score-matched cohort study. BMC Geriatr. 2019, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- Behanova, M.; Reichardt, B.; Stamm, T.A.; Zwerina, J.; Klaushofer, K.; Kocijan, R. Treatment Effects of Bisphosphonates and Denosumab on Survival and Refracture from Real-World Data of Hip-Fractured Patients. Calcif. Tissue Int. 2019, 105, 630–641. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Gamble, G.D.; Reid, I. Effect of Osteoporosis Treatment on Mortality: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2010, 95, 1174–1181. [Google Scholar] [CrossRef]
- Colón-Emeric, C.S.; Mesenbrink, P.; Lyles, K.W.; Pieper, C.F.; Boonen, S.; Delmas, P.; Eriksen, E.F.; Magaziner, J. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J. Bone Miner. Res. 2009, 25, 91–97. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, N.-C.; Wu, F.-Z.; Wu, M.-T. Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: A pilot study. Osteoporos. Int. 2020, 31, 1507–1516. [Google Scholar] [CrossRef]
| Overall Cohort | Chronic Kidney Disease Subcohort | |||||
|---|---|---|---|---|---|---|
| Characteristics | Low Adherence (n = 409) | High Adherence (n = 713) | p-Value | Low Adherence (n = 201) | High Adherence (n = 299) | p-Value |
| Age (Mean ± SD) | 75.6 ± 10.2 | 75.6 ± 9.5 | 0.95 | 78.0 ± 8.6 | 79.2 ± 8.3 | 0.12 |
| Age category (N, %) | 0.78 | 0.05 | ||||
| ≤65 | 54 (13.2%) | 86 (12.1%) | 17 (8.5%) | 17 (5.7%) | ||
| 65–75 | 119 (29.1%) | 219 (30.7%) | 53 (26.4%) | 57 (19.1%) | ||
| ≥75 | 236 (57.7%) | 408 (57.2%) | 131 (65.2%) | 225 (75.3%) | ||
| Gender (N, %) | 0.92 | 1.00 | ||||
| Male | 93 (22.7%) | 159 (22.3%) | 48 (23.9%) | 72 (24.1%) | ||
| Female | 316 (77.3%) | 554 (77.7%) | 153 (76.1%) | 227 (75.9%) | ||
| History of fracture (N, %) | 289 (70.7%) | 527 (73.9%) | 0.27 | 149 (74.1%) | 239 (79.9%) | 0.16 |
| Hip fracture | 210 (51.3%) | 348 (48.8%) | 0.45 | 113 (56.2%) | 160 (53.5%) | 0.61 |
| Non-hip fracture | 113 (27.6%) | 240 (33.7%) | 0.04 | 55 (27.4%) | 111 (37.1%) | 0.03 |
| Hip surgery history (N, %) | 60 (14.7%) | 107 (15.0%) | 0.95 | 32 (15.9%) | 51 (17.1%) | 0.83 |
| CCI score (Mean ± SD) | 2.2 ± 2.0 | 2.1 ± 2.0 | 0.45 | 2.8 ± 2.1 | 2.8 ± 2.1 | 0.86 |
| Medications | ||||||
| Diabetic drugs | 142 (34.7%) | 208 (29.2%) | 0.06 | 90 (44.8%) | 112 (37.5%) | 0.12 |
| Antihypertensive | 304 (74.3%) | 491 (68.9%) | 0.06 | 172 (85.6%) | 232 (77.6%) | 0.04 |
| Lipid-lowering drugs | 159 (38.9%) | 310 (43.5%) | 0.15 | 90 (44.8%) | 154 (51.5%) | 0.17 |
| Anticoagulants | 44 (10.8%) | 70 (9.8%) | 0.69 | 27 (13.4%) | 38 (12.7%) | 0.92 |
| Diuretics | 105 (25.7%) | 165 (23.1%) | 0.38 | 74 (36.8%) | 106 (35.5%) | 0.83 |
| Proton pump inhibitors | 86 (21.0%) | 143 (20.1%) | 0.76 | 54 (26.9%) | 68 (22.7%) | 0.34 |
| NSAIDs | 217 (53.1%) | 429 (60.2%) | 0.02 | 94 (46.8%) | 152 (50.8%) | 0.42 |
| Corticosteroids | 129 (31.5%) | 231 (32.4%) | 0.82 | 72 (35.8%) | 100 (33.4%) | 0.65 |
| Low Adherence | High Adherence | p for Independent t-Test | |
|---|---|---|---|
| Overall cohort | N = 326 | N = 582 | |
| Pre-eGFR (mL/min/1.73 m2) | 66.62 ± 27.14 | 70.63 ± 24.70 | 0.02 |
| Post-eGFR (mL/min/1.73 m2) | 64.65 ± 27.73 | 70.63 ± 25.03 | <0.01 |
| p for paired t-test | <0.01 | 0.99 | |
| CKD subcohort | N = 176 | N = 265 | |
| Pre-eGFR (mL/min/1.73 m2) | 47.65 ± 20.99 | 49.60 ± 18.57 | 0.31 |
| Post-eGFR (mL/min/1.73 m2) | 46.35 ± 22.68 | 50.44 ± 20.67 | 0.05 |
| p for paired t-test | 0.10 | 0.13 |
| Mortality | Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| No | Yes | |||
| N (%) | N (%) | Crude | Adjusted * | |
| Overall cohort | ||||
| Low adherence (n = 409) | 325 (79.5%) | 84 (20.5%) | Reference | Reference |
| High adherence (n = 713) | 615 (86.3%) | 98 (13.7%) | 0.64 (0.48−0.85) | 0.64 (0.48−0.86) |
| CKD subcohort | ||||
| Low adherence (n = 201) | 140 (69.7%) | 61 (30.3%) | Reference | Reference |
| High adherence (n = 299) | 235 (78.6%) | 64 (21.4%) | 0.64 (0.45−0.91) | 0.61 (0.43−0.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-H.; Lin, M.-Y.; Huang, T.-H.; Lee, T.-C.; Lin, S.-Y.; Chen, C.-H.; Kuo, M.-C.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J. Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease. J. Pers. Med. 2022, 12, 185. https://doi.org/10.3390/jpm12020185
Wu P-H, Lin M-Y, Huang T-H, Lee T-C, Lin S-Y, Chen C-H, Kuo M-C, Chiu Y-W, Chang J-M, Hwang S-J. Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease. Journal of Personalized Medicine. 2022; 12(2):185. https://doi.org/10.3390/jpm12020185
Chicago/Turabian StyleWu, Ping-Hsun, Ming-Yen Lin, Teng-Hui Huang, Tien-Ching Lee, Sung-Yen Lin, Chung-Hwan Chen, Mei-Chuan Kuo, Yi-Wen Chiu, Jer-Ming Chang, and Shang-Jyh Hwang. 2022. "Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease" Journal of Personalized Medicine 12, no. 2: 185. https://doi.org/10.3390/jpm12020185
APA StyleWu, P.-H., Lin, M.-Y., Huang, T.-H., Lee, T.-C., Lin, S.-Y., Chen, C.-H., Kuo, M.-C., Chiu, Y.-W., Chang, J.-M., & Hwang, S.-J. (2022). Kidney Function Change and All-Cause Mortality in Denosumab Users with and without Chronic Kidney Disease. Journal of Personalized Medicine, 12(2), 185. https://doi.org/10.3390/jpm12020185









