Pulmonary Embolism Risk Assessment Using Blood Copeptin Concentration and Pulmonary Arteries Thrombotic Burden Evaluated by Computer Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Statistical Analysis
3. Results
3.1. General Charactersistics
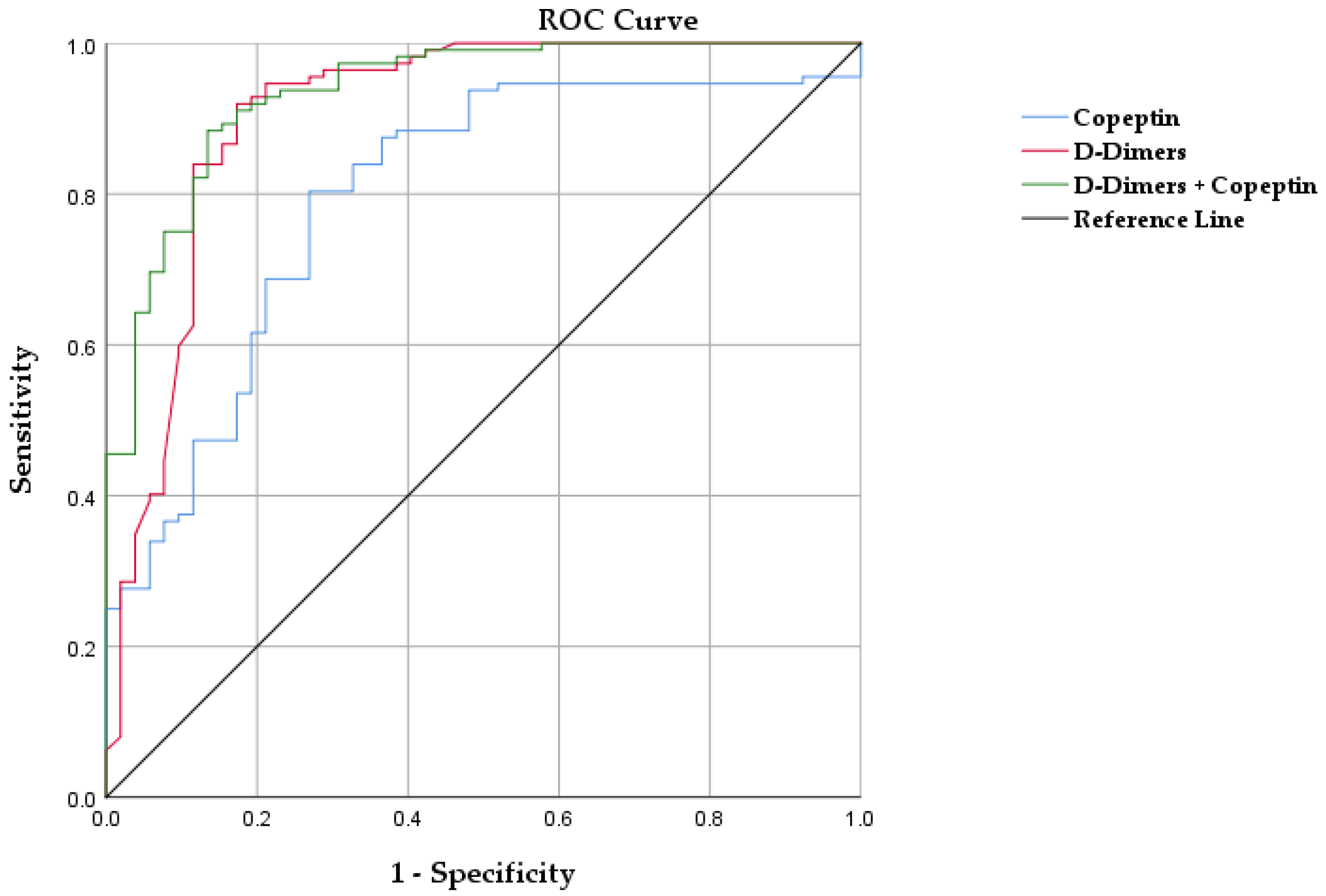
3.2. Copeptin for Diagnosis of PE
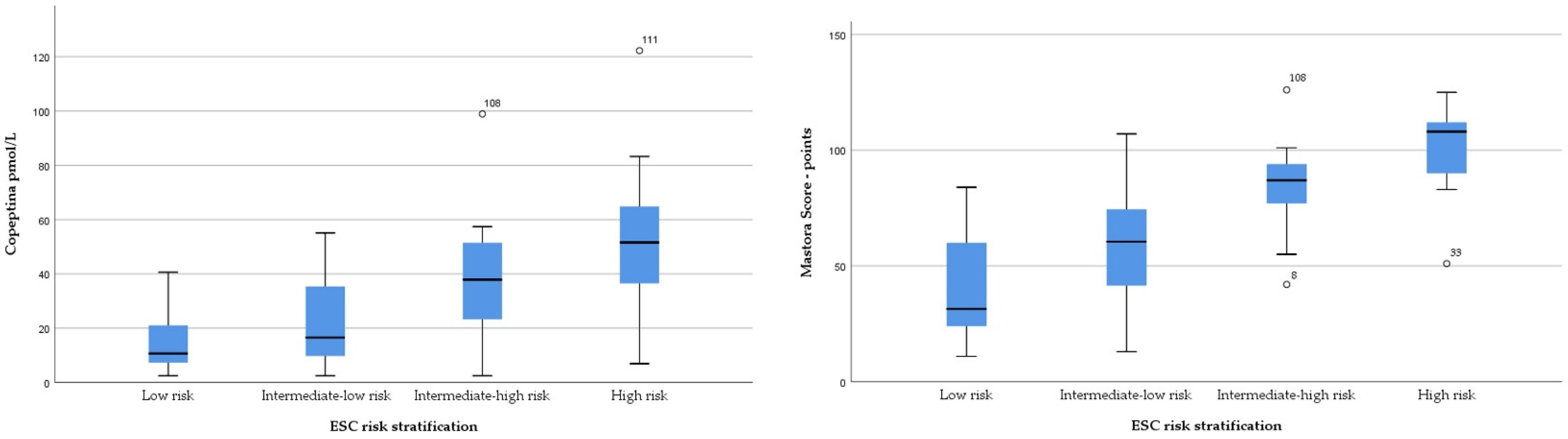
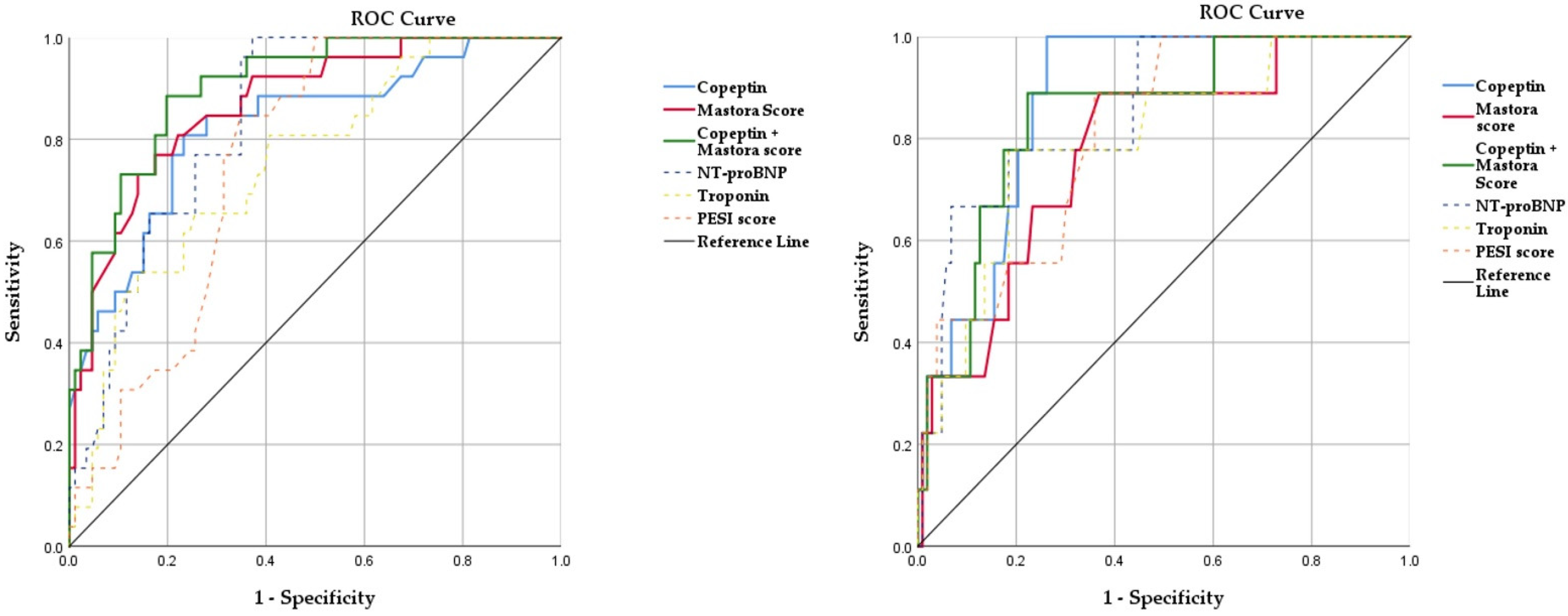
3.3. Copeptin and Mastora Score for Evaluation of PE Prognosis
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.I.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar] [PubMed]
- Jolly, M.; Phillips, J. Pulmonary embolism: Current role of catheter treatment options and operative thrombectomy. Surg. Clin. N. Am. 2018, 98, 279–292. [Google Scholar] [CrossRef]
- Li, K.; Cui, M.; Zhang, K.; Liang, K.; Liu, H.; Zhai, S. Treatment of acute pulmonary embolism using rheolytic thrombectomy. EuroIntervention 2021, 17, e158–e166. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar] [CrossRef] [Green Version]
- Giordano, N.J.; Jansson, P.S.; Young, M.N.; Hagan, K.A.; Kabrhel, C. Epidemiology, pathophysiology, stratification, and natural history of pulmonary embolism. Tech. Vasc. Interv. Radiol. 2017, 20, 135–140. [Google Scholar] [CrossRef]
- Cotugno, M.; Orgaz-Molina, J.; Rosa-Salazar, V.; Guirado-Torrecillas, L.; García-Pérez, B. Right ventricular dysfunction in acute pulmonary embolism: NT-proBNP vs. troponin T. Med. Clin. 2017, 148, 339–344. [Google Scholar] [CrossRef]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Pruszczyk, P.; Goliszek, S.; Lichodziejewska, B.; Kostrubiec, M.; Ciurzyński, M.; Kurnicka, K.; Dzikowska-Diduch, O.; Palczewski, P.; Wyzgal, A. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc. Imaging 2014, 7, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Mastora, I.; Remy-Jardin, M.; Masson, P.; Galland, E.; Delannoy, V.; Bauchart, J.-J.; Remy, J. Severity of acute pulmonary embolism: Evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur. Radiol. 2003, 13, 29–35. [Google Scholar] [CrossRef]
- Račkauskienė, J.; Gedvilaitė, V.; Matačiūnas, M.; Abrutytė, M.; Danila, E. Prognostic value of Mastora obstruction score in acute pulmonary embolism. Acta Med. Litu. 2019, 26, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apfaltrer, P.; Henzler, T.; Meyer, M.; Roeger, S.; Haghi, D.; Gruettner, J.; Süselbeck, T.; Wilson, R.B.; Schoepf, U.J.; Schoenberg, S.O.; et al. Correlation of CT angiographic pulmonary artery obstruction scores with right ventricular dysfunction and clinical outcome in patients with acute pulmonary embolism. Eur. J. Radiol. 2012, 81, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Christ-Crain, M. Vasopressin and Copeptin in health and disease. Rev. Endocr. Metab. Disord. 2019, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, C.; Deveci, O.S.; Karaaslan, M.B.; Baydar, O.; Akray, A.; Deniz, A.; Cagliyan, C.E.; Hanta, I.; Usal, A. Predictive value of plasma copeptin level for diagnosis and mortality of pulmonary embolism. Rev. Assoc. Med. Bras. 2020, 66, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, R.; Yan, L.; Lin, M.; Liu, X.; You, T. Copeptin in heart failure: Review and meta-analysis. Clin. Chim. Acta 2017, 475, 36–43. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Klug, G.; Feistritzer, H.-J.; Metzler, B.; Mair, J. Copeptin testing in acute myocardial infarction: Ready for routine use? Dis. Markers 2015, 2015, 614145. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.; Möckel, M.; Giannitsis, E.; Huber, K.; Mair, J.; Plebani, M.; Thygesen, K.; Jaffe, A.S.; Lindahl, B. ESC Study Group on biomarkers in cardiology of the acute cardiovascular care association use of copeptin for rapid rule-out of acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Hellenkamp, K.; Pruszczyk, P.; Jiménez, D.; Wyzgał, A.; Barrios, D.; Ciurzyński, M.; Morillo, R.; Hobohm, L.; Keller, K.; Kurnicka, K.; et al. Prognostic impact of copeptin in pulmonary embolism: A multicentre validation study. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Kalkan, A.K.; Ozturk, D.; Erturk, M.; Kalkan, M.E.; Cakmak, H.A.; Oner, E.; Uzun, F.; Tasbulak, O.; Yakisan, T.; Celik, A. The diagnostic value of serum copeptin levels in an acute pulmonary embolism. Cardiol. J. 2016, 23, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Di Nisio, M.; Squizzato, A.; Rutjes, A.W.S.; Büller, H.R.; Zwinderman, A.H.; Bossuyt, P.M.M. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: A systematic review. J. Thromb. Haemost. 2007, 5, 296–304. [Google Scholar] [CrossRef]
- Crawford, F.; Andras, A.; Welch, K.; Sheares, K.; Keeling, D.; Chappell, F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. 2016, CD010864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, J.A.; Williams, G.W.; Hernandez-Nino, J. D-dimer concentrations in normal pregnancy: New diagnostic thresholds are needed. Clin. Chem. 2005, 51, 825–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlson, L.; Bacchu, S.; Paneesha, S.; McManus, A.; Randall, K.; Rose, P. Elevated D-dimers are also a marker of underlying malignancy and increased mortality in the absence of venous thromboembolism. J. Clin. Pathol. 2010, 63, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Winzeler, B.; Steinmetz, M.; Refardt, J.; Cesana-Nigro, N.; Popovic, M.; Fenske, W.; Christ-Crain, M. Copeptin is not useful as a marker of malignant disease in the syndrome of inappropriate antidiuresis. Endocr. Connect. 2020, 9, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Tayyar, A.; Temel Yuksel, I.; Koroglu, N.; Tanay Tayyar, A.; Alici Davutoglu, E.; Akkaya Firat, A.; Aslan Cetin, B. Maternal copeptin levels in intrahepatic cholestasis of pregnancy. J. Matern. Fetal Neonatal Med. 2018, 31, 2066–2070. [Google Scholar] [CrossRef]
- Marek, A.; Stojko, R.; Drosdzol-Cop, A. Copeptin in patients with pregnancy-induced hypertension. Int. J. Environ. Res. Public Health 2021, 18, 6470. [Google Scholar] [CrossRef]
- Yeung, E.H.; Liu, A.; Mills, J.L.; Zhang, C.; Männistö, T.; Lu, Z.; Tsai, M.Y.; Mendola, P. Increased levels of copeptin before clinical diagnosis of preeclampsia. Hypertension 2014, 64, 1362–1367. [Google Scholar] [CrossRef]
- Gomes, D.A.; de Almeida Beltrão, R.L.; de Oliveira Junior, F.M.; da Silva Junior, J.C.; de Arruda, E.P.C.; Lira, E.C.; da Rocha, M.J.A. Vasopressin and copeptin release during sepsis and septic shock. Peptides 2021, 136, 170437. [Google Scholar] [CrossRef]
- Golembiewska, E.; Machowska, A.; Stenvinkel, P.; Lindholm, B. Prognostic value of copeptin in chronic kidney disease: From general population to end-stage renal disease. Curr. Protein Pept. Sci. 2017, 18, 1232–1243. [Google Scholar] [CrossRef]
- Yalta, K.; Yalta, T.; Sivri, N.; Yetkin, E. Copeptin and cardiovascular disease: A review of a novel neurohormone. Int. J. Cardiol. 2013, 167, 1750–1759. [Google Scholar] [CrossRef]
- Usul, E.; Ozkan, S.; Höke, M.H.; Kaya, A.E.; Ucar, F.; Cimen, T. Relationship between right ventricular dilatation and blood copeptin levels in patients with acute pulmonary embolism. Clin. Respir. J. 2020, 14, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Mos, I.C.M.; Huisman, M.V. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: A systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 2008, 178, 425–430. [Google Scholar] [CrossRef]
- Giannitsis, E.; Katus, H.A. Biomarkers for clinical decision-making in the management of pulmonary embolism. Clin. Chem. 2017, 63, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjola, V.-P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Coutance, G.; Cauderlier, E.; Ehtisham, J.; Hamon, M.; Hamon, M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: A meta-analysis. Crit. Care 2011, 15, R103. [Google Scholar] [CrossRef] [Green Version]
- Irmak, I.; Sertçelik, Ü.; Öncel, A.; Er, B.; İnam, G.; Durhan, G.; Demir, A.; Çöplü, L. Correlation of thrombosed vessel location and clot burden score with severity of disease and risk stratification in patients with acute pulmonary embolism. Anatol. J. Cardiol. 2020, 24, 247–253. [Google Scholar] [CrossRef]
- Nie, Y.; Sun, L.; Long, W.; Lv, X.; Li, C.; Wang, H.; Li, X.; Han, P.; Guo, M. Clinical importance of the distribution of pulmonary artery embolism in acute pulmonary embolism. J. Int. Med. Res. 2021, 49, 3000605211004769. [Google Scholar] [CrossRef]
- Wyzgał, A.; Koć, M.; Pacho, S.; Bielecki, M.; Wawrzyniak, R.; Kostrubiec, M.; Ciurzyński, M.; Kurnicka, K.; Goliszek, S.; Paczyńska, M.; et al. Plasma copeptin for short term risk stratification in acute pulmonary embolism. J. Thromb. Thrombolysis 2016, 41, 563–568. [Google Scholar] [CrossRef]
- Hellenkamp, K.; Schwung, J.; Rossmann, H.; Kaeberich, A.; Wachter, R.; Hasenfuß, G.; Konstantinides, S.; Lankeit, M. Risk stratification of normotensive pulmonary embolism: Prognostic impact of copeptin. Eur. Respir. J. 2015, 46, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, S.K.; Wang, S.C. Central clot score at computed tomography as a predictor of 30-day mortality after acute pulmonary embolism. Ann. Acad. Med. Singap. 2010, 39, 442–447. [Google Scholar]
- Lerche, M.; Bailis, N.; Akritidou, M.; Meyer, H.J.; Surov, A. Pulmonary vessel obstruction does not correlate with severity of pulmonary embolism. J. Clin. Med. 2019, 8, 584. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 165) | Pulmonary Embolism (n = 112) | Control Group (n = 53) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | |||||
| Age (years) | 18 | 62.04 ± 14.05 | 91 | 18 | 63.1 ± 14.10 | 91 | 30 | 59.6 ± 13.71 | 91 | 0.13 | |||
| Gender (N, %) | Male 90 (54.5%) | Female 75 (45.4%) | Male 62 (55.35%) | Female 50 (44.6%) | Male 28 (52.8%) | Female 25 (47.2%) | 0.566 | ||||||
| Systolic blood pressure (mmHg) | 50 | 127.45 ± 21.53 | 200 | 50 | 123.09 ± 21.90 | 200 | 110 | 136.66 ± 16.51 | 185 | <0.001 | |||
| Diastolic blood pressure (mmHg) | 20 | 74.05 ± 12.98 | 110 | 20 | 75.12 ± 13.80 | 91 | 60 | 71.79 ± 10.83 | 110 | 0.12 | |||
| Heart rate (bpm) | 52 | 88.57 ± 19.22.02 | 155 | 52 | 94.07 ± 23.13 | 155 | 50 | 76.94 ± 13.53 | 111 | <0.001 | |||
| Oxygen saturation in ambient air (%) | 65 | 94.21 ± 5.38 | 100 | 65 | 91.70± 4.81 | 99 | 97 | 99.45 ± 0.72 | 100 | <0.001 | |||
| Surgery within 30 days | 12 (7.27%) | 8 (7.14 %) | 4 (7.54%) | 0.328 | |||||||||
| Active cancer | 15 (9.09%) | 13 (11.6%) | 2 (3.77%) | 0.102 | |||||||||
| Post-partum | 2 (1.21%) | 1 (0.89%) | 1 (1.88%) | 0.331 | |||||||||
| Diabetes mellitus | 32 (19.4%) | 19 (16.9%) | 13 (24.5%) | 0.365 | |||||||||
| Arterial hypertension | 81 (49.09%) | 59 (52.7%) | 22 (41.5%) | 0.156 | |||||||||
| Smoking | 52 (31.5%) | 39 (34.8%) | 13 (24.5%) | 0.172 | |||||||||
| BMI (kg/m2) | 17.15 | 26.10 ± 3.75 | 40.41 | 17.15 | 26.30 ± 4.15 | 40.41 | 20.32 | 25.66 ± 2.70 | 36.45 | 0.303 | |||
| Leucocytes (×109/L) | 4.06 | 9.97 ± 3.63 | 23.9 | 4.06 | 10.51 ± 4.13 | 23.9 | 5.11 | 8.86 ± 1.79 | 15.33 | 0.006 | |||
| Hemoglobin (g/L) | 9.2 | 13.29 ± 1.58 | 17.9 | 9.2 | 13.17 ± 1.77 | 17.9 | 9.2 | 13.48 ± 1.19 | 17 | 0.249 | |||
| Thrombocytes (×109/L) | 93 | 253.51 ± 100.20 | 745 | 96 | 253.45 ± 120.16 | 745 | 93 | 253.61 ± 54.55 | 415 | 0.992 | |||
| CRP (mg/dL) | 0.02 | 5.53 ± 11.51 | 115 | 0.08 | 7.64 ± 13.44 | 115 | 0.02 | 1.09 ± 1.77 | 12 | 0.001 | |||
| Glucose (mg/dL) | 70 | 117.90 ± 41.59 | 310 | 75 | 122.92 ± 47.84 | 310 | 70 | 107.47 ± 20.21 | 160 | 0.09 | |||
| Creatinine (mg/dL) | 0.42 | 0.97 ± 0.38 | 3.71 | 0.42 | 0.97 ± 0.43 | 3.71 | 0.6 | 0.98 ± 0.27 | 1.77 | 0.715 | |||
| LVEF (%) | 15 | 51.28 ± 7.43 | 65 | 15 | 51.4 ± 7.3 | 65 | 35 | 51.09 ± 0.98 | 65 | 0.809 | |||
| Median (IQR) | Median (IQR) | Median (IQR) | |||||||||||
| D-dimer (µg/mL) | 4.6 (1.72–5.21) | 4.75 (4.32–5.56) | 1.55 (0.5–2.1) | <0.001 | |||||||||
| NT-proBNP | 86.3 (31.95–137.5) | 1376 (556–3384) | 15 (4.34–53) | <0.001 | |||||||||
| hsTnI | 6.08 (1.38–12.13) | 29 (8.98–96) | 662 (100.5–2344.5) | <0.001 | |||||||||
| Copeptin (pmol/L) | 12.82 (5.41–30.97) | 26.05 (8.69–40.23) | 9.50 (3.92–10.63) | <0.001 | |||||||||
| PE Group (No, %) | Low-Risk PE 30 (26.8%) | Intermediate-Low-Risk PE 49 (41.9%) | Intermediate-High-Risk PE 20 (17.1%) | High-Risk PE 13 (11.1%) | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | ||||||
| Age (years) | 24 | 54.5 ± 14.2 | 81 | 41 | 65.7 ± 12.1 | 88 | 18 | 66.5 ± 16.3 | 91 | 46 | 68.3 ± 9.9 | 79 | 0.001 | ||||
| Gender (Male, Female) | 12 (40%) | 18 (60%) | 33 (67.3%) | 16 (32.7%) | 11 (55%) | 9 (45%) | 6 (46.2%) | 7 (53.8%) | 0.104 | ||||||||
| BMI (kg/m2) | 20.7 | 27.4 ± 4.9 | 40.4 | 17.1 | 26.5 ± 4.1 | 36.7 | 19.5 | 24.9 ± 3.5 | 30.8 | 21.1 | 25.3 ± 3.5 | 34.6 | 0.16 | ||||
| Dyspnea (No, %) | 26 (86.7%) | 45 (91.8%) | 18 (90%) | 13 (100%) | 0.56 | ||||||||||||
| Syncope (No, %) | 3 (10%) | 3 (6.1%) | 4 (20%) | 2 (15.4%) | 0.363 | ||||||||||||
| Chest pain (No, %) | 9 (30%) | 17 (34.7%) | 6 (30%) | 5 (38.5%) | 0.93 | ||||||||||||
| Hemoptysis (No, %) | 4 (13.3%) | 8 (16.3%) | 3 (15%) | 4 (30.8%) | 0.55 | ||||||||||||
| Smoking (No, %) | 11 (36.7%) | 19 (38.8%) | 4 (20%) | 5 (38.5%) | 0.462 | ||||||||||||
| Hypertension (No, %) | 14 (46.7%) | 30 (61.2%) | 7 (35%) | 8 (61.5%) | 0.185 | ||||||||||||
| Diabetes mellitus (No, %) | 4 (13.3%) | 9 (18.4%) | 5 (25%) | 1 (7.7%) | 0.542 | ||||||||||||
| Deep vein thrombosis | 15 (50%) | 18 (36.7%) | 4 (20%) | 4 (33.3%) | 0.18 | ||||||||||||
| Systolic blood pressure (mmHg) | 105 | 130.1 ± 15.9 | 170 | 100 | 130.7 ± 18.7 | 200 | 90 | 115.75 ± 17.3 | 160 | 50 | 89.1 ± 15.6 | 125 | <0.001 | ||||
| Dyastolic blood pressure (mmHg) | 60 | 78.5 ± 11.1 | 100 | 60 | 80.2 ± 11.9 | 100 | 60 | 69.7 ± 10.1 | 90 | 20 | 56.3 ± 12.9 | 80 | <0.001 | ||||
| Heart rate (bpm) | 50 | 86.4 ± 23.8 | 130 | 42 | 94.4 ± 20.2 | 150 | 70 | 98.7 ± 25.8 | 150 | 30 | 103.3 ± 25.5 | 123 | 0.103 | ||||
| Oxygen saturation in ambient air (%) | 89 | 95 ± 2.8 | 99 | 83 | 91.5 ± 3.8 | 98 | 81 | 90 ± 4.3 | 99 | 67 | 87.5 ± 6.4 | 95 | <0.001 | ||||
| Wells score | 0 | 4.2 ± 2.4 | 10.5 | 0 | 4 ± 2.3 | 9 | 0 | 3.5 ± 2.1 | 7.5 | 1.5 | 4.3 ± 1.1 | 6 | 0.08 | ||||
| Geneva score | 2 | 8.1 ± 3.8 | 15 | 1 | 6.6 ± 3.2 | 16 | 0 | 6.2 ± 2.8 | 12 | 4 | 6.1 ± 1.2 | 9 | 0.67 | ||||
| Adverse cardiac events (No, %) | 0 (0%) | 7 (14.3%) | 9 (45%) | 10 (76.9%) | <0.001 | ||||||||||||
| Death within 30 days (No, %) | 0 (0%) | 2 (4.1%) | 2 (10%) | 5 (38.5%) | <0.001 | ||||||||||||
| PE Group (No, %) | Low-Risk PE 30 (26.8%) | Intermediate-Low-Risk PE 49 (41.9%) | Intermediate-High-Risk PE 20 (17.1%) | High-Risk PE 13 (11.1%) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | Min | Mean ± SD | Max | ||
| Leucocytes (×109/L) | 5.83 | 10.66 ± 4.08 | 22.16 | 5.39 | 10.7 ± 4.26 | 23.78 | 4.06 | 9.78 ± 4.53 | 23.9 | 6.12 | 10.5 ± 3.38 | 23.9 | 0.862 |
| Neutrophiles (×109/L) | 6.12 | 7.33 ± 3.69 | 17.84 | 2.30 | 8.03 ± 4.05 | 20.54 | 2.91 | 7.32 ± 4.18 | 20.45 | 4.05 | 8.06 ± 3.53 | 15.77 | 0.821 |
| Hemoglobin (g/L) | 9.2 | 13.1 ±1.7 | 17.6 | 9.3 | 12.9 ± 1.7 | 15.9 | 9.9 | 13.9 ± 1.9 | 17 | 9.1 | 12.8 ± 2.3 | 17.9 | 0.223 |
| Thrombocytes (×109/L) | 76 | 252.37 ± 89.02 | 420 | 46 | 236.25 ± 105.47 | 420 | 119 | 225.95 ± 97.81 | 564 | 120 | 246.9 ± 199.1 | 745 | 0.213 |
| CRP (mg/dL) | 0.11 | 5. 59 ± 7.05 | 32.8 | 0.14 | 8.93 ± 18.14 | 118 | 0.08 | 5.76 ± 7.22 | 41.2 | 0.08 | 10.54 ± 11.48 | 118 | 0.550 |
| Blood iron | 12 | 61.52 ± 40.08 | 182 | 13 | 51.19 ± 28.48 | 131 | 21 | 55 ± 26.12 | 117 | 15 | 46.62 ± 23.28 | 105 | 0.538 |
| Ferritin | 67 | 390.9 ± 644.5 | 3085 | 43 | 442.78 ± 625.5 | 3085 | 114 | 430.94 ± 480.68 | 1986 | 123 | 419.82 ± 420.22 | 1633 | 0.991 |
| LVEF (%) | 45 | 54.77 ± 6.01 | 65 | 20 | 50.45 ± 8.01 | 62 | 48 | 52.1 ± 4.45 | 65 | 15 | 49.08 ± 10.89 | 60 | 0.061 |
| D-dimer (µg/mL) | 1.54 | 4.47 ± 1.35 | 6.1 | 1.19 | 4.64 ± 1.22 | 7.12 | 3.93 | 5.09 ± 0.61 | 6.2 | 2.07 | 5.26 ± 1.79 | 8.1 | 0.154 |
| RVd (mm) | 24 | 31.27 ± 5.31 | 46 | 28 | 36.14 ± 6.07 | 50 | 27 | 37.85 ± 5.50 | 50 | 25 | 39.15 ± 7.54 | 55 | 0.002 |
| TAPSE (mm) | 15 | 21.5 ± 2.92 | 26 | 12 | 18.58 ± 3.5 | 30 | 11 | 15.33 ± 2.95 | 24 | 12 | 14.54 ± 2.57 | 22 | <0.001 |
| sPAP (mmHg) | 15 | 29.57 ± 11.13 | 50 | 15 | 37.69 ± 11.69 | 70 | 15 | 46.24 ± 12.54 | 68 | 16 | 55.08 ± 13.05 | 75 | <0.001 |
| RV/LV > 1 (No, %) | 4 (11%) | 17 (35.4%) | 10 (47.6%) | 10 (76.9%) | <0.001 | ||||||||
| Mastora score (points) | 11 | 41.93 ± 21.03 | 84 | 13 | 59.63±24.05 | 107 | 42 | 83.57±17.34 | 126 | 51 | 101.23 ± 20.62 | 125 | <0.001 |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||||||||||
| hsTnI (ng/L) | 22 (2.54–42.2) | 26 (15–92.25) | 112 (30.1–185.5) | 101 (60–346) | 0.003 | ||||||||
| NT-proBNP (pg/mL) | 442 (200–662) | 1377.5 (647–3334.25) | 2488 (1615.5–3886) | 5265 (4233–8328) | <0.001 | ||||||||
| Copeptin (pmol/L) | 9.86 (6.89–17.30) | 18.63 (9.31–35.76) | 37.89 (25.04–51.01) | 51.45 (36.5–75.09) | <0.001 | ||||||||
| Parameter | Copeptin | Mastora Score | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Age | 0.101 | 0.289 | 0.193 | 0.61 |
| Sex | 0.032 | 0.74 | 0.039 | 0.680 |
| Diabetes mellitus | 0.058 | 0.583 | 0.084 | 0.376 |
| Arterial hypertension | 0.057 | 0.549 | 0.063 | 0.511 |
| Cancer | 0.11 | 0.910 | −0.011 | 0.907 |
| BMI | −0.79 | 0.41 | −0.64 | 0.50 |
| SBP | −0.25 | 0.006 | −0.282 | 0.003 |
| HR | 0.332 | 0.001 | 0.237 | 0.12 |
| Oxygen saturation | −0.338 | <0.001 | −0.382 | 0.001 |
| Hemoglobin | 0.064 | 0.504 | 0.19 | 0.06 |
| Leukocytes | 0.137 | 0.15 | 0.04 | 0.675 |
| Thrombocytes | −0.34 | 0.70 | −0.65 | 0.5 |
| CRP | 0.235 | 0.013 | 0.030 | 0.757 |
| RVd | 0.312 | 0.001 | 0.416 | 0.04 |
| TAPSE | −0.48 | 0.001 | −0.473 | <0.001 |
| sPAP | 0.523 | 0.072 | 0.639 | 0.064 |
| RV/LV ratio > 1 | 0.442 | <0.001 | 0.282 | 0.03 |
| LVEF | −0.015 | 0.877 | −0.079 | 0.409 |
| hs cTnI | 0.484 | <0.001 | 0.324 | 0.056 |
| D-dimers | 0.326 | <0.001 | 0.341 | 0.01 |
| NT-proBNP | 0.461 | <0.001 | 0.37 | 0.04 |
| Copeptin | 1 | 0 | 0.535 | 0.011 |
| Mastora score | 0.535 | 0.011 | 1 | 0 |
| Adverse events | 0.467 | 0.001 | 0.542 | 0.001 |
| Death | 0.361 | <0.001 | 0.287 | 0.002 |
| Parameter | Adverse Events | Death | ||||
|---|---|---|---|---|---|---|
| Area Under Curve (AUC) | p-Value | 95% Confidence Interval | Area Under Curve (AUC) | p-Value | 95% Confidence Interval | |
| Mastora score | 0.871 | <0.001 | 0.796–0.945 | 0.804 | 0.003 | 0.653–0.955 |
| Copeptin | 0.820 | <0.001 | 0.725–0.914 | 0.883 | <0.001 | 0.809–0.957 |
| Copeptin + Mastora score | 0.902 | <0.001 | 0.841–0.962 | 0.850 | 0.001 | 0.724–0.967 |
| Nt-proBNP | 0.853 | <0.001 | 0.783–0.922 | 0.855 | <0.001 | 0.739–0.971 |
| hsTnI | 0.736 | 0.002 | 0.629–0.843 | 0.797 | 0.003 | 0.644–0.949 |
| PESI score | 0.742 | 0.001 | 0.649–0.834 | 0.810 | 0.002 | 0.688–0.932 |
| Biomarker | Adverse Events | Death | ||||
|---|---|---|---|---|---|---|
| Exp B (Odds Ratio) | 95% CI for Exp B | p-Value | Exp B (Odds Ratio) | 95% CI for Exp B | p-Value | |
| Copeptin | 1.049 | 1.009–1.090 | 0.015 | 1.048 | 1.004–1.094 | 0.032 |
| Mastora score | 1.065 | 1.026–1.105 | 0.001 | 1.016 | 0.979–1.055 | 0.396 |
| hsTnI | 0.999 | 0.993–1.004 | 0.568 | 1.002 | 0.998–1.005 | 0.364 |
| NT-proBNP | 1.023 | 1.002–1.075 | 0.048 | 1.001 | 0.997–1.025 | 0.593 |
| PESI score | 0.997 | 0.974–1.021 | 0.818 | 1.024 | 0.995–1.054 | 0.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haba, M.Ș.C.; Tudorancea, I.; Miftode, R.Ș.; Popa, I.P.; Mitu, O.; Mihai, C.T.; Haba, R.M.; Onofrei, V.A.; Petris, A.O.; Costache, I.I.; et al. Pulmonary Embolism Risk Assessment Using Blood Copeptin Concentration and Pulmonary Arteries Thrombotic Burden Evaluated by Computer Tomography. J. Pers. Med. 2022, 12, 2084. https://doi.org/10.3390/jpm12122084
Haba MȘC, Tudorancea I, Miftode RȘ, Popa IP, Mitu O, Mihai CT, Haba RM, Onofrei VA, Petris AO, Costache II, et al. Pulmonary Embolism Risk Assessment Using Blood Copeptin Concentration and Pulmonary Arteries Thrombotic Burden Evaluated by Computer Tomography. Journal of Personalized Medicine. 2022; 12(12):2084. https://doi.org/10.3390/jpm12122084
Chicago/Turabian StyleHaba, Mihai Ștefan Cristian, Ionut Tudorancea, Radu Ștefan Miftode, Irene Paula Popa, Ovidiu Mitu, Cosmin Teodor Mihai, Raluca Maria Haba, Viviana Aursulesei Onofrei, Antoniu Octavian Petris, Irina Iuliana Costache, and et al. 2022. "Pulmonary Embolism Risk Assessment Using Blood Copeptin Concentration and Pulmonary Arteries Thrombotic Burden Evaluated by Computer Tomography" Journal of Personalized Medicine 12, no. 12: 2084. https://doi.org/10.3390/jpm12122084
APA StyleHaba, M. Ș. C., Tudorancea, I., Miftode, R. Ș., Popa, I. P., Mitu, O., Mihai, C. T., Haba, R. M., Onofrei, V. A., Petris, A. O., Costache, I. I., Haba, D., & Șorodoc, L. (2022). Pulmonary Embolism Risk Assessment Using Blood Copeptin Concentration and Pulmonary Arteries Thrombotic Burden Evaluated by Computer Tomography. Journal of Personalized Medicine, 12(12), 2084. https://doi.org/10.3390/jpm12122084








