2. Methods
2.1. The Multidisciplinary Approach
Established in January 2018, the multidisciplinary center at the Second Xiangya Hospital of Central South University is one of the largest bone and soft tissue tumor centers in China that meets international standards. Approximately 300 patients with bone and soft tissue tumors were potential subjects of multidisciplinary discussion during the past four consecutive years. Experts in various specialties are all skilled in the diagnosis and treatment of these tumors.
An MDT panel on the diagnosis and treatment of bone and soft tissue tumors includes orthopedic surgeons, oncologists, radiologists, pathologists, thoracic surgeons, and non-core members, such as clinical pharmacists, nutritionists, psychiatrists, and nurses. Orthopedics is the core of the team, and orthopedic surgeons are mainly responsible for patient consultations regarding bone and soft tissue diseases, hospital admission, preliminary diagnosis, and follow-up. The MDT discussion was held weekly. Outpatients and inpatients submitted their applications for discussion at the MDT center. Prior to the panel discussion, the physicians retrieved the medical history and compiled the imaging data and other relevant information. Information about the patient to be discussed was provided to the MDT experts one day in advance. Generally, the discussion was organized as follows: The medical history of patients, clinical symptoms, physical examination, imaging characteristics, histological findings, and preliminary diagnosis were initially reviewed. Then, the experts who participated in the MDT discussion expressed their views on diagnosis and treatment. Finally, an orthopedic oncologist made a summary, including consensus on the diagnosis, relapse diagnosis, treatment of the disease, features and management of pulmonary nodules, and the potential need for extended resection and unplanned surgery.
2.2. Study Population and Design
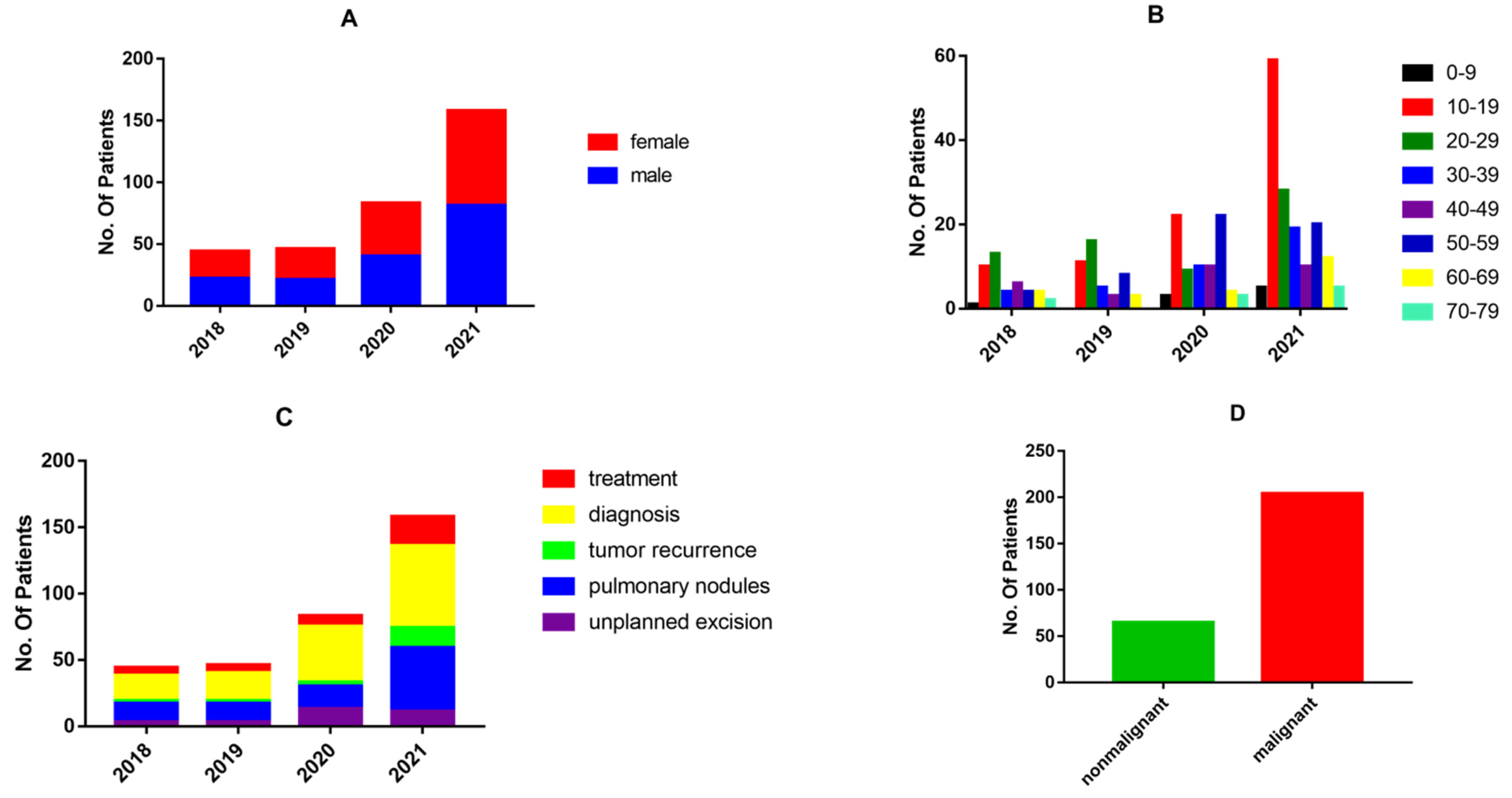
This study was conducted at the Second Xiangya Hospital of Central South University in Changsha, Hunan Province, China. A total of 269 patients were subjects of MDT discussions from January 2018 (inception) to December 2021. The basic information for all patients, including clinical symptoms, imaging, and pathological findings, was obtained from electronic medical records. MDT discussions concerning these recruited patients were retrospectively examined. This study was approved by the Ethics Committee of Central South University and conducted in accordance with the Declaration of Helsinki. An audit was commenced in January 2018. A total of 331 panel discussions were held regarding 269 patients, as some patients were discussed several times before reaching a final consensus. To reflect the involvement of the MDT in the diagnosis and treatment of bone and soft tissue sarcomas, the period from January 2018 to December 2021 was further divided into four parts.
The 269 patients were sorted into different categories by type of disease. Demographic data were obtained, such as age, gender, tumor site, Enneking stage, theme of discussion, benign or malignant tumors, whether metastasis occurred, and survival. Further data include the accuracy of diagnosis before and after MDT discussion, the accuracy of relapse diagnosis, unplanned excision decisions, and pulmonary nodule management. Tumor treatment effects, namely complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), were also assessed. For osteosarcoma patients with pulmonary nodules, we conducted a comparative survival analysis between patients who were discussed by the MDT versus undiscussed patients. The experimental group (n = 42) consisted of patients who were discussed by an MDT, and 40 osteosarcoma patients with complete information who did not apply for MDT discussion served as the controls. Multiple parameters, including age, gender, tumor location, Enneking stage, metastasis occurrence, and survival, were compared between the two groups.
2.3. Standards and Endpoints
Follow-up began in January 2018. The first postoperative day was used as the initial time point for follow-up. The follow-up schedule was as follows: every three months for years one and two; every four months for year three; every six months for years four and five; and yearly thereafter. Patients with pulmonary nodules had their lung CT reviewed at every follow-up visit and submitted a request for MDT discussion when there were changes in the lung nodules. The starting point for survival analysis was the day of surgery, and the survival analysis endpoint was on 31 December 2021, or upon the patient’s death.
2.4. Statistical Analysis
Statistical analysis was performed using the SPSS Statistics 25 package. A p < 0.05 was considered to be statistically significant. Categorical variables were expressed as percentages. The rate of age, gender, tumor location, Enneking stage, and metastasis were compared between the experimental and control groups using the chi-square (χ2) test and Fisher’s exact test. Kaplan-Meier estimation was used to create graphs of the observed survival curves, while the log-rank test was used to compare curves from different groups.
4. Discussion
Bone and soft tissue sarcomas are a highly heterogeneous group of mesenchymal malignancies, including malignant bone tumors [
4]. Primary malignant bone tumors are extremely rare neoplasms accounting for fewer than 0.2% of all malignancies, although the actual incidence is difficult to determine [
5]. Soft tissue sarcomas contribute to 1% of all adult malignancies, with more than 100 histologic subtypes that have developed from or within muscle, fat, nerve, cartilage, and bone tissues [
6]. The low incidence of bone and soft tissue tumors is further complicated by their variable histological, imaging, and clinical manifestations. The same type of tumor may have different radiographic characteristics, and different tumors may show similar imaging features. The early clinical features of bone and soft tissue tumors are generally not obvious. Even if bone and soft tissue tumors have typical imaging features, physicians can only make a preliminary diagnosis based on imaging results, and pathological examination is required to establish the final diagnosis. Histological examination is usually considered the most accurate diagnostic method, but these pathologic observations are only one of the diagnostic criteria for bone and soft tissue tumors. Pathologic tissue biopsy may also be affected by many factors, such as the biopsy site and preservation of specimens, which may bring certain difficulties to pathological diagnosis and increase the misdiagnosis rate. Accordingly, the diagnosis and treatment of bone and soft tissue tumors cannot be made by one discipline alone. Therefore, a multidisciplinary collaboration among orthopedic surgeons, radiologists, pathologists, oncologists, thoracic surgeons, and other specialists is required to reduce the rate of misdiagnosis, underdiagnosis, and mistreatment so that patients can receive standardized and precise treatment.
To the best of our knowledge, this is the first study to investigate the role of an MDT in the management of diagnosis and treatment of bone and soft tissue tumors.
Diagnosis and pulmonary nodules were the main topics of the MDT discussions. The number of cases discussed by the MDT increased every year, with the number of cases in 2021 being almost twice as high as in 2020. This indicates that MDT discussions are becoming increasingly widespread in the diagnosis and treatment of bone and soft tissue tumors.
In recent studies, the diagnostic accuracy of bone and soft tissue tumors based on core needle biopsies ranged from 74% to 98% [
7,
8,
9,
10,
11]. In line with these results, we identified an overall diagnostic accuracy of 90.84% in bone and soft tissue tumors without MDT discussion. However, the MDT discussion increased the diagnostic accuracy to 95.42%. Diagnosis of bone and soft tissue tumors based on imaging and pathological observations is complex and difficult. The pathological tissue subtypes are diverse and have overlapping histological features. The advantage of the MDT discussions in improving diagnostic accuracy is evident. For certain difficult cases, through preoperative MDT discussions, radiologists and pathologists can communicate with orthopedic surgeons and advise the most suitable site for biopsy, which may reduce the probability of invalid specimens and improve the quality of specimens. For cases in which the pathological diagnosis is still unclear after biopsy, an MDT discussion can clarify whether to perform a repeat biopsy, switch from core-needle to open biopsy, or proceed to the next step of treatment, which improves the efficiency of diagnosis and the quality of treatment.
Despite effective treatment, local recurrence or distant metastases of bone and soft tissue sarcomas occur in up to 60% of patients who initially receive treatment [
12,
13]. Recurrent or refractory bone and soft tissue sarcomas have a very poor prognosis in children. Second-line chemotherapy regimens or newly developed agents have not improved the outcome. OS rates have been reported to be less than 40% after relapse in most series [
14,
15,
16,
17,
18,
19,
20,
21,
22]. Survival rates for osteosarcoma and Ewing sarcoma after relapse are less than 30% [
17,
19,
23] and 23% [
17,
22], respectively. Soft tissue sarcoma local recurrences occur in approximately 10% of patients treated with margin-negative resection. Most recurrences occur within the first two years [
24]. Given the high risk of recurrence in this patient population, the accurate and early detection of local or recurrent metastatic lesions is highly warranted because early treatment can prolong survival. The diagnostic sensitivity and specificity using contrast-enhanced CT alone were 78% and 67%, respectively, with an accuracy of 73%. In contrast, the diagnostic sensitivity and specificity of
18F-FDG PET/CT were 94% and 92%, respectively, with an accuracy of 93%.
18F-FDG PET/CT was particularly superior regarding the detection of local recurrence of soft tissue lesions (sensitivity and specificity: 83% and 100% vs. 50% and 100%, respectively) or bone metastases (100% and 100% vs. 85% and 88%, respectively) [
25]. In our study, 16 patients were included in the discussion of a recurrence diagnosis. Of the 11 patients considered for recurrence after MDT discussion, eight cases underwent reoperation, and all had positive postoperative pathology. The three patients who did not undergo reoperation had worsening lesions upon re-examination. In the five cases not considered as recurrence, no significant imaging changes were observed upon re-examination. The accuracy of relapse diagnosis by the MDT reached 100%, and the MDT improved the accuracy of a recurrence diagnosis of bone and soft tissue tumors compared to the available literature.
Unplanned resection usually refers to inappropriate surgical resection of a soft tissue sarcoma misdiagnosed as a benign tumor, resulting in a positive tumor specimen margin or residual tumor. Unplanned resection, which continues to be a major issue for musculoskeletal oncologists, is common in bone and soft tissue sarcomas. Osseous malignancies with a non-classic presentation are frequently misdiagnosed; therefore, they receive inappropriate surgical management. Those referred for secondary treatment after an unplanned resection are more likely to have a sarcoma with a diameter of <5 cm that is superficially located and painless [
26,
27,
28,
29]. Lesions with the above manifestations are regarded as benign tumors. In addition, there is currently no effective referral system in China. Patients with bone and soft tissue tumors, especially soft tissue tumors, are treated in orthopedics, general surgery, and even other departments. Even when treatment is provided in orthopedic departments, it is often not administered by an orthopedic oncologist. In addition, up to one-third of all soft tissue sarcomas arise superficially to the investing muscular fascia, and they can range in size from a few millimeters to several tens of centimeters [
27]. Reported rates of residual sarcoma in the re-resected specimen after unplanned resections are uniformly high, ranging from 24% to 74% [
26,
28,
30,
31,
32,
33,
34,
35]. In contrast, the residual rate of re-excision after MDT discussion at our center was 81.2%, higher than that reported in the literature. These data may indicate to some extent that the MDT plays an active role in the decision to perform re-excision after unplanned resection.
The lung is the most common site for sarcoma metastasis. It is reported that approximately 15–20% of osteosarcoma patients suffered from distant metastases at the time of presentation, with more than 85% of these metastases being pulmonary [
36]. Of patients with extremity sarcomas, approximately 20% may proceed with isolated pulmonary metastatic disease at some point in the course of their disease [
37]. Since CT examination is more sensitive than chest plain film, it has been regarded as the best method to diagnose metastatic pulmonary tumors. However, there are many studies showing that CT cannot detect the exact number of pulmonary metastases accurately [
38,
39]. It is well recognized that not all pulmonary nodules identified on a chest CT in patients with sarcoma represent metastases [
40,
41] and that a chest CT is relatively insensitive compared to lung palpation/thoracotomy in the identification of pulmonary metastases [
42,
43,
44]. CT has become the standard for detecting and monitoring pulmonary lesions, but it frequently identifies nodules of uncertain clinical significance. Often the assumption is that, especially in children, these nodules represent metastatic disease. However, up to 60% of pulmonary nodules in adults and 33% in children may be non-malignant [
45,
46].
Differentiating benign and malignant lesions is essential for planning treatment and determining prognosis. When nodules are detected, invasive procedures may be necessary to establish the histopathologic diagnosis, but in many cases, the nodules are simply monitored with periodic CT scans. From the patients’ perspective, these nodules may cause psychological distress due to the uncertain origin and biological features of the nodules. Therefore, the diagnosis of pulmonary nodules requires multidisciplinary cooperation so that the strengths of each department can be better utilized to offer patients individualized treatment. The pulmonary nodules were broadly classified into four types: benign, metastatic, indeterminate, and inflammatory. After the MDT discussion, inflammatory, benign, and indeterminate pulmonary nodules were monitored with CT scans. In our MDT, the accuracy of diagnosis of benign pulmonary nodules achieved 95.8%. Of the 24 patients, only one showed progressive lesions after review, five showed a disappearance of pulmonary nodules, and 18 showed no significant changes. Four cases of indeterminate pulmonary nodules without significant changes on multiple re-examinations were reported. Among the five cases of inflammatory nodules, one case showed progressive lesions, three cases showed no significant changes, and one case of pulmonary nodules disappeared (
Table 5). The 29 cases considered to be metastatic pulmonary nodules were managed in three ways, metastasectomy, biopsy, and targeted therapy, according to the condition and resectability of the pulmonary nodules. We found that postoperative pathology was positive in all pulmonary nodules undergoing metastasectomy, while in three of the four cases for which the MDT recommended puncture biopsy, postoperative pathology was negative. Of the 14 patients for whom targeted therapy was recommended by the MDT, 11 cases had progressive lung nodule lesions, nodules disappeared in two cases, and one case had no change (
Table 6). The possible causes of the three pulmonary nodules without progressive lesions were either effective treatment or incorrect MDT judgement. Therefore, the overall accuracy of the MDT in determining the nature of pulmonary nodules was 87.1–91.9%. In contrast, the literature reports that between 68% and 74% of nodules were correctly classified by the reporting CT radiologists [
47]. This suggests that an MDT can improve the diagnostic accuracy of pulmonary nodules and better guide treatment.
Indeterminate pulmonary nodules present a continued diagnostic dilemma for a large range of solid cancers, and there is a clear paucity in the literature guiding best practices in their management [
48,
49]. Regarding the need for a multidisciplinary approach in the treatment of rare diseases such as metastatic soft tissue sarcomas [
50], cooperation between orthopedic surgeons, pathologists, oncologists, and thoracic surgeons is of utmost importance. In the current literature, the resection of pulmonary nodules remains a vital cornerstone in treating patients with bone and soft tissue sarcoma metastasis. The thoracic surgeon has therefore become an integral part of the sarcoma team. The accurate diagnosis of pulmonary metastases, specifically differentiating them from infection or non-specific pulmonary nodules, requires close cooperation with oncologists and radiologists.
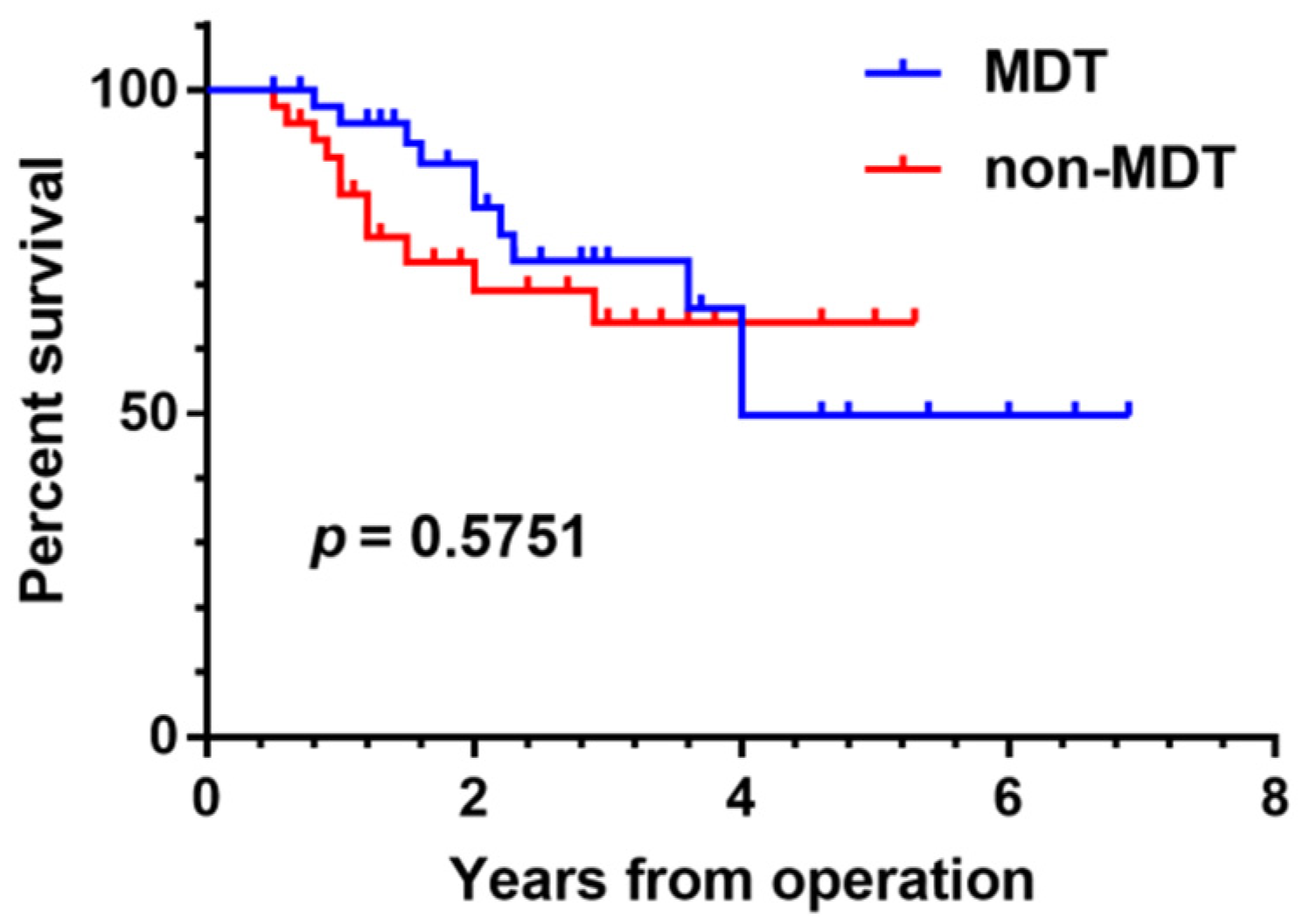
We conducted a comparative survival analysis between groups of patients with osteosarcoma presenting with pulmonary nodules who were discussed by the MDT versus undiscussed patients. There was no significant difference in age, gender, tumor location, Enneking stage, metastasis, and survival between the two groups (
p > 0.05). Our study did not demonstrate any statistically significant survival benefit from MDT management of osteosarcoma patients with pulmonary nodules (
p = 0.5751) (
Figure 4). However, in the first four years of follow-up, it can be seen that the survival time curve for patients who were subjects of the MDT discussions is superior to that of undiscussed patients. It remains to be further validated if early survival rates are higher in patients discussed by the MDT. Possible explanations for the absence of any significant survival difference between the two groups include the insufficient number of cases, the comparatively short follow-up time, and the potential bias of selected cases.
Despite significant improvements in modern multimodality treatment, the outcomes and OS rates remain poor for bone and soft tissue sarcoma patients with advanced, refractory, metastatic, or relapsed lesions. The prognosis of those patients has risen from one year in the 1980s and 1990s to 18 months in the last two decades [
51,
52]. The MDT discussed the treatment of a total of 32 patients with a clear diagnosis of bone and soft tissue malignancies that were either postoperative or metastatic. Among these patients, three cases (9.37%) obtained a CR, two cases (6.25%) achieved a PR, eight cases (25%) experienced SD, and 19 cases (59.38%) had progressive disease. Of the 19 patients in the progressive stage, 15 (78.9%) died. Most of the patients discussed by the MDT for treatment were those with multiple metastases, pelvic malignancies, and tumors of high malignancy; therefore, the prognosis was poor. Those patients with CR, such as isolated fibrous tumors, bone metastases from breast cancer, and chondrosarcoma, are less malignant or have a better prognosis. All but one of the patients in the progressive stage died within two years, and the majority died within one year, which is generally consistent with what has been reported in the literature [
51,
52,
53,
54,
55]. An MDT may not significantly effect survival in advanced bone and soft tissue tumors.
Our study has several limitations, including the inherent limitations of a unicentric and retrospective study. Moreover, the MDT was not blinded and had access to the initial clinical plan, which may have introduced potential bias. Additionally, in our study, the follow-up time was not very long, and some cases were excluded.
In conclusion, the rarity of bone and soft tissue sarcomas and the difficulty in interpreting the imaging and histology results make diagnosis and treatment difficult and complex. Precise and standardized diagnosis and treatment require systematic MDT management of patients with these diseases. MDT discussion can play an important role in the diagnosis and treatment of bone and soft tissue tumors.