High Ultrafiltration Rate Is Associated with Increased All-Cause Mortality in Incident Hemodialysis Patients with a High Cardiothoracic Ratio
Abstract
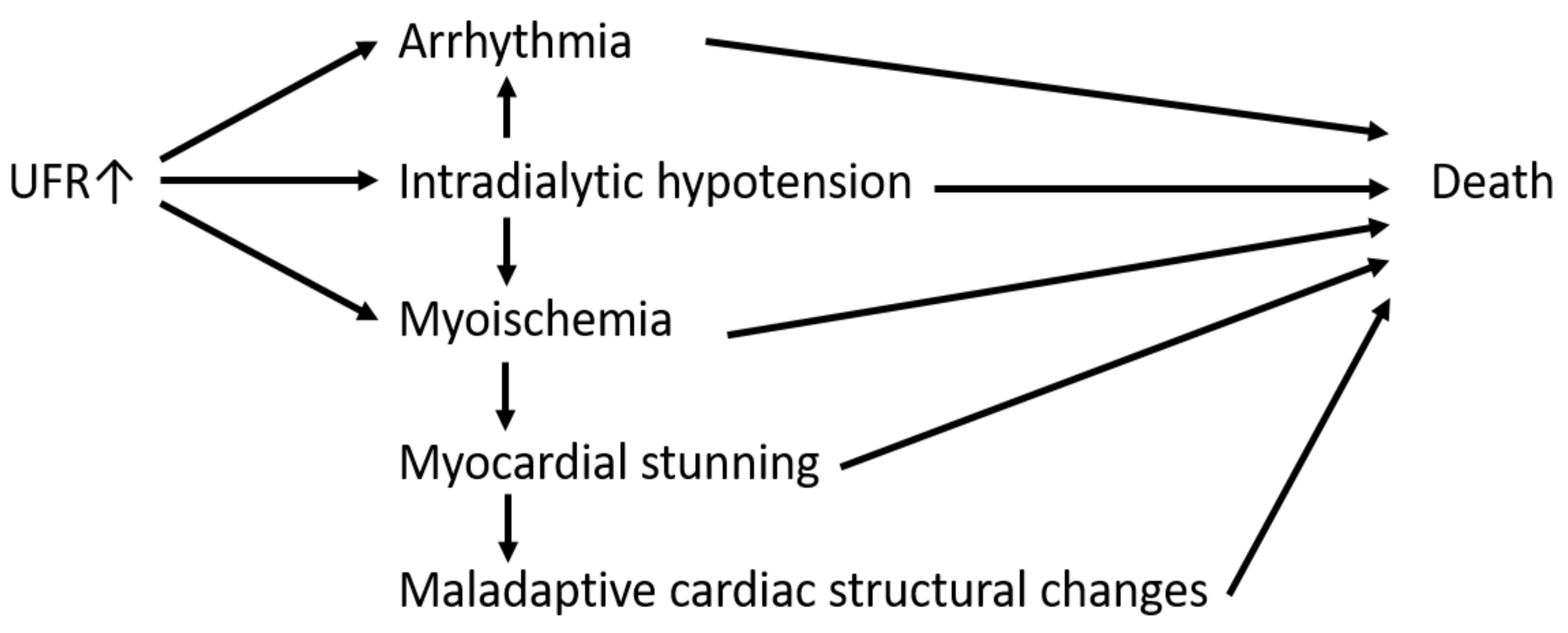
:1. Introduction
2. Patients and Methods
2.1. Participants and Design
2.2. Measurements
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Hemodialysis Patients
3.2. Factors Associated with UFR
3.3. Association between UFR and Mortality
3.4. Echocardiographic Parameters Associated with CTR
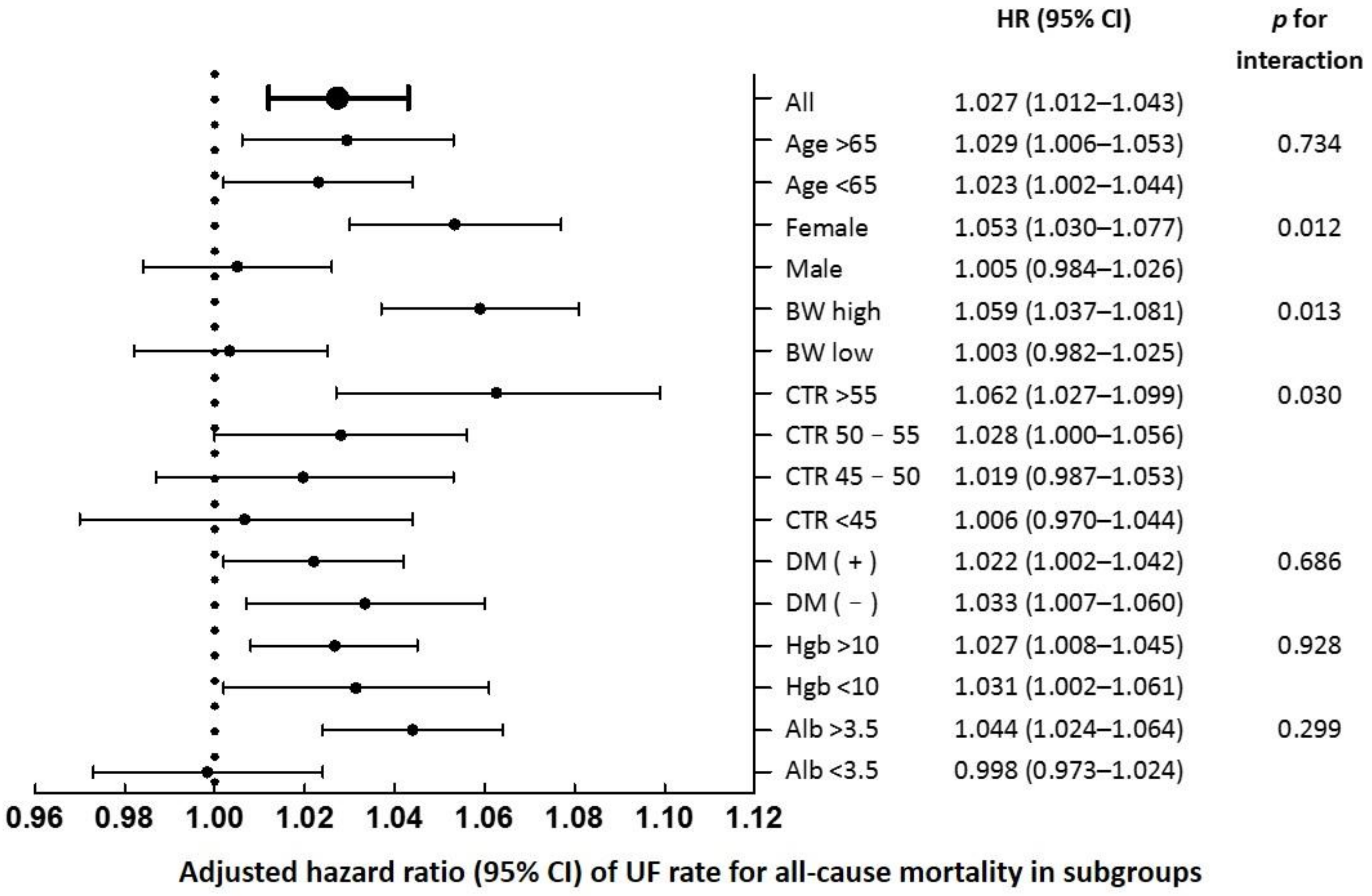
3.5. Subgroup Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, T.; Tsubakihara, Y.; Fujii, M.; Imai, E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004, 66, 1212–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, L.W. Symptomatic intradialytic hypotension and mortality: An opinionated review. Semin. Dial. 2012, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Liu, S.; Montez-Rath, M.E.; Winkelmayer, W.C.; Chang, T.I. Ultrafiltration rate and incident atrial fibrillation among older individuals initiating hemodialysis. Nephrol. Dial. Transpl. 2021, 36, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, X.; Li, Y.; Wang, Y.; Liu, Z.; Shen, B.; Teng, J.; Zou, J.; Ding, X. High ultrafiltration rate induced intradialytic hypotension is a predictor for cardiac remodeling: A 5-year cohort study. Ren. Fail. 2021, 43, 40–48. [Google Scholar] [CrossRef]
- Kim, J.K.; Song, Y.R.; Park, G.; Kim, H.J.; Kim, S.G. Impact of rapid ultrafiltration rate on changes in the echocardiographic left atrial volume index in patients undergoing haemodialysis: A longitudinal observational study. BMJ Open 2017, 7, e013990. [Google Scholar] [CrossRef]
- Tok, D.; Gullu, H.; Erdogan, D.; Topcu, S.; Ciftci, O.; Yildirim, I.; Muderrisoglu, H. Impaired coronary flow reserve in hemodialysis patients: A transthoracic Doppler echocardiographic study. Nephron Clin. Pract. 2005, 101, c200–c206. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; Sniderman, A.D.; Barre, P.E.; Vasilevsky, M.; Alam, A. High Ultrafiltration Rates Increase Troponin Levels in Stable Hemodialysis Patients. Am. J. Nephrol. 2016, 43, 173–178. [Google Scholar] [CrossRef]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Jadoul, M.; Thumma, J.; Fuller, D.S.; Tentori, F.; Li, Y.; Morgenstern, H.; Mendelssohn, D.; Tomo, T.; Ethier, J.; Port, F.; et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin. J. Am. Soc. Nephrol. 2012, 7, 765–774. [Google Scholar] [CrossRef]
- Eldehni, M.T.; Odudu, A.; McIntyre, C.W. Brain white matter microstructure in end-stage kidney disease, cognitive impairment, and circulatory stress. Hemodial. Int. 2019, 23, 356–365. [Google Scholar] [CrossRef]
- Seong, E.Y.; Zheng, Y.; Winkelmayer, W.C.; Montez-Rath, M.E.; Chang, T.I. The Relationship between Intradialytic Hypotension and Hospitalized Mesenteric Ischemia: A Case-Control Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 1517–1525. [Google Scholar] [CrossRef] [Green Version]
- Saran, R.; Bragg-Gresham, J.L.; Levin, N.W.; Twardowski, Z.J.; Wizemann, V.; Saito, A.; Kimata, N.; Gillespie, B.W.; Combe, C.; Bommer, J.; et al. Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int. 2006, 69, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Flythe, J.E.; Kimmel, S.E.; Brunelli, S.M. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011, 79, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Movilli, E.; Gaggia, P.; Zubani, R.; Camerini, C.; Vizzardi, V.; Parrinello, G.; Savoldi, S.; Fischer, M.S.; Londrino, F.; Cancarini, G. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis—A 5-year prospective observational multicentre study. Nephrol. Dial. Transplant. 2007, 22, 3547–3552. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Chang, T.I.; Kim, T.H.; Chou, J.A.; Soohoo, M.; Ravel, V.A.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Association of Ultrafiltration Rate with Mortality in Incident Hemodialysis Patients. Nephron 2018, 139, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Okuda, Y.; Sy, J.; Lee, Y.K.; Obi, Y.; Cho, S.; Chen, J.L.T.; Jin, A.; Rhee, C.M.; Kalantar-Zadeh, K.; et al. Ultrafiltration Rate, Residual Kidney Function, and Survival among Patients Treated with Reduced-Frequency Hemodialysis. Am. J. Kidney Dis. 2020, 75, 342–350. [Google Scholar] [CrossRef]
- Harnett, J.D.; Foley, R.N.; Kent, G.M.; Barre, P.E.; Murray, D.; Parfrey, P.S. Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int. 1995, 47, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Antlanger, M.; Aschauer, S.; Kopecky, C.; Hecking, M.; Kovarik, J.J.; Werzowa, J.; Mascherbauer, J.; Genser, B.; Säemann, M.D.; Bonderman, D. Heart Failure with Preserved and Reduced Ejection Fraction in Hemodialysis Patients: Prevalence, Disease Prediction and Prognosis. Kidney Blood Press. Res. 2017, 42, 165–176. [Google Scholar] [CrossRef]
- Banerjee, D.; Ma, J.Z.; Collins, A.J.; Herzog, C.A. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin. J. Am. Soc. Nephrol. 2007, 2, 1186–1190. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Cohn, J.N.; Johnson, G.R.; Shabetai, R.; Loeb, H.; Tristani, F.; Rector, T.; Smith, R.; Fletcher, R. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation 1993, 87 (Suppl. S6), VI5–V16. [Google Scholar] [PubMed]
- Philbin, E.F.; Garg, R.; Danisa, K.; Denny, D.M.; Gosselin, G.; Hassapoyannes, C.; Horney, A.; Johnstone, D.E.; Lang, R.M.; Ramanathan, K.; et al. The Relationship Between Cardiothoracic Ratio and Left Ventricular Ejection Fraction in Congestive Heart Failure. Arch. Intern. Med. 1998, 158, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truszkiewicz, K.; Macek, P.; Poręba, M.; Poręba, R.; Gać, P. Radiological Cardiothoracic Ratio as a Potential Marker of Left Ventricular Hypertrophy Assessed by Echocardiography. Radiol. Res. Pract. 2022, 2022, 4931945. [Google Scholar] [CrossRef]
- Ogata, H.; Kumasawa, J.; Fukuma, S.; Mizobuchi, M.; Kinugasa, E.; Fukagawa, M.; Fukuhara, S.; Akizawa, T. The cardiothoracic ratio and all-cause and cardiovascular disease mortality in patients undergoing maintenance hemodialysis: Results of the MBD-5D study. Clin. Exp. Nephrol. 2017, 21, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yotsueda, R.; Taniguchi, M.; Tanaka, S.; Eriguchi, M.; Fujisaki, K.; Torisu, K.; Masutani, K.; Hirakata, H.; Kitazono, T.; Tsuruya, K. Cardiothoracic Ratio and All-Cause Mortality and Cardiovascular Disease Events in Hemodialysis Patients: The Q-Cohort Study. Am. J. Kidney Dis. 2017, 70, 84–92. [Google Scholar] [CrossRef]
- Abudiab, M.M.; Redfield, M.M.; Melenovsky, V.; Olson, T.P.; Kass, D.A.; Johnson, B.D.; Borlaug, B.A. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2013, 15, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, R.; Dhakal, B.P.; Eisman, A.S.; Pappagianopoulos, P.P.; Dress, A.; Weiner, R.B.; Baggish, A.L.; Semigran, M.J.; Lewis, G.D. Pulmonary Vascular Distensibility Predicts Pulmonary Hypertension Severity, Exercise Capacity, and Survival in Heart Failure. Circ. Heart Fail. 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- London, G.M.; Guerin, A.P.; Marchais, S.J. Pathophysiology of left ventricular hypertrophy in dialysis patients. Blood Purif. 1994, 12, 277–283. [Google Scholar] [CrossRef]
- Dasselaar, J.J.; Slart, R.H.; Knip, M.; Pruim, J.; Tio, R.A.; McIntyre, C.W.; de Jong, P.E.; Franssen, C.F. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol. Dial. Transpl. 2009, 24, 604–610. [Google Scholar] [CrossRef]
- Assimon, M.M.; Wenger, J.B.; Wang, L.; Flythe, J.E. Ultrafiltration Rate and Mortality in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2016, 68, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Swaraj, S.; Kozor, R.; Arnott, C.; Di Bartolo, B.A.; G, A.F. Heart Failure with Reduced Ejection Fraction-Does Sex Matter? Curr. Hear. Fail. Rep. 2021, 18, 345–352. [Google Scholar] [CrossRef]
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.P.; Kaye, D.M. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Larson, M.; Levy, D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am. J. Cardiol. 1993, 72, 310–313. [Google Scholar] [CrossRef]
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Xu, H.; Cupples, L.A.; Stokes, A.; Liu, C.-T. Association of Obesity With Mortality Over 24 Years of Weight History: Findings from the Framingham Heart Study. JAMA Netw. Open 2018, 1, e184587. [Google Scholar] [CrossRef] [Green Version]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef]
- Merlo, M.; Cannatà, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Mascia, G.; Crotti, L.; Groppelli, A.; Canepa, M.; Merlo, A.C.; Benenati, S.; Di Donna, P.; Della Bona, R.; Soranna, D.; Zambon, A.; et al. Syncope in hypertrophic cardiomyopathy (part I): An updated systematic review and meta-analysis. Int. J. Cardiol. 2022, 357, 88–94. [Google Scholar] [CrossRef]
- Shafi, T.; Jaar, B.G.; Plantinga, L.C.; Fink, N.E.; Sadler, J.H.; Parekh, R.S.; Powe, N.R.; Coresh, J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2010, 56, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.J.; Yang, W.C.; Lin, M.Y.; Mau, L.W.; Chen, H.C. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: A national cohort study in Taiwan. Nephrol. Dial. Transpl. 2010, 25, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
| Variables | Ultrafiltration Rate | ||||||
|---|---|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | Q5 | p (Anova) | |
| UFR (mL/kg/h) | <6.6 | 6.6–8.6 | 8.6–10.3 | 10.3–12.3 | >12.3 | ||
| No. of patients | 2615 | 523 (20.0%) | 523 (20.0%) | 523 (20.0%) | 523 (20.0%) | 523 (20.0%) | |
| Age at dialysis (year) | 59.1 (14.2) | 64.0 (12.9) | 60.2 (13.8) | 58.6 (13.6) | 57.7 (14.0) | 54.9 (15.0) | <0.001 |
| Gender (female) | 1317 (50.4%) | 263 (50.3%) | 257 (49.1%) | 259 (49.5%) | 270 (51.6%) | 268 (51.2%) | 0.916 |
| Comorbidities | |||||||
| Diabetes mellitus | 1261 (48.2%) | 225 (43.0%) | 218 (41.7%) | 264 (50.5%) | 286 (54.7%) | 268 (51.2%) | <0.001 |
| Hypertension | 1831 (70.0%) | 340 (65.0%) | 367 (70.2%) | 391 (74.8%) | 375 (71.7%) | 358 (68.5%) | 0.010 |
| Heart failure | 850 (32.5%) | 143 (27.3%) | 166 (31.7%) | 168 (32.1%) | 181 (34.6%) | 192 (36.7%) | 0.018 |
| Stroke | 194 (7.4%) | 33 (6.3%) | 41 (7.8%) | 43 (8.2%) | 45 (8.6%) | 32 (6.1%) | 0.417 |
| Cancer | 161 (6.2%) | 50 (9.6%) | 33 (6.3%) | 37 (7.1%) | 24 (4.6%) | 17 (3.3%) | <0.001 |
| Dialysis status | |||||||
| Dry body weight (kg) | 56.8 (11.7) | 57.7 (11.6) | 58.3 (12.5) | 58.1 (11.9) | 56.4 (11.5) | 52.9 (9.5) | <0.001 |
| Kt/V (Gotch) | 1.30 (0.23) | 1.30 (0.23) | 1.30 (0.24) | 1.30 (0.24) | 1.30 (0.22) | 1.30 (0.23) | 0.995 |
| Kt/V < 1.4 | 1805 (69.0%) | 325 (62.1%) | 347 (66.3%) | 364 (69.6%) | 374 (71.5%) | 395 (75.5%) | <0.001 |
| nPCR | 1.2 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 0.006 |
| UFR (mL/kg/h) | 9.6 (3.8) | 4.8 (1.5) | 7.6 (0.6) | 9.4 (0.5) | 11.2 (0.6) | 15.0 (3.1) | <0.001 |
| UF/BW ratio (%) | 3.8 (1.5) | 1.9 (0.6) | 3.1 (0.2) | 3.8 (0.2) | 4.5 (0.2) | 5.9 (1.3) | <0.001 |
| Dialysis length (min) | 239.0 (6.8) | 239.2 (5.8) | 239.7 (6.0) | 239.1 (7.1) | 239.4 (4.4) | 237.5 (9.3) | <0.001 |
| CTR (%) | 50.3 (6.5) | 50.9 (6.3) | 50.2 (6.4) | 50.0 (6.4) | 50.0 (6.8) | 50.5 (6.5) | 0.128 |
| Laboratory Results | |||||||
| WBC (×1000/μL) | 7.0 (2.3) | 7.2 (2.7) | 6.9 (2.1) | 6.9 (2.1) | 7.0 (2.2) | 6.9 (2.2) | 0.300 |
| Hemoglobin (g/dL) | 9.9 (1.2) | 10.0 (1.3) | 9.9 (1.2) | 10.0 (1.2) | 9.8 (1.2) | 9.6 (1.2) | <0.001 |
| Albumin (gm/dL) | 3.7 (0.4) | 3.7 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 3.7 (0.4) | 3.7 (0.4) | 0.013 |
| Creatinine (mg/dL) | 9.2 (2.8) | 8.8 (2.7) | 9.5 (2.9) | 9.5 (2.7) | 9.2 (2.9) | 9.3 (3.0) | <0.001 |
| Glucose [AC] (mg/dL) | 136.4 (60.8) | 126.2 (50.8) | 130.6 (53.9) | 135.5 (60.6) | 142.6 (63.8) | 147.2 (70.5) | <0.001 |
| Cholesterol (mg/dL) | 184 (156–215) | 181 (152–211) | 186 (158–214) | 182 (157–215) | 186 (155–220) | 185 (156–216) | 0.238 |
| Triglyceride (mg/dL) | 142 (99–210) | 143 (102–206) | 145 (99–224) | 141 (98–207) | 146 (97–226) | 136 (100–196) | 0.252 |
| Sodium (meq/L) | 138.1 (3.5) | 138.8 (3.4) | 138.7 (3.2) | 137.9 (3.4) | 137.8 (3.5) | 137.6 (3.7) | <0.001 |
| Calcium (mg/dL) | 9.3 (0.8) | 9.4 (0.8) | 9.3 (0.7) | 9.3 (0.8) | 9.3 (0.8) | 9.2 (0.8) | 0.001 |
| Phosphate (mg/dL) | 5.0 (1.2) | 4.6 (1.2) | 4.9 (1.1) | 5.0 (1.2) | 5.1 (1.3) | 5.2 (1.3) | <0.001 |
| intact-PTH (pg/mL) | 176 (64–359) | 179 (60–360) | 176 (59–352) | 173 (67–351) | 167 (66–339) | 179 (67–392) | 0.944 |
| Iron saturation (%) | 32.4 (20.5) | 31.8 (15.6) | 32.5 (17.1) | 32.4 (14.4) | 33.6 (25.8) | 31.6 (26.5) | 0.513 |
| Ferritin (ng/mL) | 519 (318–798) | 534 (345–803) | 528 (327–805) | 507 (336–764) | 536 (282–830) | 483 (287–797) | 0.384 |
| Outcomes | |||||||
| Follow-up days | 2246 (1087–3596) | 2144 (985–3480) | 2378 (1187–3585) | 2394 (1171–3708) | 2090 (1017–3391) | 2268 (1049–3732) | 0.068 |
| Mortality | 1247 (47.7%) | 264 (50.5%) | 230 (44.0%) | 232 (44.4%) | 253 (48.4%) | 268 (51.2%) | 0.047 |
| CV mortality | 384 (14.7%) | 90 (17.2%) | 66 (12.6%) | 70 (13.4%) | 69 (13.2%) | 89 (17.0%) | 0.035 |
| Variables | β | 95% CI of β | p-Value |
|---|---|---|---|
| Age at dialysis | −0.069 | −0.081 to −0.057 | <0.001 |
| Gender (female vs. male) | −0.418 | −0.771 to −0.066 | 0.020 |
| Entry year (late vs. early) | −0.289 | −0.611 to 0.034 | 0.079 |
| Diabetes | 0.834 | 0.496 to 1.173 | <0.001 |
| Hypertension | 0.205 | −0.118 to 0.527 | 0.214 |
| Heart failure | 0.383 | 0.064 to 0.701 | 0.018 |
| Stroke | 0.240 | −0.296 to 0.777 | 0.380 |
| Cancer | −0.577 | −1.146 to −0.008 | 0.047 |
| Hepatitis | −0.512 | −0.909 to −0.116 | 0.011 |
| Hemoglobin (g/dL) | −0.052 | −0.180 to 0.076 | 0.424 |
| Dry body weight (kg) | −0.089 | −0.104 to −0.073 | <0.001 |
| Kt/V (Gotch) | −0.282 | −1.125 to 0.560 | 0.512 |
| nPCR | 0.057 | −0.500 to 0.614 | 0.840 |
| Cardiothoracic ratio (%) | −0.004 | −0.028 to 0.019 | 0.716 |
| Albumin (gm/dL) | −0.278 | −0.721 to 0.165 | 0.219 |
| WBC (×1000/μL) | −0.060 | −0.130 to 0.009 | 0.088 |
| Cholesterol | −0.179 | −1.654 to 1.297 | 0.812 |
| Glucose (mg/dL) | 0.008 | 0.005 to 0.010 | <0.001 |
| Phosphate (mg/dL) | 0.502 | 0.366 to 0.637 | <0.001 |
| Iron saturation | 0.003 | −0.004 to 0.009 | 0.461 |
| Ultrafiltration Rate (1 mL/kg/h) | Ultrafiltration Rate | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| HR for all-cause mortality | ||||||
| Unadjusted | 1.007 (0.991–1.022) | 1.23 (1.03–1.46) | 1 (reference) | 1.00 (0.84–1.20) | 1.18 (0.99–1.41) | 1.19 (1.00–1.42) |
| Model 1 a | 1.036 (1.021–1.050) * | 0.99 (0.83–1.19) | 1 (reference) | 1.03 (0.86–1.24) | 1.24 (1.03–1.48) * | 1.50 (1.25–1.79) * |
| Model 2 b | 1.027 (1.012–1.043) * | 1.03 (0.86–1.23) | 1 (reference) | 1.13 (0.94–1.36) | 1.24 (1.03–1.49) * | 1.44 (1.20–1.74) * |
| Model 3 c | 1.016 (1.003–1.028) * | 1.22 (0.98–1.53) | 1 (reference) | 1.21 (0.95–1.54) | 1.36 (1.07–1.74) * | 1.38 (1.09–1.74) * |
| HR for CV mortality | ||||||
| Unadjusted | 1.000 (0.972–1.028) | 1.40 (1.02–1.92) | 1 (reference) | 1.07 (0.76–1.49) | 1.07 (0.76–1.50) | 1.35 (0.98–1.86) |
| Model 1 a | 1.002 (0.973–1.030) | 1.35 (0.98–1.85) | 1 (reference) | 1.08 (0.79–1.49) | 1.07 (0.75–1.51) | 1.36 (0.98–1.86) |
| Model 2 b | 1.003 (0.975–1.032) | 1.27 (0.92–1.76) | 1 (reference) | 1.10 (0.78–1.55) | 1.00 (0.71–1.41) | 1.38 (0.99–1.92) |
| Model 3 c | 1.003 (0.974–1.033) | 1.29 (0.93–1.78) | 1 (reference) | 1.21 (0.86–1.71) | 1.14 (0.80–1.62) | 1.37 (0.98–1.90) |
| Ultrafiltration Rate | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for Interaction | |
| CTR a | 0.026 | |||||
| CTR < 45% | 0.82 (0.53–1.29) | 1 (reference) | 0.73 (0.47–1.16) | 1.07 (0.70–1.65) | 1.10 (0.70–1.75) | |
| CTR 45–50% | 0.99 (0.69–1.41) | 1 (reference) | 1.10 (0.78–1.54) | 1.10 (0.77–1.59) | 1.22 (0.85–1.75) | |
| CTR 50–55% | 1.00 (0.72–1.38) | 1 (reference) | 1.31 (0.93–1.85) | 1.20 (0.84–1.71) | 1.48 (1.04–2.12) | |
| CTR > 55% | 1.18 (0.82–1.71) | 1 (reference) | 1.17 (0.79–1.75) | 1.39 (0.96–2.04) | 1.91 (1.30–2.82) | |
| Gender b | 0.071 | |||||
| Female | 0.96 (0.75–1.25) | 1 (reference) | 1.15 (0.88–1.50) | 1.20 (0.92–1.57) | 1.73 (1.31–2.28) | |
| Male | 1.08 (0.84–1.40) | 1 (reference) | 1.05 (0.81–1.37) | 1.25 (0.96–1.62) | 1.21 (0.93–1.57) | |
| Dry BW c | 0.095 | |||||
| High d | 0.90 (0.68–1.17) | 1 (reference) | 1.21 (0.93–1.57) | 1.50 (1.14–1.96) | 1.83 (1.38–2.43) | |
| Low | 1.08 (0.84–1.38) | 1 (reference) | 0.98 (0.75–1.28) | 1.00 (0.77–1.29) | 1.18 (0.92–1.52) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-J.; Chao, Y.-L.; Kuo, I.-C.; Niu, S.-W.; Hung, C.-C.; Chiu, Y.-W.; Chang, J.-M. High Ultrafiltration Rate Is Associated with Increased All-Cause Mortality in Incident Hemodialysis Patients with a High Cardiothoracic Ratio. J. Pers. Med. 2022, 12, 2059. https://doi.org/10.3390/jpm12122059
Yang L-J, Chao Y-L, Kuo I-C, Niu S-W, Hung C-C, Chiu Y-W, Chang J-M. High Ultrafiltration Rate Is Associated with Increased All-Cause Mortality in Incident Hemodialysis Patients with a High Cardiothoracic Ratio. Journal of Personalized Medicine. 2022; 12(12):2059. https://doi.org/10.3390/jpm12122059
Chicago/Turabian StyleYang, Lii-Jia, Yu-Lin Chao, I-Ching Kuo, Sheng-Wen Niu, Chi-Chih Hung, Yi-Wen Chiu, and Jer-Ming Chang. 2022. "High Ultrafiltration Rate Is Associated with Increased All-Cause Mortality in Incident Hemodialysis Patients with a High Cardiothoracic Ratio" Journal of Personalized Medicine 12, no. 12: 2059. https://doi.org/10.3390/jpm12122059
APA StyleYang, L.-J., Chao, Y.-L., Kuo, I.-C., Niu, S.-W., Hung, C.-C., Chiu, Y.-W., & Chang, J.-M. (2022). High Ultrafiltration Rate Is Associated with Increased All-Cause Mortality in Incident Hemodialysis Patients with a High Cardiothoracic Ratio. Journal of Personalized Medicine, 12(12), 2059. https://doi.org/10.3390/jpm12122059







