Mucin (MUC) Family Influence on Acute Lymphoblastic Leukemia in Cancer and Non-Cancer Native American Populations from the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics
2.2. Selection of Genes
2.3. Extraction of the DNA and Preparation of the Exomes
2.4. Bioinformatics and Statistical Analysis
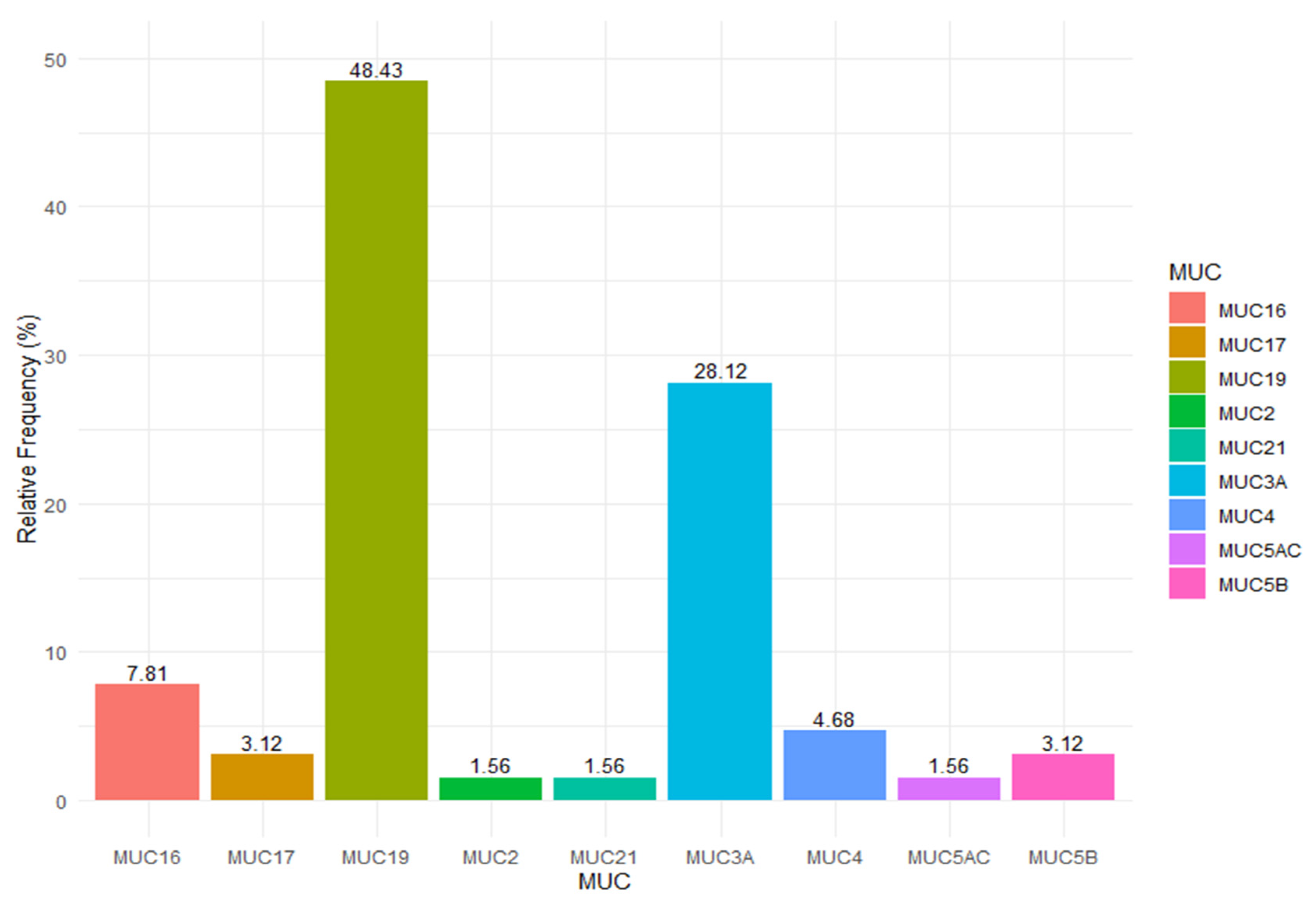
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.-H.; Choi, S.; Park, Y.; Jin, H. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals 2021, 14, 1053. [Google Scholar] [CrossRef]
- Xu, M.; Wang, D.C.; Wang, X.; Zhang, Y. Correlation between mucin biology and tumor heterogeneity in lung cancer. Semin. Cell Dev. Biol. 2017, 64, 73–78. [Google Scholar] [CrossRef]
- Choi, Y.J.; Ohn, J.H.; Kim, N.; Kim, W.; Park, K.; Won, S.; Sael, L.; Shin, C.M.; Lee, S.M.; Lee, S.; et al. Family-based exome sequencing combined with linkage analyses identifies rare susceptibility variants of MUC4 for gastric cancer. PLoS ONE 2020, 15, e0236197. [Google Scholar] [CrossRef]
- Hsu, H.-P.; Lai, M.-D.; Lee, J.-C.; Yen, M.-C.; Weng, T.-Y.; Chen, W.-C.; Fang, J.-H.; Chen, Y.-L. Mucin 2 silencing promotes colon cancer metastasis through interleukin-6 signaling. Sci. Rep. 2017, 7, 5823. [Google Scholar] [CrossRef]
- Jia, Y.; Persson, C.; Hou, L.; Zheng, Z.; Yeager, M.; Lissowska, J.; Chanock, S.J.; Chow, W.-H.; Ye, W. A comprehensive analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes and risk of stomach cancer. Cancer Causes Control 2010, 21, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Jonckheere, N.; Van Seuningen, I. Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20 signature is associated with poor survival in human carcinomas. J. Transl. Med. 2018, 16, 259. [Google Scholar] [CrossRef]
- Shete, S.; Liu, H.; Wang, J.; Yu, R.; Sturgis, E.M.; Li, G.; Dahlstrom, K.R.; Liu, Z.; Amos, C.I.; Wei, Q. A Genome-Wide Association Study Identifies Two Novel Susceptible Regions for Squamous Cell Carcinoma of the Head and Neck. Cancer Res. 2020, 80, 2451–2460. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.W.T.; Lloyd, K.O. Molecular Cloning of the CA125 Ovarian Cancer Antigen: Identification as a New MUCIN, MUC16 *. J. Biol. Chem. 2001, 276, 27371–27375. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.W.T.; Dnistrian, A.; Lloyd, K.O. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int. J. Cancer 2002, 98, 737–740. [Google Scholar] [CrossRef]
- Cao, Y.; Karsten, U. Binding patterns of 51 monoclonal antibodies to peptide and carbohydrate epitopes of the epithelial mucin (MUC1) on tissue sections of adenolymphomas of the parotid (Warthin’s tumours): Role of epitope masking by glycans. Histochem. Cell Biol. 2001, 115, 349–356. [Google Scholar] [CrossRef]
- Rezaei, M.; Tan, J.; Zeng, C.; Li, Y.; Ganjalikhani-Hakemi, M. TIM-3 in Leukemia; Immune Response and Beyond. Front. Oncol. 2021, 11, 753677. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Stroopinsky, D.; Kufe, D.; Avigan, D. MUC1 in hematological malignancies. Leuk. Lymphoma 2016, 57, 2489–2498. [Google Scholar] [CrossRef]
- Tagde, A.; Rajabi, H.; Stroopinsky, D.; Gali, R.; Alam, M.; Bouillez, A.; Kharbanda, S.; Stone, R.; Avigan, D.; Kufe, D. MUC1-C induces DNA methyltransferase 1 and represses tumor suppressor genes in acute myeloid leukemia. Oncotarget 2016, 7, 38974–38987. [Google Scholar] [CrossRef] [Green Version]
- Onciu, M. Acute Lymphoblastic Leukemia. Hematol./Oncol. Clin. N. Am. 2009, 23, 655–674. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, S.; Campo, E.; Harris, N.; Jaffe, E.; Pileri, S.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2. [Google Scholar]
- Pui, C.-H.; Pei, D.; Campana, D.; Bowman, W.P.; Sandlund, J.T.; Kaste, S.C.; Ribeiro, R.C.; Rubnitz, J.E.; Coustan-Smith, E.; Jeha, S.; et al. Improved Prognosis for Older Adolescents With Acute Lymphoblastic Leukemia. JCO 2011, 29, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.H.; Colt, J.S.; Deziel, N.C.; Whitehead, T.P.; Reynolds, P.; Gunier, R.B.; Nishioka, M.; Dahl, G.V.; Rappaport, S.M.; Buffler, P.A.; et al. Residential Levels of Polybrominated Diphenyl Ethers and Risk of Childhood Acute Lymphoblastic Leukemia in California. Environ. Health Perspect. 2014, 122, 1110–1116. [Google Scholar] [CrossRef]
- American Cancer Society Key Statistics for Acute Lymphocytic Leukemia (ALL). Available online: https://www.cancer.org/cancer/acute-lymphocytic-leukemia/about/key-statistics.html (accessed on 24 March 2022).
- De Oliveira Santos, M. Estimativa/2020—Incidência de Câncer no Brasil. Rev. Brasileira. Cancerol. 2020, 66, 25–26. [Google Scholar] [CrossRef]
- Carvalho, D.C.; Wanderley, A.V.; Amador, M.A.T.; Fernandes, M.R.; Cavalcante, G.C.; Pantoja, K.B.C.C.; Mello, F.A.R.; de Assumpção, P.P.; Khayat, A.S.; Ribeiro-dos-Santos, Â.; et al. Amerindian genetic ancestry and INDEL polymorphisms associated with susceptibility of childhood B-cell Leukemia in an admixed population from the Brazilian Amazon. Leuk. Res. 2015, 39, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.J.; Puumala, S.E.; Mueller, B.A.; Carozza, S.E.; Fox, E.E.; Horel, S.; Johnson, K.J.; McLaughlin, C.C.; Reynolds, P.; Von Behren, J.; et al. Childhood cancer in relation to parental race and ethnicity: A 5-state pooled analysis. Cancer 2010, 116, 3045–3053. [Google Scholar] [CrossRef]
- De Carvalho, D.C.; Wanderley, A.V.; Dos Santos, A.M.R.; Fernandes, M.R.; Cohen Lima de Castro, A.D.N.; Leitão, L.P.C.; De Carvalho, J.A.N.; De Souza, T.P.; Khayat, A.S.; Dos Santos, S.E.B.; et al. Pharmacogenomics and variations in the risk of toxicity during the consolidation/maintenance phases of the treatment of pediatric B-cell leukemia patients from an admixed population in the Brazilian Amazon. Leuk. Res. 2018, 74, 10–13. [Google Scholar] [CrossRef]
- Quiroz, E.; Aldoss, I.; Pullarkat, V.; Rego, E.; Marcucci, G.; Douer, D. The emerging story of acute lymphoblastic leukemia among the Latin American population—Biological and clinical implications. Blood Rev. 2019, 33, 98–105. [Google Scholar] [CrossRef]
- Shoag, J.M.; Barredo, J.C.; Lossos, I.S.; Pinheiro, P.S. Acute lymphoblastic leukemia mortality in Hispanic Americans. Leuk. Lymphoma 2020, 61, 2674–2681. [Google Scholar] [CrossRef]
- de Carvalho, D.C.; Wanderley, A.V.; dos Santos, A.M.R.; Moreira, F.C.; de Sá, R.B.A.; Fernandes, M.R.; Modesto, A.A.C.; de Souza, T.P.; Cohen-Paes, A.; Leitão, L.P.C.; et al. Characterization of pharmacogenetic markers related to Acute Lymphoblastic Leukemia toxicity in Amazonian native Americans population. Sci. Rep. 2020, 10, 10292. [Google Scholar] [CrossRef]
- Gervasini, G.; Vagace, J.M. Impact of genetic polymorphisms on chemotherapy toxicity in childhood acute lymphoblastic leukemia. Front. Gene. 2012, 3, 249. [Google Scholar] [CrossRef] [Green Version]
- Santos, N.P.C.; Ribeiro-Rodrigues, E.M.; Ribeiro-dos-Santos, Â.K.C.; Pereira, R.; Gusmão, L.; Amorim, A.; Guerreiro, J.F.; Zago, M.A.; Matte, C.; Hutz, M.H.; et al. Assessing individual interethnic admixture and population substructure using a 48-insertion-deletion (INSEL) ancestry-informative marker (AIM) panel. Hum. Mutat. 2010, 31, 184–190. [Google Scholar] [CrossRef]
- Azofeifa, J.; Ruiz-Narváez, E.A.; Leal, A.; Gerlovin, H.; Rosero-Bixby, L. Amerindian ancestry and extended longevity in Nicoya, Costa Rica. Am. J. Hum. Biol. 2018, 30, e23055. [Google Scholar] [CrossRef]
- Spangenberg, L.; Fariello, M.I.; Arce, D.; Illanes, G.; Greif, G.; Shin, J.-Y.; Yoo, S.-K.; Seo, J.-S.; Robello, C.; Kim, C.; et al. Indigenous Ancestry and Admixture in the Uruguayan Population. Front. Genet. 2021, 12, 733195. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Ahmed, S.; Sabir, M.S.; Blauwendraat, C.; Adarmes-Gómez, A.D.; Bernal-Bernal, I.; Bonilla-Toribio, M.; Buiza-Rueda, D.; Carrillo, F.; Carrión-Claro, M.; et al. The Genetic Architecture of Parkinson Disease in Spain: Characterizing Population-Specific Risk, Differential Haplotype Structures, and Providing Etiologic Insight. Mov. Disord. 2019, 34, 1851–1863. [Google Scholar] [CrossRef]
- Pesquisa Envolvendo Povos Indígenas e Suas Terras. Available online: https://cep.prpi.ufg.br/p/38832-pesquisa-envolvendo-povos-indigenas-e-suas-terras (accessed on 24 March 2022).
- Shin, J.H. Nucleic Acid Extraction Techniques. In Advanced Techniques in Diagnostic Microbiology; Springer: New York, NY, USA, 2012; pp. 209–225. [Google Scholar] [CrossRef]
- Price, C.W.; Leslie, D.C.; Landers, J.P. Nucleic acid extraction techniques and application to the microchip. Lab Chip 2009, 9, 2484–2494. [Google Scholar] [CrossRef]
- Tan, S.C.; Yiap, B.C. DNA, RNA, and Protein Extraction: The Past and The Present. J. Biomed. Biotechnol. 2009, 2009, 574398. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-dos-Santos, A.M.; Vidal, A.F.; Vinasco-Sandoval, T.; Guerreiro, J.; Santos, S.; Ribeiro-dos-Santos, Â.; de Souza, S.J. Exome Sequencing of Native Populations From the Amazon Reveals Patterns on the Peopling of South America. Front. Genet. 2020, 11, 548507. [Google Scholar] [CrossRef]
- Rodrigues, J.C.G.; Souza, T.P.D.; Pastana, L.F.; Ribeiro dos Santos, A.M.; Fernandes, M.R.; Pinto, P.; Wanderley, A.V.; De Souza, S.J.; Kroll, J.E.; Pereira, A.L.; et al. Identification of NUDT15 gene variants in Amazonian Amerindians and admixed individuals from northern Brazil. PLoS ONE 2020, 15, e0231651. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Franco, J.; Ayón-Pérez, M.F.; Durán-Avelar, M.D.J.; Vibanco-Pérez, N.; Sánchez-Jasso, D.E.; Bañuelos-Aguayo, D.G.; Sánchez-Meza, J.; Pimentel-Gutiérrez, H.J.; Zambrano-Zaragoza, J.F.; Agraz-Cibrián, J.M.; et al. High frequency of the risk allele of rs4132601 and rs11978267 from the IKZF1 gene in indigenous Mexican population. Mol. Genet. Genom. Med. 2021, 9, e1589. [Google Scholar] [CrossRef]
- Franke, A.; McGovern, D.P.B.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- The International IBD Genetics Consortium (IIBDGC); Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Huang, L.; Xu, Q.; Lv, Y.; Wang, Z.; Zhan, P.; Han, H.; Shao, Y.; Lin, D.; Lv, T.; et al. Association of MUC19 Mutation with Clinical Benefits of Anti-PD-1 Inhibitors in Non-small Cell Lung Cancer. Front. Oncol. 2021, 11, 596542. [Google Scholar] [CrossRef]
- Song, L.; Xiao, Y. Downregulation of hsa_circ_0007534 suppresses breast cancer cell proliferation and invasion by targeting miR-593/MUC19 signal pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2603–2610. [Google Scholar] [CrossRef]
- Fransson, S.; Martinez-Monleon, A.; Johansson, M.; Sjöberg, R.-M.; Björklund, C.; Ljungman, G.; Ek, T.; Kogner, P.; Martinsson, T. Whole-genome sequencing of recurrent neuroblastoma reveals somatic mutations that affect key players in cancer progression and telomere maintenance. Sci. Rep. 2020, 10, 22432. [Google Scholar] [CrossRef]
- Buisine, M.P.; Colombel, J.F.; Lecomte-Houcke, M.; Gower, P.; Aubert, J.P.; Porchet, N.; Janin, A. Abnormal mucus in cap polyposis. Gut 1998, 42, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Pinzón Martín, S.; Seeberger, P.H.; Varón Silva, D. Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators during Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019, 7, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; You, Y.; Chen, D.; Qian, J.-M.; Li, J. Cronkhite-Canada syndrome complicated with three malignant tumors: A case report and whole exome sequencing analysis. Chin. Med. J. 2019, 132, 3001–3002. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tomoshige, K.; Meister, M.; Muley, T.; Fukazawa, T.; Tsuchiya, T.; Karns, R.; Warth, A.; Fink-Baldauf, I.M.; Nagayasu, T.; et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol. Med. 2017, 9, 462–481. [Google Scholar] [CrossRef]
- Bouanene, H.; Hadj Kacem, H.; Ben Fatma, L.; Ben Limem, H.; Ben Ahmed, S.; Yakoub, S.; Miled, A. Polymorphisms in the MUC16 Gene: Potential Implication in Epithelial Ovarian Cancer. Pathol. Oncol. Res. 2011, 17, 295–299. [Google Scholar] [CrossRef]
- Fingerlin, T.E.; Murphy, E.; Zhang, W.; Peljto, A.L.; Brown, K.K.; Steele, M.P.; Loyd, J.E.; Cosgrove, G.P.; Lynch, D.; Groshong, S.; et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 2013, 45, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Loeb, M.; Eskandarian, S.; Rupp, M.; Fishman, N.; Gasink, L.; Patterson, J.; Bramson, J.; Hudson, T.J.; Lemire, M. Genetic Variants and Susceptibility to Neurological Complications Following West Nile Virus Infection. J. Infect. Dis. 2011, 204, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, I.; Salfity, S.; Seshacharyulu, P.; Rachagani, S.; Thomas, A.; Das, S.; Majhi, P.D.; Nimmakayala, R.K.; Vengoji, R.; Lele, S.M.; et al. MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by suppressing p53. Clin. Cancer Res. 2017, 23, 3906–3917. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Kanwal, M.; Li, G.; Yang, J.; Niu, H.; Li, Z.; Ding, X. MUC16 in non-small cell lung cancer patients affected by familial lung cancer and indoor air pollution: Clinical characteristics and cell behaviors. Transl. Lung Cancer Res. 2019, 8, 476–488. [Google Scholar] [CrossRef]
- Muniyan, S.; Haridas, D.; Chugh, S.; Rachagani, S.; Lakshmanan, I.; Gupta, S.; Seshacharyulu, P.; Smith, L.M.; Ponnusamy, M.P.; Batra, S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016, 7, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gum, J.R.; Crawley, S.C.; Hicks, J.W.; Szymkowski, D.E.; Kim, Y.S. MUC17, a Novel Membrane-Tethered Mucin. Biochem. Biophys. Res. Commun. 2002, 291, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Moehle, C.; Ackermann, N.; Langmann, T.; Aslanidis, C.; Kel, A.; Kel-Margoulis, O.; Schmitz-Madry, A.; Zahn, A.; Stremmel, W.; Schmitz, G. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J. Mol. Med. 2006, 84, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, A.; Hu, Y.; Tao, C.; Wang, J.M.; Lu, Y.; Xing, R. Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop. J. Exp. Clin. Cancer Res. 2019, 38, 283. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, C.; Ren, Y.; Yi, H.; Luo, T.; Xing, F.; Bai, X.; Cui, L.; Zhu, L.; Ouyang, J.; et al. Genomic characterization of Chinese ovarian clear cell carcinoma identifies driver genes by whole exome sequencing. Neoplasia 2020, 22, 399–430. [Google Scholar] [CrossRef]
- Midha, M.K.; Huang, Y.-F.; Yang, H.-H.; Fan, T.-C.; Chang, N.-C.; Chen, T.-H.; Wang, Y.-T.; Kuo, W.-H.; Chang, K.-J.; Shen, C.-Y.; et al. Comprehensive Cohort Analysis of Mutational Spectrum in Early Onset Breast Cancer Patients. Cancers 2020, 12, 2089. [Google Scholar] [CrossRef]
- McDonald, M.E.; Salinas, E.A.; Devor, E.J.; Newtson, A.M.; Thiel, K.W.; Goodheart, M.J.; Bender, D.P.; Smith, B.J.; Leslie, K.K.; Gonzalez-Bosquet, J. Molecular Characterization of Non-responders to Chemotherapy in Serous Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 1175. [Google Scholar] [CrossRef]
| MUC | Rs | Region Detailed | Var Type | Impact | ALL | NAM | p-Value |
|---|---|---|---|---|---|---|---|
| MUC19 | rs5797672 | FRAME_SHIFT | INDEL | HIGH | 0.6 | 0 | <0.01 |
| MUC19 | rs60568788 | FRAME_SHIFT | INDEL | HIGH | 0.6 | 0.71 | <0.01 |
| MUC19 | rs1444222 | INTRON | SNV | MODIFIER | 0.6 | 0 | <0.01 |
| MUC19 | rs4768284 | INTRON | SNV | MODIFIER | 0.6 | 0 | <0.01 |
| MUC19 | rs994798 | INTRON | SNV | MODIFIER | 0.6 | 0 | <0.01 |
| MUC19 | rs6581732 | INTRON | SNV | MODIFIER | 0.8 | 0 | <0.01 |
| MUC19 | rs111256342 | INTRON | INDEL | MODIFIER | 0.8 | 0 | <0.01 |
| MUC19 | rs1444215 | INTRON | SNV | MODIFIER | 0.9 | 0 | <0.01 |
| MUC19 | rs2029615 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC19 | rs2638879 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC19 | rs2638875 | NON_SYN | SNV | MODERATE | 0.6 | 0 | <0.01 |
| MUC19 | rs2638865 | NON_SYN | SNV | MODERATE | 0.6 | 0 | <0.01 |
| MUC19 | rs2588401 | NON_SYN | SNV | MODERATE | 0.62 | 0 | <0.01 |
| MUC19 | rs1492322 | NON_SYN | SNV | MODERATE | 0.6 | 0 | <0.01 |
| MUC19 | rs1492313 | NON_SYN | SNV | MODERATE | 0.6 | 0 | <0.01 |
| MUC19 | rs2251431 | NON_SYN | SNV | MODERATE | 0.7 | 0 | <0.01 |
| MUC19 | rs2638866 | NON_SYN | SNV | MODERATE | 0.7 | 0 | <0.01 |
| MUC19 | rs2933354 | NON_SYN | SNV | MODERATE | 0.8 | 0 | <0.01 |
| MUC19 | rs2638868 | NON_SYN | SNV | MODERATE | 0.8 | 0 | <0.01 |
| MUC19 | rs2405077 | NON_SYN | SNV | MODERATE | 0.8 | 0 | <0.01 |
| MUC19 | rs7300780 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs75899846 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs73269929 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs78409264 | NON_SYN | SNV | MODERATE | 0.4 | 0 | <0.01 |
| MUC19 | rs78204462 | NON_SYN | SNV | MODERATE | 0.4 | 0 | <0.01 |
| MUC19 | rs73269926 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs7133943 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs6581782 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs79829730 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC19 | rs73112046 | NON_SYN | SNV | MODERATE | 0.3 | 0 | <0.01 |
| MUC3A | rs75254397 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs75196671 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs78724937 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs78470577 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs73398800 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs75547895 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC3A | rs73714242 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs78538898 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs76249962 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs78684063 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs28515787 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs74460367 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs78826835 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs79233494 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs73163757 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC3A | rs73398732 | NON_SYN | SNV | MODERATE | 0.4 | 0 | <0.01 |
| MUC3A | rs78584246 | NON_SYN | SNV | MODERATE | 0.5 | 0.046 | <0.05 |
| MUC3A | rs75517157 | NON_SYN | SNV | MODERATE | 0.5 | 0.064 | <0.05 |
| MUC5B | rs2857476 | INTRON | SNV | MODIFIER | 0.8 | 0 | <0.01 |
| MUC5B | rs77287508 | NON_SYN | SNV | MODERATE | 0.5 | 0.02 | <0.01 |
| MUC17 | rs6966570 | INTRON | SNV | MODIFIER | 0.4 | 0 | <0.01 |
| MUC17 | rs10246021 | INTRON | SNV | MODIFIER | 0.5 | 0 | <0.01 |
| MUC2 | rs12416873 | INTRON | SNV | MODIFIER | 0.6 | 0 | <0.01 |
| MUC4 | rs13095016 | NON_SYN | SNV | MODERATE | 0.3 | 0 | <0.01 |
| MUC4 | rs2259419 | INTRON | SNV | MODIFIER | 0.3 | 0 | <0.01 |
| MUC4 | rs729593 | NON_SYN | SNV | MODERATE | 0.5 | 0 | <0.01 |
| MUC5AC | rs1132434 | NON_SYN | SNV | MODERATE | 0.6 | 0 | <0.01 |
| MUC21 | rs2517418 | INTRON | SNV | MODIFIER | 0.3 | 0 | <0.01 |
| MUC16 | rs3764552 | INTRON | SNV | MODIFIER | 0.3 | 0 | <0.01 |
| MUC16 | rs2547065 | NON_SYN | SNV | MODERATE | 0.3 | 0.85 | <0.01 |
| MUC16 | rs2591592 | NON_SYN | SNV | MODERATE | 0.6 | 0.14 | <0.05 |
| MUC16 | rs10422567 | NEXT-PROT | SNV | MODERATE | 0.7 | 0.97 | <0.05 |
| MUC16 | rs1559172 | NON_SYN | SNV | MODERATE | 0.7 | 0.96 | <0.05 |
| MUC | Chro | Position | Region | Region Detailed | Var Type | Impact | Refer | Variant | Freq |
|---|---|---|---|---|---|---|---|---|---|
| MUC17 | chr7 | 101036821 | CDS | FRAME_SHIFT | INDEL | HIGH | CT | C | 0.018 |
| MUC16 | chr19 | 8949879 | CDS | FRAME_SHIFT | INDEL | HIGH | TTGGA | T | 0.009 |
| MUC5B | chr11 | 1258072 | intronic | INTRON | SNV | MODIFIER | C | A | 0.036 |
| MUC5B | chr11 | 1260321 | intronic | INTRON | SNV | MODIFIER | G | C | 0.042 |
| MUC16 | chr19 | 8949763 | CDS | NON_SYNONYMOUS | SNV | MODERATE | T | G | 0.009 |
| MUC16 | chr19 | 8952957 | CDS | NON_SYNONYMOUS | SNV | MODERATE | G | T | 0.009 |
| MUC21 | chr6 | 30987157 | CDS | CODON_CHANGE + CODON_INSERTION | INDEL | MODERATE | A | AGCA | 0.009 |
| MUC5AC | chr11 | 1168745 | CDS | NON_SYNONYMOUS | SNV | MODERATE | G | C | 0.009 |
| MUC16 | chr19 | 8956261 | CDS | NON_SYNONYMOUS | SNV | MODERATE | G | C | 0.019 |
| MUC19 | chr12 | 40429590 | CDS | SYNONYMOUS_CODING | SNV | LOW | A | G | 0.009 |
| MUC6 | chr11 | 1032052 | OTHER | SPLICE_SITE + SYNONYMOUS | SNV | LOW | G | A | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcântara, A.L.d.; Pastana, L.F.; Gellen, L.P.A.; Vieira, G.M.; Dobbin, E.A.F.; Silva, T.A.; Pereira, E.E.B.; Rodrigues, J.C.G.; Guerreiro, J.F.; Fernandes, M.R.; et al. Mucin (MUC) Family Influence on Acute Lymphoblastic Leukemia in Cancer and Non-Cancer Native American Populations from the Brazilian Amazon. J. Pers. Med. 2022, 12, 2053. https://doi.org/10.3390/jpm12122053
Alcântara ALd, Pastana LF, Gellen LPA, Vieira GM, Dobbin EAF, Silva TA, Pereira EEB, Rodrigues JCG, Guerreiro JF, Fernandes MR, et al. Mucin (MUC) Family Influence on Acute Lymphoblastic Leukemia in Cancer and Non-Cancer Native American Populations from the Brazilian Amazon. Journal of Personalized Medicine. 2022; 12(12):2053. https://doi.org/10.3390/jpm12122053
Chicago/Turabian StyleAlcântara, Angélica Leite de, Lucas Favacho Pastana, Laura Patrícia Albarello Gellen, Giovana Miranda Vieira, Elizabeth Ayres Fragoso Dobbin, Thays Amâncio Silva, Esdras Edgar Batista Pereira, Juliana Carla Gomes Rodrigues, João Farias Guerreiro, Marianne Rodrigues Fernandes, and et al. 2022. "Mucin (MUC) Family Influence on Acute Lymphoblastic Leukemia in Cancer and Non-Cancer Native American Populations from the Brazilian Amazon" Journal of Personalized Medicine 12, no. 12: 2053. https://doi.org/10.3390/jpm12122053
APA StyleAlcântara, A. L. d., Pastana, L. F., Gellen, L. P. A., Vieira, G. M., Dobbin, E. A. F., Silva, T. A., Pereira, E. E. B., Rodrigues, J. C. G., Guerreiro, J. F., Fernandes, M. R., Assumpção, P. P. d., Cohen-Paes, A. d. N., Santos, S. E. B. D., & Santos, N. P. C. d. (2022). Mucin (MUC) Family Influence on Acute Lymphoblastic Leukemia in Cancer and Non-Cancer Native American Populations from the Brazilian Amazon. Journal of Personalized Medicine, 12(12), 2053. https://doi.org/10.3390/jpm12122053








