Diversity of Circulating NKT Cells in Defense against Carbapenem-Resistant Klebsiella Pneumoniae Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples Collection
2.2. Single-Cell RNA Sequencing
2.3. Single-Cell Data Processing
2.4. RNA Velocity Analyses
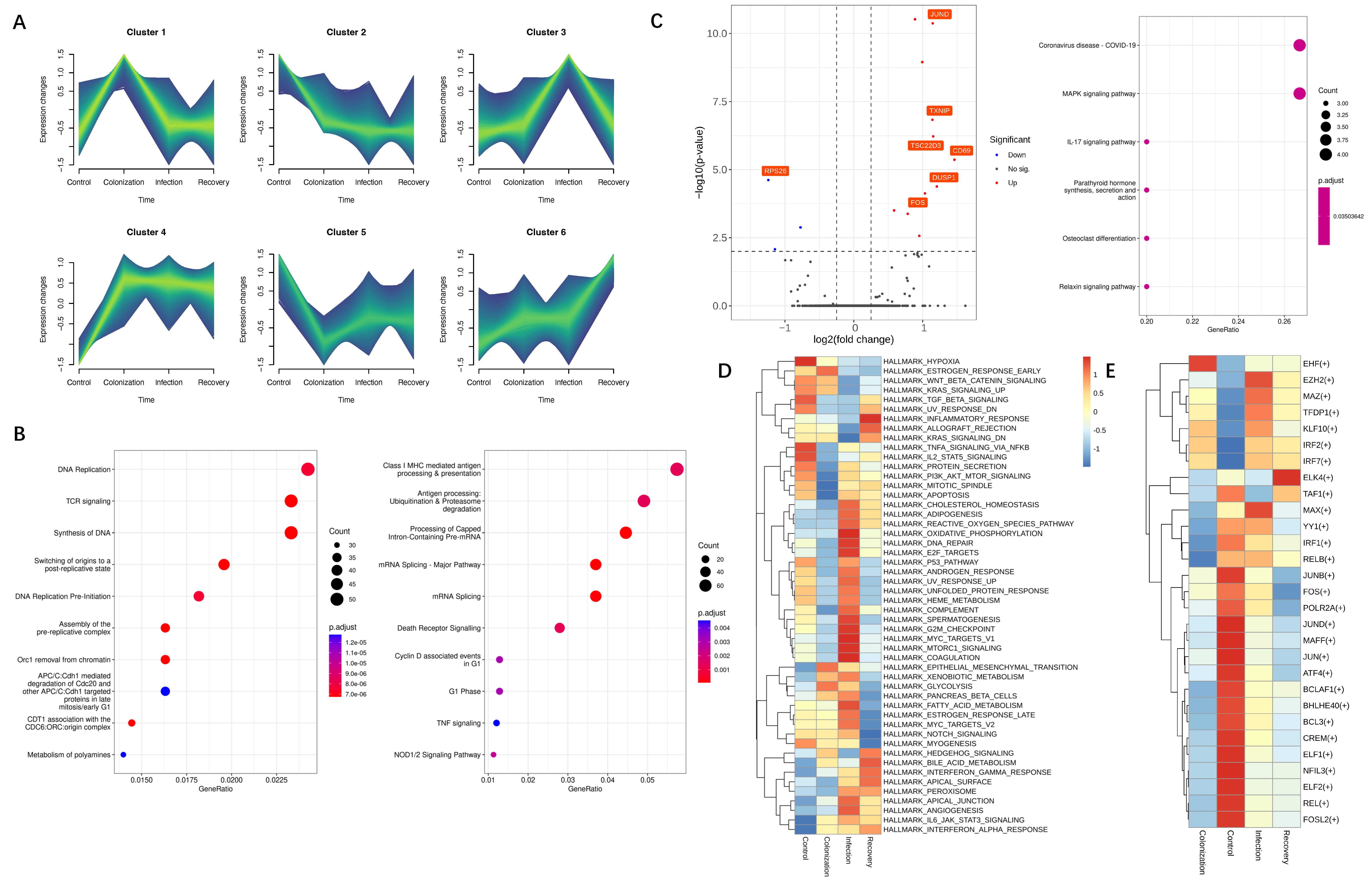
2.5. Time Dependent Transcriptional Analysis
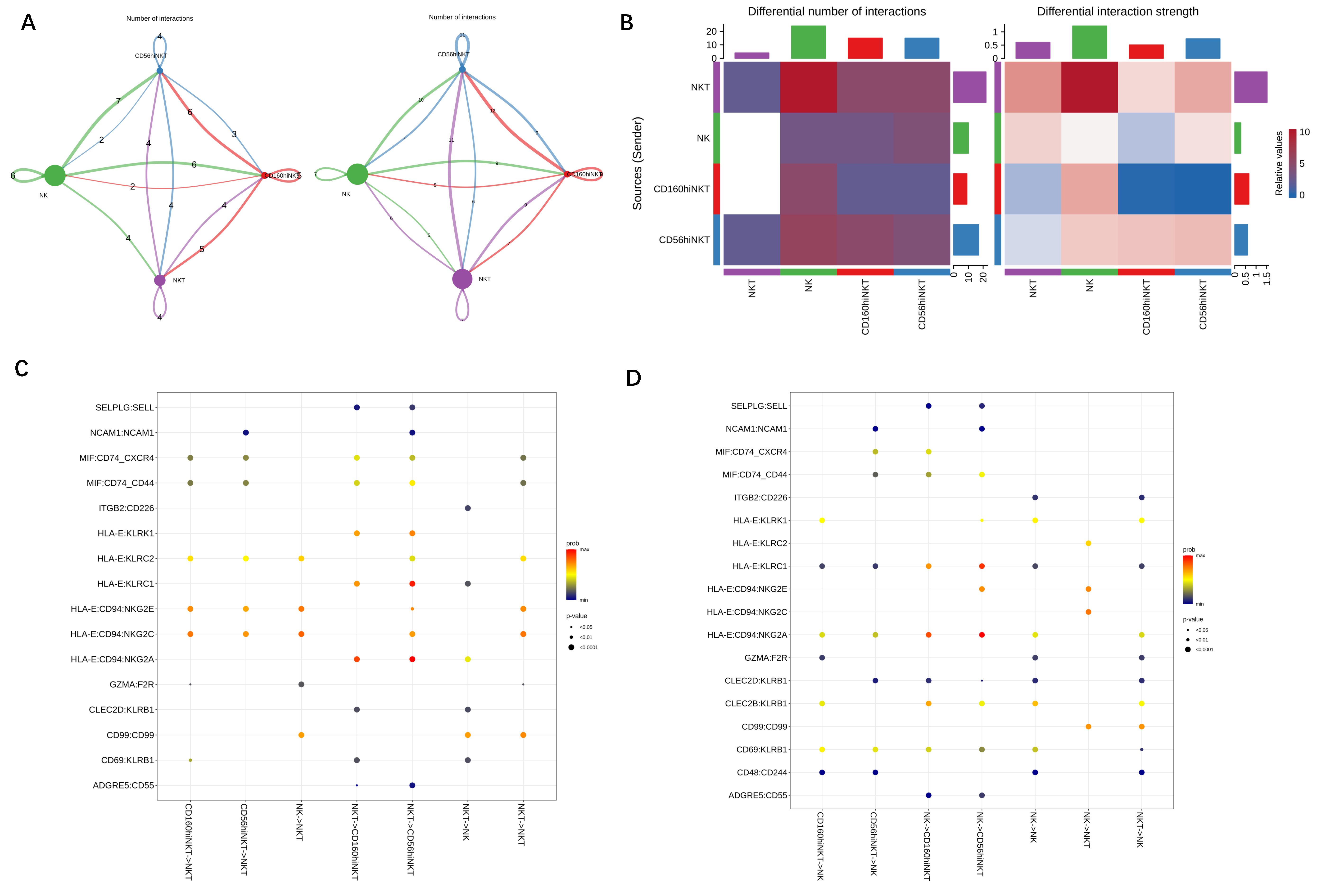
2.6. Cell–Cell Communication Analysis
2.7. Statistical Analysis
3. Results
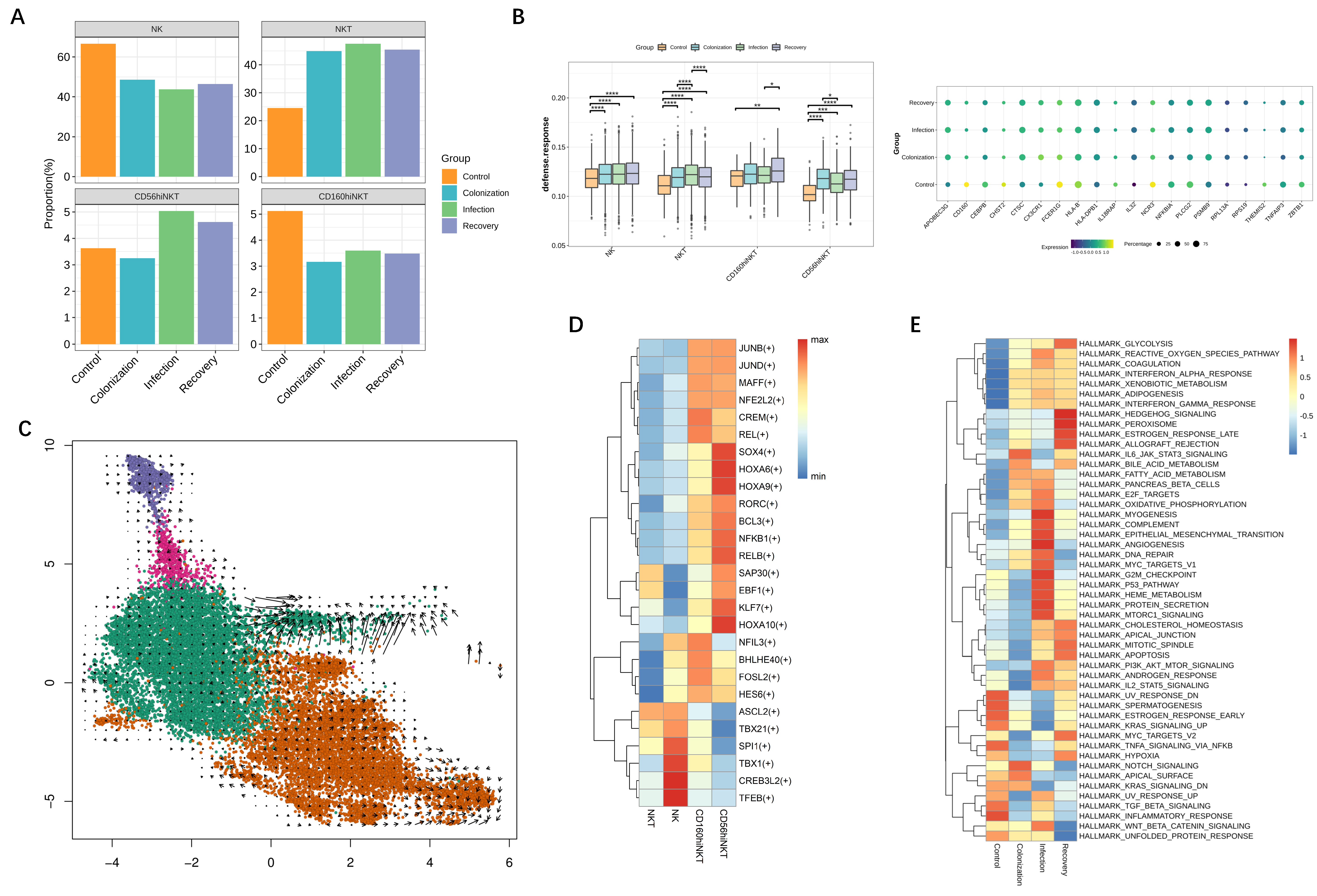
3.1. NK and NKT Subset Distribution in Patients during CRKP Infection
3.2. Transcriptional Alterations of NK and NKT Subsets during CRKP Infection
3.3. The Regulatory Effect of NKT on NK Function during CRKP Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, F.; Zhu, D.; Wang, F.; Wang, M. Current Status and Trends of Antibacterial Resistance in China. Clin. Infect. Dis. 2018, 67, S128–S134. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Wu, S.; Hao, M.; Zhu, J.; Ding, B.; Yang, Y.; Xu, X.; Wang, M.; Yang, F.; Hu, F. The Colonization of Carbapenem-Resistant Klebsiella pneumoniae: Epidemiology, Resistance Mechanisms, and Risk Factors in Patients Admitted to Intensive Care Units in China. J. Infect. Dis. 2020, 221, S206–S214. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Iosifidis, E.; Antachopoulos, C.; Karapanagiotou, A.; Karyoti, A.; Gritsi-Gerogianni, N.; Tsakris, A.; Roilides, E. Impact of active surveillance and infection control measures on carbapenem-resistant Gram-negative bacterial colonization and infections in intensive care. J. Hosp. Infect. 2018, 99, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Geladari, A.; Simitsopoulou, M.; Antachopoulos, C.; Roilides, E. Immunomodulatory effects of colistin on host responses against carbapenem-resistant Klebsiella pneumoniae biofilms. Int. J. Antimicrob. Agents 2020, 56, 106182. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Bartoletti, M.; Morelli, M.C.; Tedeschi, S.; Cristini, F.; Tumietto, F.; Pasqualini, E.; Danese, I.; Campoli, C.; Lauria, N.D.; et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: The importance of pre- and posttransplant colonization. Am. J. Transpl. 2015, 15, 1708–1715. [Google Scholar] [CrossRef]
- Giannella, M.; Trecarichi, E.M.; De Rosa, F.G.; Del Bono, V.; Bassetti, M.; Lewis, R.E.; Losito, A.R.; Corcione, S.; Saffioti, C.; Bartoletti, M.; et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin. Microbiol. Infect. 2014, 20, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, N.; Sandquist, I.; Wanek, A.; McCombs, J.; Song, K.; Kolls, J.K. Host immunology and rational immunotherapy for carbapenem-resistant Klebsiella pneumoniae infection. JCI Insight 2020, 5, e135591. [Google Scholar] [CrossRef]
- Vogt, S.; Mattner, J. NKT Cells Contribute to the Control of Microbial Infections. Front. Cell Infect. Microbiol. 2021, 11, 718350. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.; Yap, H.; Ng, M.L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef]
- Kim, E.Y.; Ner-Gaon, H.; Varon, J.; Cullen, A.M.; Guo, J.; Choi, J.; Barragan-Bradford, D.; Higuera, A.; Pinilla-Vera, M.; Short, S.A.; et al. Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-γ in NK cells. J. Clin. Investig. 2020, 130, 3238–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, J.; Cochain, C.; Zernecke, A.; Ley, K. Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-Seq. Cardiovasc. Res. 2021, 117, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Rosati, E.; Martini, G.R.; Pogorelyy, M.V.; Minervina, A.A.; Degenhardt, F.; Wendorff, M.; Sari, S.; Mayr, G.; Fazio, A.; Dowds, C.M.; et al. A novel unconventional T cell population enriched in Crohn’s disease. Gut 2022, 71, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Gribov, A.; Sill, M.; Lück, S.; Rücker, F.; Döhner, K.; Bullinger, L.; Benner, A.; Unwin, A. SEURAT: Visual analytics for the integrated analysis of microarray data. BMC Med. Genom. 2010, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolock, S.L.; Lopez, R.; Klein, A.M. Scrublet: Computational Identification of Cell Doublets in Single-Cell Transcriptomic Data. Cell Syst. 2019, 8, 281–291.e289. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Van de Sande, B.; Flerin, C.; Davie, K.; De Waegeneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saelens, W.; Cannoodt, R.; Rouchon, Q.; et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020, 15, 2247–2276. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lönnerberg, P.; Furlan, A.; et al. RNA velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef]
- Futschik, M.E.; Carlisle, B. Noise-robust soft clustering of gene expression time-course data. J. Bioinform. Comput. Biol. 2005, 3, 965–988. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.C.A.; Pallis, P.; Bielecki, P.; Low, J.S.; Zhao, J.; Harman, C.C.D.; Kroehling, L.; Jackson, R.; Bailis, W.; Licona-Limón, P.; et al. Effector T(H)17 Cells Give Rise to Long-Lived T(RM) Cells that Are Essential for an Immediate Response against Bacterial Infection. Cell 2019, 178, 1176–1188.e1115. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Goldstein, E.J.; Wise, J.; Bilker, W.B.; Tolomeo, P.; Lautenbach, E. Epidemiology of Carbapenem-Resistant Klebsiella pneumoniae in a Network of Long-Term Acute Care Hospitals. Clin. Infect. Dis. 2017, 64, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; He, L.; Miao, J.; Yang, W.; Wang, X.; Ma, J.; Wu, N.; Cao, Y.; Wang, L.; Lu, G.; et al. Actively surveillance and appropriate patients placements’ contact isolation dramatically decreased Carbapenem-Resistant Enterobacteriaceae infection and colonization in pediatric patients in China. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Kritsotakis, E.I.; Tsioutis, C.; Roumbelaki, M.; Christidou, A.; Gikas, A. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-{beta}-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: Results of a double case-control study. J. Antimicrob. Chemother. 2011, 66, 1383–1391. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Bergen, P.J.; Rao, G.G.; Nation, R.L.; Landersdorfer, C.B. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents 2020, 55, 105833. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef]
- Constantinides, M.G. Interactions between the microbiota and innate and innate-like lymphocytes. J. Leukoc. Biol. 2018, 103, 409–419. [Google Scholar] [CrossRef]
- Scorpio, D.G.; Choi, K.S.; Dumler, J.S. Anaplasma phagocytophilum-Related Defects in CD8, NKT, and NK Lymphocyte Cytotoxicity. Front. Immunol. 2018, 9, 710. [Google Scholar] [CrossRef]
- Berzins, S.P.; Smyth, M.J.; Baxter, A.G. Presumed guilty: Natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Burugupalli, S.; Almeida, C.F.; Smith DG, M.; Shah, S.; Patel, O.; Rossjohn, J.; Uldrich, A.P.; Godfrey, D.I.; Williams, S.J. α-Glucuronosyl and α-glucosyl diacylglycerides, natural killer T cell-activating lipids from bacteria and fungi. Chem. Sci. 2020, 11, 2161–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lalani, S.; Parekh, V.V.; Vincent, T.L.; Wu, L.; Van Kaer, L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J. Clin. Investig. 2008, 118, 2301–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wei, C.; Xu, H.; Jia, J.; Wei, Z.; Guo, R.; Jia, Y.; Wu, Y.; Li, Y.; Qi, X.; et al. The Immunoregulation of Th17 in Host against Intracellular Bacterial Infection. Mediators Inflamm. 2018, 2018, 6587296. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022. [Google Scholar] [CrossRef]
- Zheng, X.; Qian, Y.; Fu, B.; Jiao, D.; Jiang, Y.; Chen, P.; Shen, Y.; Zhang, H.; Sun, R.; Tian, Z.; et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019, 20, 1656–1667. [Google Scholar] [CrossRef]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Zhang, Y.; Solinas, A.; Cairo, S.; Evert, M.; Chen, X.; Calvisi, D.F. Molecular Mechanisms of Hepatoblastoma. Semin. Liver Dis. 2021, 41, 28–41. [Google Scholar] [CrossRef]
- Bou-Tayeh, B.; Laletin, V.; Salem, N.; Just-Landi, S.; Fares, J.; Leblanc, R.; Balzano, M.; Kerdiles, Y.M.; Bidaut, G.; Hérault, O.; et al. Chronic IL-15 Stimulation and Impaired mTOR Signaling and Metabolism in Natural Killer Cells During Acute Myeloid Leukemia. Front. Immunol. 2021, 12, 730970. [Google Scholar] [CrossRef]
- Oricchio, E.; Katanayeva, N.; Donaldson, M.C.; Sungalee, S.; Pasion, J.P.; Béguelin, W.; Battistello, E.; Sanghvi, V.R.; Jiang, M.; Jiang, Y.; et al. Genetic and epigenetic inactivation of SESTRIN1 controls mTORC1 and response to EZH2 inhibition in follicular lymphoma. Sci. Transl. Med. 2017, 9, eaak9969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, J.; Liew, C.S.; Riethoven, J.M.; Sillman, S.; Vu, H.L.X. Porcine Reproductive and Respiratory Syndrome Virus Infection Upregulates Negative Immune Regulators and T-Cell Exhaustion Markers. J. Virol. 2021, 95, e0105221. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zang, F.; Ye, X.; Li, Z.; Zhu, W.; Cao, X.; Cai, X.; Ma, X.; Xu, L.; Zhang, Y.; et al. Diversity of Circulating NKT Cells in Defense against Carbapenem-Resistant Klebsiella Pneumoniae Infection. J. Pers. Med. 2022, 12, 2025. https://doi.org/10.3390/jpm12122025
Wang Y, Zang F, Ye X, Li Z, Zhu W, Cao X, Cai X, Ma X, Xu L, Zhang Y, et al. Diversity of Circulating NKT Cells in Defense against Carbapenem-Resistant Klebsiella Pneumoniae Infection. Journal of Personalized Medicine. 2022; 12(12):2025. https://doi.org/10.3390/jpm12122025
Chicago/Turabian StyleWang, Yidi, Feng Zang, Xiangyu Ye, Zhanjie Li, Wenhao Zhu, Xiaoxiao Cao, Xuehong Cai, Xinyan Ma, Lei Xu, Yongxiang Zhang, and et al. 2022. "Diversity of Circulating NKT Cells in Defense against Carbapenem-Resistant Klebsiella Pneumoniae Infection" Journal of Personalized Medicine 12, no. 12: 2025. https://doi.org/10.3390/jpm12122025
APA StyleWang, Y., Zang, F., Ye, X., Li, Z., Zhu, W., Cao, X., Cai, X., Ma, X., Xu, L., Zhang, Y., Bi, L., Yu, R., & Huang, P. (2022). Diversity of Circulating NKT Cells in Defense against Carbapenem-Resistant Klebsiella Pneumoniae Infection. Journal of Personalized Medicine, 12(12), 2025. https://doi.org/10.3390/jpm12122025






