Greek Guidelines for the Management of COPD, a Proposal of a Holistic Approach Based on the needs of the Greek Community
Abstract
1. Introduction
2. Methodology
3. Risk Factors
4. Diagnosis
- Dyspnea (progressively deteriorating, persisting, worsening with fatigue);
- Chronic cough;
- Increased sputum production.
- History of exposure to smoking (commonly > 10 pack-years);
- History of exposure to environmental or occupational air pollutants, smoke, dust, and chemicals;
- Frequent respiratory tract infections;
- Family history of COPD.
5. Diagnostic Tests
5.1. Spirometry
5.2. Other Tests and Examinations
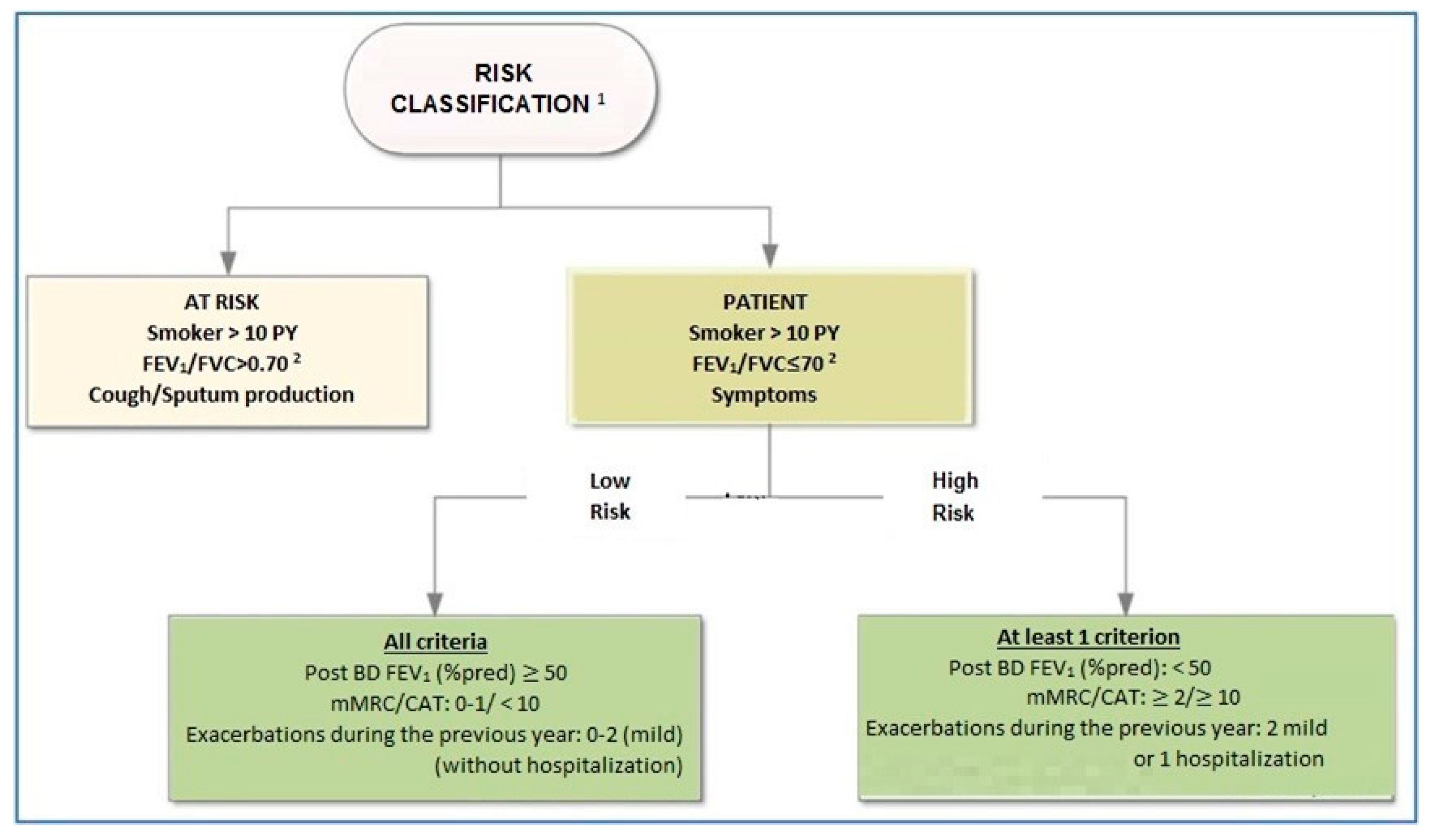
6. Classification of Severity and Assessment of Risk
7. Special Diagnostic Considerations
7.1. Diagnostic Difficulty
- In borderline FEV1/FVC or LLN.
- In technical inability to perform spirometry, i.e., tracheostomy.
- In concomitant diseases that may influence critical spirometric values, e.g., congestive heart disease or kyphoscoliosis.
7.2. Coexistence of Asthma and COPD
8. Classification of Disease Outcome Risk
9. Treatment of COPD
- Decelerate disease progression;
- Symptom control;
- Improve exercise tolerance;
- Improve QoL;
- Prevent disease complications;
- Prevent/treat exacerbations;
- Reduce mortality.
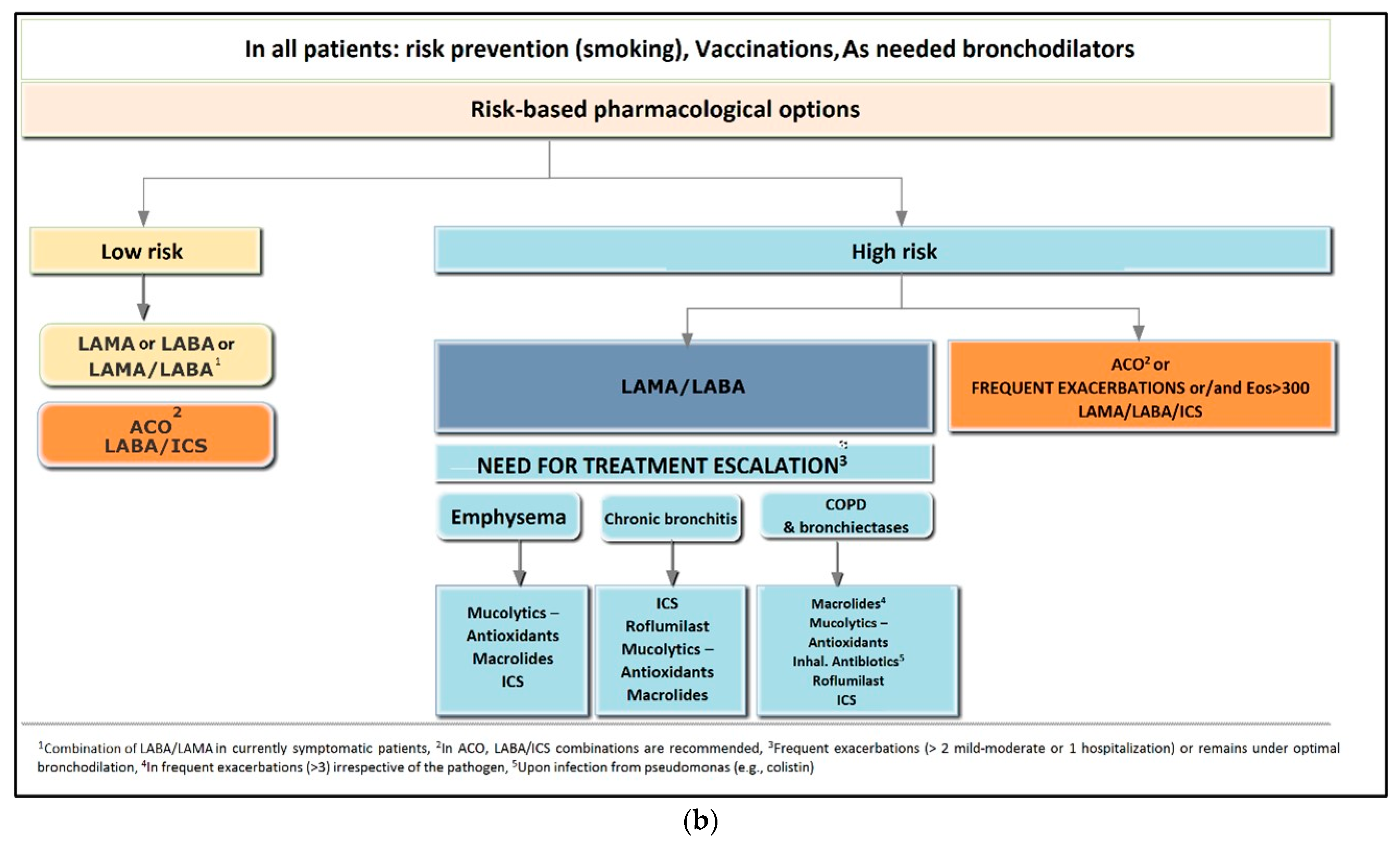
9.1. Treatment of Stable COPD
9.2. Pharmacological Treatment of Low-Risk Patients
9.3. Treatment of High-Risk Patients
10. Vaccinations
11. Precautions
- (A)
- To protect themselves and their patients;
- (B)
- To limit the transmission of influenza in healthcare and social care facilities;
- (C)
- To protect individuals who may have had a reduced immune response to their vaccinations.
12. Smoking Cessation in COPD
Pharmacological Treatment for Smoking Cessation
- Nicotine replacement therapy is available in various forms (patches, sublingual tablets, gums, spray, inhaler) and dosing regimens.
- Varenicline.
- Bupropion.
13. Pulmonary Rehabilitation
14. Diagnosis and Treatment of COPD Exacerbation
14.1. Definition
14.2. Classification of COPD Exacerbations
- A.
- Mild: Exacerbations requiring an increase in the use of bronchodilators for <2 days outside the hospital.
- B.
- Moderate: Exacerbations requiring administration of antibiotics with or without oral corticosteroids.
- C.
- Severe: Exacerbations requiring hospitalization.
14.3. Assessment of COPD Exacerbation
- Symptoms severity (dyspnea, confusion, etc.).
- Clinical signs of COPD and/or comorbidities.
- Oxygen saturation.
- Comorbidities (cardiovascular diseases, diabetes mellitus, chronic kidney disease, etc.).
- Respiratory function and symptoms (cough, dyspnea, etc.) compared to the patient’s stable state.
- History of previous exacerbations and their treatment.
- History of hospitalizations for exacerbations.
- Patient’s current stable treatment.
14.4. Cause of Exacerbations
14.5. Indications for Referral to the Hospital
- Serious symptom deterioration: sudden development of resting dyspnea, tachypnoea, confusion, etc.
- Appearance of new physical signs (e.g., cyanosis, peripheral edema).
- Oxygen desaturation with SaO2 < 90% in patients not previously on oxygen therapy at home or worsening of pre-existing respiratory failure.
- Presence of serious comorbidities (e.g., congestive heart failure, chronic kidney disease).
- Inability to identify the cause of the exacerbation.
- Failure of an exacerbation to respond to initial management.
- Insufficient home care.
14.6. Indications for Hospitalization
- Presence of severe signs/symptoms (resting dyspnea, tachypnoea, labored breathing, confusion), despite initial management of the exacerbation in the ED.
- Persistent respiratory failure requiring high oxygen mixtures and/or severe/worsening respiratory acidosis requiring non-invasive mechanical ventilation (NIV).
- Hemodynamic instability.
- Appearance of new physical signs (e.g., cyanosis, peripheral edema).
- Presence of serious comorbidities (e.g., heart failure, arrhythmia).
- Insufficient home care.
14.7. Indications for HDU/ICU Admission
- Persistent or worsening respiratory failure and/or severe/worsening respiratory acidosis (pH < 7.35), despite oxygen therapy and NIV.
- Change in mental status: confusion, coma.
- Need for invasive mechanical ventilation.
- Hemodynamic instability, requirement of vasoconstrictors.
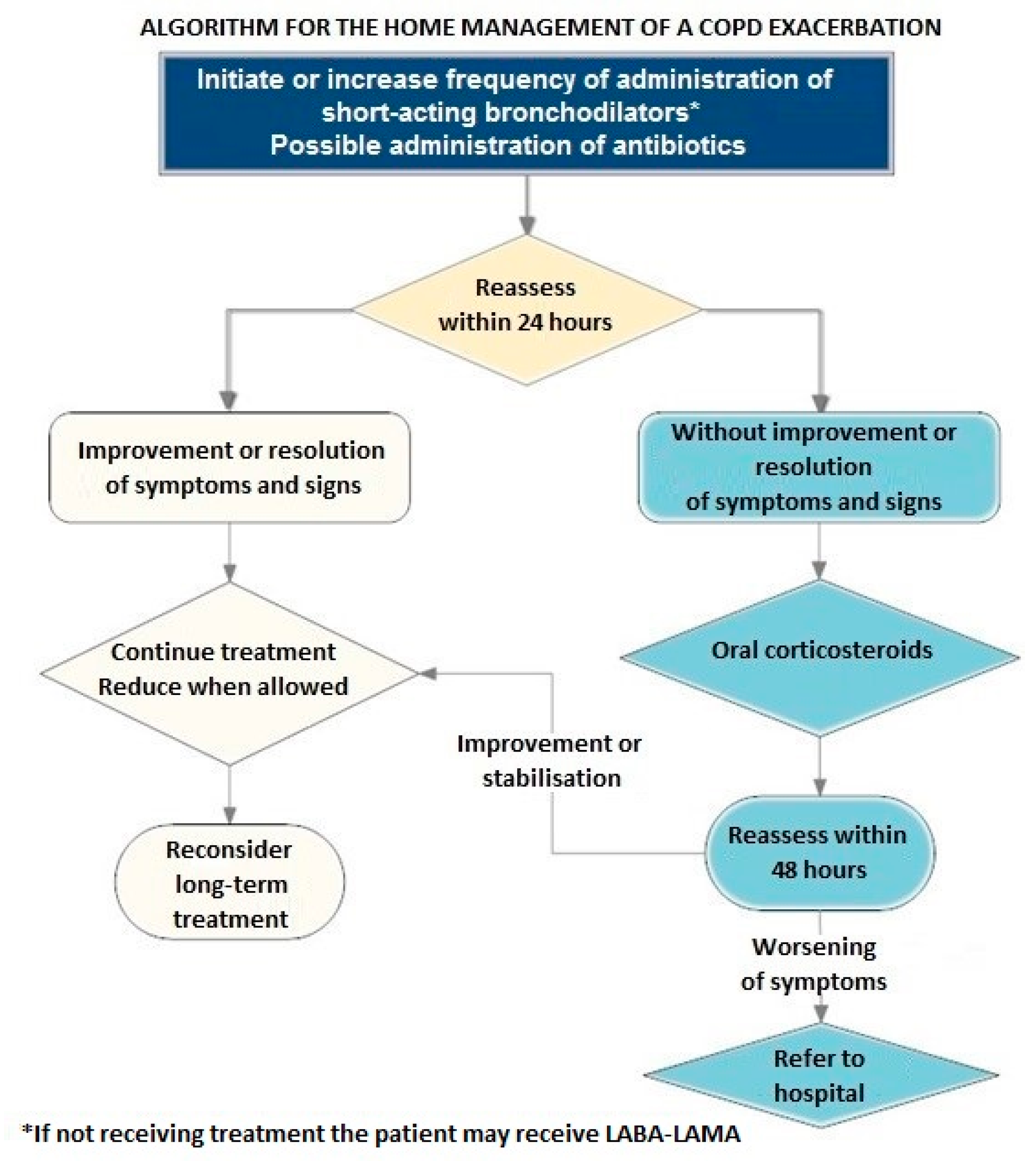
14.8. At-Home and In-Hospital Treatment of an Exacerbation
14.9. Steps in the Treatment of a COPD Exacerbation
14.9.1. A: Antibiotics
14.9.2. B: Bronchodilators
14.9.3. C: Glucocorticosteroids
14.9.4. D: Other Supportive Measures
- Both at home or in the hospital, early mobilization and sufficient management and treatment of concomitant diseases should be addressed.
- In hospitalized patients, the following should be considered:
- In those with respiratory failure, oxygen therapy should be administered, and ABG should be regularly monitored.
- Careful balance of fluids.
- Low-molecular-weight heparin should be administered in prophylactic doses (excluding patients treated with anticoagulants for other reasons, e.g., atrial fibrillation who should receive a full dose).
- Finally, the differential diagnosis and recognition of potential concomitant diseases that mimic exacerbation (e.g., pulmonary embolism, pneumothorax, pneumonia, etc.) are required.
14.9.5. E: Patient Education
14.9.6. Additional Management
14.9.7. Oxygen Therapy
14.9.8. Non-Invasive Mechanical Ventilation
14.9.9. Indications for NIV
- Respiratory acidosis (PCO2 > 45 mmHg and arterial blood pH ≤ 7.35).
- Severe dyspnea with clinical signs suggestive of respiratory muscle fatigue, increased work of breathing, or both, such as the use of respiratory accessory muscles, paradoxical motion of the abdomen, or retraction of the intercostal spaces.
- Persistent hypoxemia despite high-concentration oxygen therapy.
- Ιn cases in which NIV fails or is contraindicated, intubation and invasive ventilation should take place.
14.9.10. High-Flow Nasal Canula
14.9.11. Laboratory Testing and Imaging
- 1.
- Complete blood count;
- 2.
- Biochemistry;
- 3.
- Chest X-ray;
- 4.
- Quantitative CRP;
- 5.
- Complete blood count;
- 6.
- Biochemistry;
- 7.
- Quantitative CRP;
- 8.
- Procalcitonin
- 9.
- D-dimers (only when pulmonary embolism is suspected);
- 10.
- Troponin and NT-pro BNP (if the deterioration of congestive heart failure and/or suspicion of a coronary event must be assessed)
- 11.
- ECG;
- 12.
- Echocardiogram, if indicated;
- 13.
- Sputum culture for common pathogens before the initiation of antibiotics;
- 14.
- Chest X-ray (F&P)
- 15.
- Chest CT or other imaging modality, e.g., CTPA, only if indicated.
15. Oxygen Therapy at Home (Long-Term Oxygen Therapy)
- In patients with hypoxemia during sleep, LTOT is not currently recommended, as it does not seem to improve mortality [84].
- Patients with hypoxemia during physical activity may benefit from the use of portable oxygen delivery during activity if they qualify for LTOT at rest. Portable oxygen delivery in these patients appears to increase compliance and works towards achieving the target of ≥15 h/day.
- Oxygen delivery flow is titrated as follows:
- Initiation of LTOT and flow titration in chronic hypercapnic patients should be performed under close monitoring and with measurements of ABG performed after any change in the oxygen flow, including final titration [83].
16. Non-Invasive Ventilation in Stable COPD
- Daytime hypercapnia with PaCO2 ≥ 50 mmHg (absence of acute exacerbations for ≥3 weeks).
- Nocturnal hypercapnia with PaCO2 ≥ 55 mmHg (absence of acute exacerbations for ≥3 weeks).
- Ideally, titration should be performed in a hospital setting and, in any case, under strict medical supervision.
- Initial titration for daily use.
- Initially low EPAP (3–4 cmH2O) and IPAP (14–16 cmH2O) with a low backup respiratory rate.
- Gradual IPAP increase to the maximum tolerated level (usually ~30 cmH2O, but this value may differ from patient to patient; range: 20–40 cmH2O).
- Respiratory rate should increase up to the patient’s stable respiratory rate.
- Perform a slight increase of EPAP at 4–6 cmH2O, to avoid hyperinflation.
- Higher EPAP values are necessary in cases of concomitant obstructive sleep apnea (OSA).
- The application of NIV at night should only be started after a period of adjustment for the patient to get used to its daytime use.
- During this period, changes in the settings may be necessary to maximize patient comfort and compliance.
- In case of poor tolerance to nighttime use or suspicion of concomitant OSA, polysomnography may be required for the titration of pressures.
17. Surgical and Bronchoscopic Treatment of Severe Pulmonary Emphysema
- A.
- Reversible airway obstruction techniques leading to target segments (placement of one-way valves at the segment or lobe level) given that there is no collateral ventilation and the fissure between the lobes is complete.
- B.
- Irreversible techniques directly reducing the parenchyma of the target lobe (coil placement, vapor ablation in the case of incomplete fissure and the presence of collateral ventilation. Adequate representation of lung parenchyma is required for the use of endobronchial coils.
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABG | Arterial blood gases |
| ED | Emergency department |
| HDU | High dependency unit |
| ICU | Intensive care unit |
| LABA | Long-acting beta2-agonists |
| LAMA | Long-acting muscarinic agonists |
| LLN | Lower limits of normal |
| LTOT | Long-term oxygen therapy |
| NIV | Non-invasive mechanical ventilation |
| OSA | Obstructive sleep apnea |
| PCV13 | 13-valent pneumococcal conjugate vaccine 2 |
| PPSV23 | 3-valent polysaccharide vaccine |
| QIV | Quadrivalent influenza vaccine |
| QoL | Quality of life |
| SPT | Skin prick test |
| TIV | Trivalent influenza vaccine |
References
- Buist, A.S.; Vollmer, W.M.; McBurnie, M.A. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int. J. Tuberc. Lung Dis. 2008, 12, 703–708. [Google Scholar] [PubMed]
- Tzanakis, N.; Anagnostopoulou, U.; Filaditaki, V.; Christaki, P.; Siafakas, N.; COPD group of the Hellenic Thoracic Society. Prevalence of COPD in Greece. Chest 2004, 125, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Kourlaba, G.; Hillas, G.; Vassilakopoulos, T.; Maniadakis, N. The disease burden of chronic obstructive pulmonary disease in Greece. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2179–2189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Souliotis, K.; Kousoulakou, H.; Hillas, G.; Tzanakis, N.; Toumbis, M.; Vassilakopoulos, T. The direct and indirect costs of managing chronic obstructive pulmonary disease in Greece. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Stafyla, E.; Geitona, M.; Kerenidi, T.; Economou, A.; Daniil, Z.; Gourgoulianis, K.I. The annual direct costs of stable COPD in Greece. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 309–315. [Google Scholar] [CrossRef]
- Gunen, H.; Kilinc, O.; Polatli, M.; Suerdem, M.; Uzaslan, E. Modification of the GOLD recommendations for chronic obstructive pulmonary disease to broaden their usage in Turkey. Expert Rev. Respir. Med. 2016, 10, 625–628. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Aisanov, Z.; Avdeev, S.; Arkhipov, V.; Belevskiy, A.; Chuchalin, A.; Leshchenko, I.; Ovcharenko, S.; Shmelev, E.; Miravitlles, M. Russian guidelines for the management of COPD: Algorithm of pharmacologic treatment. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 183–187. [Google Scholar] [CrossRef]
- Köktürk, N.; Gurgun, A.; Sen, E.; Kocabas, A.; Polatli, M.; Nayci, S.A.; Coplu, L.; Tellioglu, E.; Elmas, F.; Erdinc, E.; et al. The View of the Turkish Thoracic Society on the Report of the GOLD 2017 Global Strategy for the Diagnosis, Management, and Prevention of COPD. Turk. Thorac. J. 2017, 18, 57–64. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluña, J.J. GOLD in 2017: A View From the Spanish COPD Guidelines (GesCOPD). Arch Bronconeumol 2017, 53, 89–90. [Google Scholar] [CrossRef]
- Vukoja, M.; Kopitovic, I.; Lazic, Z.; Milenkovic, B.; Stankovic, I.; Zvezdin, B.; Ilic, A.D.; Cekerevac, I.; Vukcevic, M.; Zugic, V.; et al. Diagnosis and management of chronic obstructive pulmonary disease in Serbia: An expert group position statement. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, N.; Koulouris, N.; Dimakou, K.; Gourgoulianis, K.; Kosmas, E.; Chasapidou, G.; Konstantinidis, A.; Kyriakopoulos, C.; Kontakiotis, T.; Rapti, A.; et al. Classification of COPD patients and compliance to recommended treatment in Greece according to GOLD 2017 report: The RELICO study. BMC Pulm. Med. 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Tsiligianni, I.; Kampouraki, M.; Ierodiakonou, D.; Poulonirakis, I.; Papadokostakis, P. COPD patients’ characteristics, usual care, and adherence to guidelines: The Greek UNLOCK study. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Rachiotis, G.; Barbouni, A.; Katsioulis, A.; Antoniadou, E.; Kostikas, K.; Merakou, K.; Kourea, K.; Khoury, R.N.; Tsouros, A.; Kremastinou, J.; et al. Prevalence and determinants of current and secondhand smoking in Greece: Results from the Global Adult Tobacco Survey (GATS) study. BMJ Open 2017, 7, e013150. [Google Scholar] [CrossRef] [PubMed]
- Sichletidis, L.; Spyratos, D.; Tsiotsios, A.; Haidich, A.-B.; Chloros, D.; Ganidis, I.; Michailidis, D.; Triantafyllou, G.; Kottakis, G.; Melas, D. Exposure to PM10 as a risk factor for the development of nasal obstruction and chronic obstructive pulmonary disease. Int. J. Occup. Environ. Health 2014, 20, 9–15. [Google Scholar] [CrossRef][Green Version]
- Li, L.S.; Paquet, C.; Johnston, K.; Williams, M.T. “What are my chances of developing COPD if one of my parents has the disease?” A systematic review and meta-analysis of prevalence of co-occurrence of COPD diagnosis in parents and offspring. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 403–415. [Google Scholar] [CrossRef]
- Stoller, J.K.; Aboussouan, L.S. Alpha1-antitrypsin deficiency. Lancet 2005, 365, 2225–2236. [Google Scholar] [CrossRef]
- Beran, D.; Zar, H.J.; Perrin, C.; Menezes, A.M.; Burney, P. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir. Med. 2015, 3, 159–170. [Google Scholar] [CrossRef]
- GOLD. Global Strategy for Prevention, Diagnosis and Management of Copd: 2022 Report; Global Strategy for Prevention, Diagnosis and Management of Copd, Inc. 2022. Available online: https://goldcopd.org/.
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef]
- Knudson, R.J.; Slatin, R.C.; Lebowitz, M.D.; Burrows, B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am. Rev. Respir. Dis. 1976, 113, 587–600. [Google Scholar]
- Postma, D.S.; Rabe, K.F. The Asthma-COPD Overlap Syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Cusack, R.P.; Chaudhary, N.; Satia, I.; Kurmi, O.P. Under- and over-diagnosis of COPD: A global perspective. Breathe 2019, 15, 24–35. [Google Scholar] [CrossRef] [PubMed]
- GIfAG. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2019. 2020. Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed on 19 June 2020).
- GSFPDAMOC. Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report). 2020. Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed on 19 June 2020).
- Lange, P.; Colak, Y.; Ingebrigtsen, T.S.; Vestbo, J.; Marott, J.L. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: A prospective population-based analysis. Lancet Respir. Med. 2016, 4, 454–462. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Montes de Oca, M.; Perez-Padilla, R.; Nadeau, G.; Wehrmeister, F.C.; Lopez-Varela, M.V.; Muiño, A.; Jardim, J.R.B.; Valdivia, G.; Tálamo, C.; et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014, 145, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mindus, S.; Malinovschi, A.; Ekerljung, L.; Forsberg, B.; Gíslason, T.; Jõgi, R.; Franklin, K.A.; Holm, M.; Johannessen, A.; Middelveld, R.; et al. Asthma and COPD overlap (ACO) is related to a high burden of sleep disturbance and respiratory symptoms: Results from the RHINE and Swedish GA2LEN surveys. PLoS ONE 2018, 13, e0195055. [Google Scholar] [CrossRef]
- Samitas, K.; Zervas, E.; Gaga, M. T2-low asthma: Current approach to diagnosis and therapy. Curr. Opin. Pulm. Med. 2017, 23, 48–55. [Google Scholar] [CrossRef]
- Singh, D.; Kolsum, U.; Brightling, C.; Locantore, N.; Agusti, A.; Tal-Singer, R. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef]
- Siva, R.; Green, R.H.; Brightling, C.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef]
- Zhang, L.; He, L.; Gong, J.; Liu, C. Risk Factors Associated with Irreversible Airway Obstruction in Asthma: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 9868704. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Celli, B.; Decramer, M.; Liu, D.; Burkhart, D.; Cassino, C.; Kesten, S. Bronchodilator responsiveness in patients with COPD. Eur. Respir. J. 2008, 31, 742–750. [Google Scholar] [CrossRef]
- Alcázar-Navarrete, B.; Romero-Palacios, P.J.; Ruiz-Sancho, A.; Ruiz-Rodriguez, O. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide Biol. Chem. 2016, 54, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Lamprecht, B.; Ramírez, A.S.; Martinez-Camblor, P.; Kaiser, B.; Alfageme, I.; Almagro, P.; Casanova, C.; Esteban, C.; Soler-Cataluña, J.J.; et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: A pooled analysis of individual patient data. Lancet Respir. Med. 2015, 3, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Kazerooni, E.A.; Lynch, D.A.; Liu, L.X.; Murray, S.; Curtis, J.L.; Criner, G.J.; Kim, V.; Bowler, R.P.; Hanania, N.A.; et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: Associated radiologic phenotypes. Radiology 2011, 261, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kempsford, R.; Norris, V.; Siederer, S. Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. Pulm. Pharmacol. Ther. 2013, 26, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kew, K.M.; Mavergames, C.; Walters, J.A. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013, 10, CD010177. [Google Scholar] [CrossRef]
- Koch, A.; Pizzichini, E.; Hamilton, A.; Hart, L.; Korducki, L.; De Salvo, M.C.; Paggiaro, P. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat(R) versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: Results from two replicate 48-week studies. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 697–714. [Google Scholar] [CrossRef]
- van der Molen, T.; Cazzola, M. Beyond lung function in COPD management: Effectiveness of LABA/LAMA combination therapy on patient-centred outcomes. Prim. Care Respir. J. 2012, 21, 101–108. [Google Scholar] [CrossRef]
- Mackay, A.J.; Kostikas, K.; Roche, N.; Frent, S.-M.; Olsson, P.; Pfister, P.; Gupta, P.; Patalano, F.; Banerji, D.; Wedzicha, J.A. Impact of baseline symptoms and health status on COPD exacerbations in the FLAME study. Respir. Res. 2020, 21, 93. [Google Scholar] [CrossRef]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.-M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Poole, P.; Sathananthan, K.; Fortescue, R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 5, CD001287. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A.D., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.; Barnacle, H.; Birk, R.; Brealey, N.; Locantore, N.; Lomas, D.A.; Ludwig-Sengpiel, A.; Mohindra, R.; Tabberer, M.; Zhu, C.-Q.; et al. FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 438–446. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Papi, A.; Corradi, M.; Pavlisova, I.; Montagna, I.; Francisco, C.; Cohuet, G.; Vezzoli, S.; Scuri, M.; Vestbo, J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): A double-blind, parallel group, randomised controlled trial. Lancet 2016, 388, 963–973. [Google Scholar] [CrossRef]
- Harskamp, R.E.; van Ginkel, M.W. Acute respiratory tract infections: A potential trigger for the acute coronary syndrome. Ann. Med. 2008, 40, 121–128. [Google Scholar] [CrossRef]
- Garrastazu, R.; García-Rivero, J.L.; Ruiz, M.; Helguera, J.M.; Arenal, S.; Bonnardeux, C.; León, C.; Llorca, J.; Santibañez, M. Prevalence of Influenza Vaccination in Chronic Obstructive Pulmonary Disease Patients and Impact on the Risk of Severe Exacerbations. Arch. Bronconeumol. 2016, 52, 88–95. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Kopsaftis, Z.; Wood-Baker, R.; Poole, P. Influenza vaccine for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2018, 6, CD002733. [Google Scholar] [CrossRef]
- Poole, P.J.; Chacko, E.; Wood-Baker, R.W.; Cates, C.J. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2006, 1, CD002733. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.N.; Gurtman, A.; Frenck, R.W.; Strout, C.; Jansen, K.U.; Trammel, J.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60–64 years of age. Vaccine 2014, 32, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.A.; Tang, J.N.; Poole, P.; Wood-Baker, R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 1, CD001390. [Google Scholar] [CrossRef]
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2016, 3, CD008286. [Google Scholar] [CrossRef]
- Maciosek, M.V.; Coffield, A.B.; Edwards, N.M.; Flottemesch, T.J.; Goodman, M.J.; Solberg, L.I. Priorities among effective clinical preventive services: Results of a systematic review and analysis. Am. J. Prev. Med. 2006, 31, 52–61. [Google Scholar] [CrossRef]
- Fiore, M.; Bailey, W.; Cohen, S. Treating Tobacco Use and Dependence: 2008 Update; U.S. Department of Health and Human Services, Public Health Service: Rockville, MD, USA, 2008.
- Garvey, C.; Bayles, M.P.; Hamm, L.F.; Hill, K.; Holland, A.; Limberg, T.M.; Spruit, M.A. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: An official statement from the american association of cardiovascular and pulmonary rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 75–83. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Nesme-Meyer, P.; Chanez, P.; Caillaud, D.; Carré, P.; Perez, T.; Roche, N. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009, 135, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Barnes, P.J. Exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 29, 1224–1238. [Google Scholar] [CrossRef]
- Seemungal, T.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef]
- Vijayasaratha, K.; Stockley, R.A. Reported and unreported exacerbations of COPD: Analysis by diary cards. Chest 2008, 133, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.; Hershfield, E.S.; Harding, G.K.; Nelson, N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef]
- Quon, B.S.; Gan, W.Q.; Sin, D.D. Contemporary management of acute exacerbations of COPD: A systematic review and metaanalysis. Chest 2008, 133, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhang, S.M.; Li, X.H.; Zhang, Y.; Xu, Z.Y.; Cao, B. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: A prospective randomized controlled trial. Int. J. Infect. Dis. 2016, 48, 40–45. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, W.H.; Douma, W.R.; Slebos, D.J.; Kerstjens, H.A. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst. Rev. 2016, 8, CD011826. [Google Scholar] [CrossRef]
- Davies, L.; Angus, R.M.; Calverley, P.M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: A prospective randomised controlled trial. Lancet 1999, 354, 456–460. [Google Scholar] [CrossRef]
- Leuppi, J.D.; Schuetz, P.; Bingisser, R.; Bodmer, M.; Briel, M.; Drescher, T.; Duerring, U.; Henzen, C.; Leibbrandt, Y.; Maier, S.; et al. Short-term vs. conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The REDUCE randomized clinical trial. JAMA 2013, 309, 2223–2231. [Google Scholar] [CrossRef]
- Jennings, J.H.; Thavarajah, K.; Mendez, M.P.; Eichenhorn, M.; Kvale, P.; Yessayan, L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: A randomized controlled trial. Chest 2015, 147, 1227–1234. [Google Scholar] [CrossRef]
- McKeever, T.M.; Hearson, G.; Housley, G.; Reynolds, C.; Kinnear, W.; Harrison, T.W.; Kelly, A.-M.; Shaw, D.E. Using venous blood gas analysis in the assessment of COPD exacerbations: A prospective cohort study. Thorax 2016, 71, 210–215. [Google Scholar] [CrossRef]
- Osadnik, C.R.; Tee, V.S.; Carson-Chahhoud, K.V.; Picot, J.; Wedzicha, J.A.; Smith, B.J. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 7, CD004104. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Longhini, F.; Madotto, F.; Groff, P.; Scala, R.; Crimi, C.; Carlucci, A.; Bruni, A.; Garofalo, E.; Raineri, S.M.; et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: A multicenter non-inferiority randomized trial. Crit. Care 2020, 24, 692. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, S.; Ergan, B.; Bos, L.; Chatwin, M.; Ferrer, M.; Gregoretti, C.; Heunks, L.; Frat, J.-P.; Longhini, F.; Nava, S.; et al. ERS clinical practice guidelines: High-flow nasal cannula in acute respiratory failure. Eur. Respir. J. 2021, 59, 2101574. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.I.; Shafuddin, E.; King, P.T.; Chang, C.L.; Bardin, P.G.; Hancox, R.J. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir. Med. 2016, 4, 138–148. [Google Scholar] [CrossRef]
- Kostikas, K.; Bakakos, P.; Papiris, S.; Stolz, D.; Celli, B.R. Systemic biomarkers in the evaluation and management of COPD patients: Are we getting closer to clinical application? Curr. Drug Targets 2013, 14, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann. Intern. Med. 1980, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981, 1, 681–686. [Google Scholar]
- Ahmadi, Z.; Sundh, J.; Bornefalk-Hermansson, A.; Ekstrom, M. Long-Term Oxygen Therapy 24 vs. 15 h/day and Mortality in Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0163293. [Google Scholar] [CrossRef]
- Hardinge, M.; Annandale, J.; Bourne, S.; Cooper, B.; Evans, A.; Freeman, D.; Green, A.; Hippolyte, S.; Knowles, V.; MacNee, W.; et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015, 70, i1–i43. [Google Scholar] [CrossRef]
- Chaouat, A.; Weitzenblum, E.; Kessler, R.; Charpentier, C.; Enrhart, M.; Schott, R.; Levi-Valensi, P.; Zielinski, J.; Delaunois, L.; Cornudella, R.; et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur. Respir. J. 1999, 14, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, D.; Gorzelak, K.; Sliwinski, P.; Tobiasz, M.; Zielinski, J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax 1997, 52, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ringbaek, T.; Martinez, G.; Lange, P. The long-term effect of ambulatory oxygen in normoxaemic COPD patients: A randomised study. Chronic Respir. Dis. 2013, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chaney, J.C.; Jones, K.; Grathwohl, K.; Olivier, K.N. Implementation of an oxygen therapy clinic to manage users of long-term oxygen therapy. Chest 2002, 122, 1661–1667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guyatt, G.H.; Nonoyama, M.; Lacchetti, C.; Goeree, R.; McKim, D.; Heels-Ansdell, D.; Goldstein, R. A randomized trial of strategies for assessing eligibility for long-term domiciliary oxygen therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Levi-Valensi, P.; Weitzenblum, E.; Pedinielli, J.L.; Racineux, J.L.; Duwoos, H. Three-month follow-up of arterial blood gas determinations in candidates for long-term oxygen therapy. A multicentric study. Am. Rev. Respir. Dis. 1986, 133, 547–551. [Google Scholar] [PubMed]
- Carlin, B.W.; Clausen, J.L.; Ries, A.L. The use of cutaneous oximetry in the prescription of long-term oxygen therapy. Chest 1988, 94, 239–241. [Google Scholar] [CrossRef]
- Restrick, L.J.; Paul, E.A.; Braid, G.M.; Cullinan, P.; Moore-Gillon, J.; Wedzicha, J.A. Assessment and follow up of patients prescribed long term oxygen treatment. Thorax 1993, 48, 708–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calverley, P.M.; Leggett, R.J.; McElderry, L.; Flenley, D.C. Cigarette smoking and secondary polycythemia in hypoxic cor pulmonale. Am. Rev. Respir. Dis. 1982, 125, 507–510. [Google Scholar] [CrossRef]
- Chang, T.T.; Lipinski, C.A.; Sherman, H.F. A hazard of home oxygen therapy. J. Burn. Care Rehabil. 2001, 22, 71–74, discussion 0–1. [Google Scholar] [CrossRef]
- Clini, E.; Sturani, C.; Rossi, A.; Viaggi, S.; Corrado, A.; Donner, C.; Ambrosino, N. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur. Respir. J. 2002, 20, 529–538. [Google Scholar] [CrossRef]
- Duiverman, M.L.; Wempe, J.B.; Bladder, G.; Vonk, J.M.; Zijlstra, J.G.; Kerstjens, H.A.M.; Wijkstra, P.J. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: A randomized controlled trial. Respir. Res. 2011, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Pierce, R.J.; Hillman, D.; Esterman, A.; Ellis, E.E.; Catcheside, P.G.; O’Donoghue, F.J.; Barnes, D.J.; Grunstein, R.R.; Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax 2009, 64, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Terzoni, S.; Gaeta, M.; Sorgente, A.; Destrebecq, A.; Gigliotti, F. Physical training and noninvasive ventilation in COPD patients: A meta-analysis. Respir. Care 2014, 59, 709–717. [Google Scholar] [CrossRef]
- Tsolaki, V.; Pastaka, C.; Karetsi, E.; Zygoulis, P.; Koutsokera, A.; Gourgoulianis, K.I.; Kostikas, K. One-year non-invasive ventilation in chronic hypercapnic COPD: Effect on quality of life. Respir. Med. 2008, 102, 904–911. [Google Scholar] [CrossRef]
- Diaz, O.; Bégin, P.; Andresen, M.; Prieto, M.E.; Castillo, C.; Jorquera, J.; Lisboa, C. Physiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPD. Eur. Respir. J. 2005, 26, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Celli, B.R.; Tost, L.; Soriano, E.; Abreu, J.; Velasco, V.; Santolaria, F. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000, 118, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Struik, F.M.; Lacasse, Y.; Goldstein, R.S.; Kerstjens, H.A.; Wijkstra, P.J. Nocturnal noninvasive positive pressure ventilation in stable COPD: A systematic review and individual patient data meta-analysis. Respir. Med. 2014, 108, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Haenel, M.; Storre, J.H.; Dreher, M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int. J. Med. Sci. 2009, 6, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Kostic, S.; Dreher, M.; Virchow, J.C., Jr.; Sorichter, S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of Pa(CO2). Chest 2005, 128, 657–662. [Google Scholar] [CrossRef]
- Windisch, W.; Quality of life in home mechanical ventilation study g. Impact of home mechanical ventilation on health-related quality of life. Eur. Respir. J. 2008, 32, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Vogel, M.; Sorichter, S.; Hennings, E.; Bremer, H.; Hamm, H.; Matthys, H.; Virchow, J. Normocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir. Med. 2002, 96, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Storre, J.H.; Schmoor, C.; Windisch, W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: A randomised crossover trial. Thorax 2010, 65, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ankjaergaard, K.L.; Maibom, S.L.; Wilcke, J.T. Long-term non-invasive ventilation reduces readmissions in COPD patients with two or more episodes of acute hypercapnic respiratory failure. Eur. Clin. Respir. J. 2016, 3, 28303. [Google Scholar] [CrossRef]
- Funk, G.-C.; Breyer, M.-K.; Burghuber, O.C.; Kink, E.; Kirchheiner, K.; Kohansal, R.; Schmidt, I.; Hartl, S. Long-term non-invasive ventilation in COPD after acute-on-chronic respiratory failure. Respir. Med. 2011, 105, 427–434. [Google Scholar] [CrossRef]
- Windisch, W.; Walterspacher, S.; Siemon, K.; Geiseler, J.; Sitter, H.; German Society for Pneumology. Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Published by the German Society for Pneumology (DGP). Pneumologie 2010, 64, 640–652. [Google Scholar] [CrossRef]
- Crimi, C.; Noto, A.; Princi, P.; Cuvelier, A.; Masa, J.F.; Simonds, A.; Elliott, M.W.; Wijkstra, P.; Windisch, W.; Nava, S. Domiciliary Non-invasive Ventilation in COPD: An International Survey of Indications and Practices. Copd 2016, 13, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; Carlucci, A.; Chatwin, M.; Clini, E.; Elliott, M.; Gonzalez-Bermejo, J.; Hart, N.; Luján, M.; et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur. Respir. J. 2019, 54, 1901003. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D. The history of surgical procedures for emphysema. Ann. Thorac. Surg. 1997, 63, 312–319. [Google Scholar] [CrossRef]

| Diagnosis | Main Characteristics |
|---|---|
| COPD |
|
| Asthma |
|
| Bronchiectasis |
|
| Chronic heart failure |
|
| Bronchiolitis (infectious or autoimmune) |
|
| Tuberculosis |
|
| Stages of Severity (FEV1 % Pred.) | Symptoms | Exacerbations | Comorbidities |
|---|---|---|---|
| Stage 1 (>80) | Dyspnea with moderate physical exertion, little/no effect on physical activity, cough and/or sputum production | Frequency and severity increase per stage | Observed at all stages |
| Stage 2 (79–50) | Increased dyspnea, e.g., after walking 100 m on level ground, decreased physical activity, cough and sputum production, recurrent respiratory tract infections | ||
| Stage 3 (49–30) | Dyspnea with little physical exertion, daily cough and sputum production, a significant decrease in daily activity, and symptoms of frequent infections; Stage 4 patients usually have severe hypoxemia and/or respiratory failure | ||
| Stage 4 (<30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzanakis, N.; Kosmas, E.; Papaioannou, A.I.; Hillas, G.; Zervas, E.; Loukides, S.; Bakakos, P.; Katsaounou, P.; Boutou, A.; Perlikos, P.; et al. Greek Guidelines for the Management of COPD, a Proposal of a Holistic Approach Based on the needs of the Greek Community. J. Pers. Med. 2022, 12, 1997. https://doi.org/10.3390/jpm12121997
Tzanakis N, Kosmas E, Papaioannou AI, Hillas G, Zervas E, Loukides S, Bakakos P, Katsaounou P, Boutou A, Perlikos P, et al. Greek Guidelines for the Management of COPD, a Proposal of a Holistic Approach Based on the needs of the Greek Community. Journal of Personalized Medicine. 2022; 12(12):1997. https://doi.org/10.3390/jpm12121997
Chicago/Turabian StyleTzanakis, Nikolaos, Epameinontas Kosmas, Andriana I. Papaioannou, Georgios Hillas, Eleftherios Zervas, Stelios Loukides, Petros Bakakos, Paraskevi Katsaounou, Afroditi Boutou, Photis Perlikos, and et al. 2022. "Greek Guidelines for the Management of COPD, a Proposal of a Holistic Approach Based on the needs of the Greek Community" Journal of Personalized Medicine 12, no. 12: 1997. https://doi.org/10.3390/jpm12121997
APA StyleTzanakis, N., Kosmas, E., Papaioannou, A. I., Hillas, G., Zervas, E., Loukides, S., Bakakos, P., Katsaounou, P., Boutou, A., Perlikos, P., Rovina, N., Dimakou, K., Steiropoulos, P., Stratakos, G., Emmanouil, P., Tryfon, S., & Koulouris, N. (2022). Greek Guidelines for the Management of COPD, a Proposal of a Holistic Approach Based on the needs of the Greek Community. Journal of Personalized Medicine, 12(12), 1997. https://doi.org/10.3390/jpm12121997











