Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders
Abstract
1. Introduction
2. AAV-Mediated Gene Therapy—Considerations for Designing a Therapeutic Approach
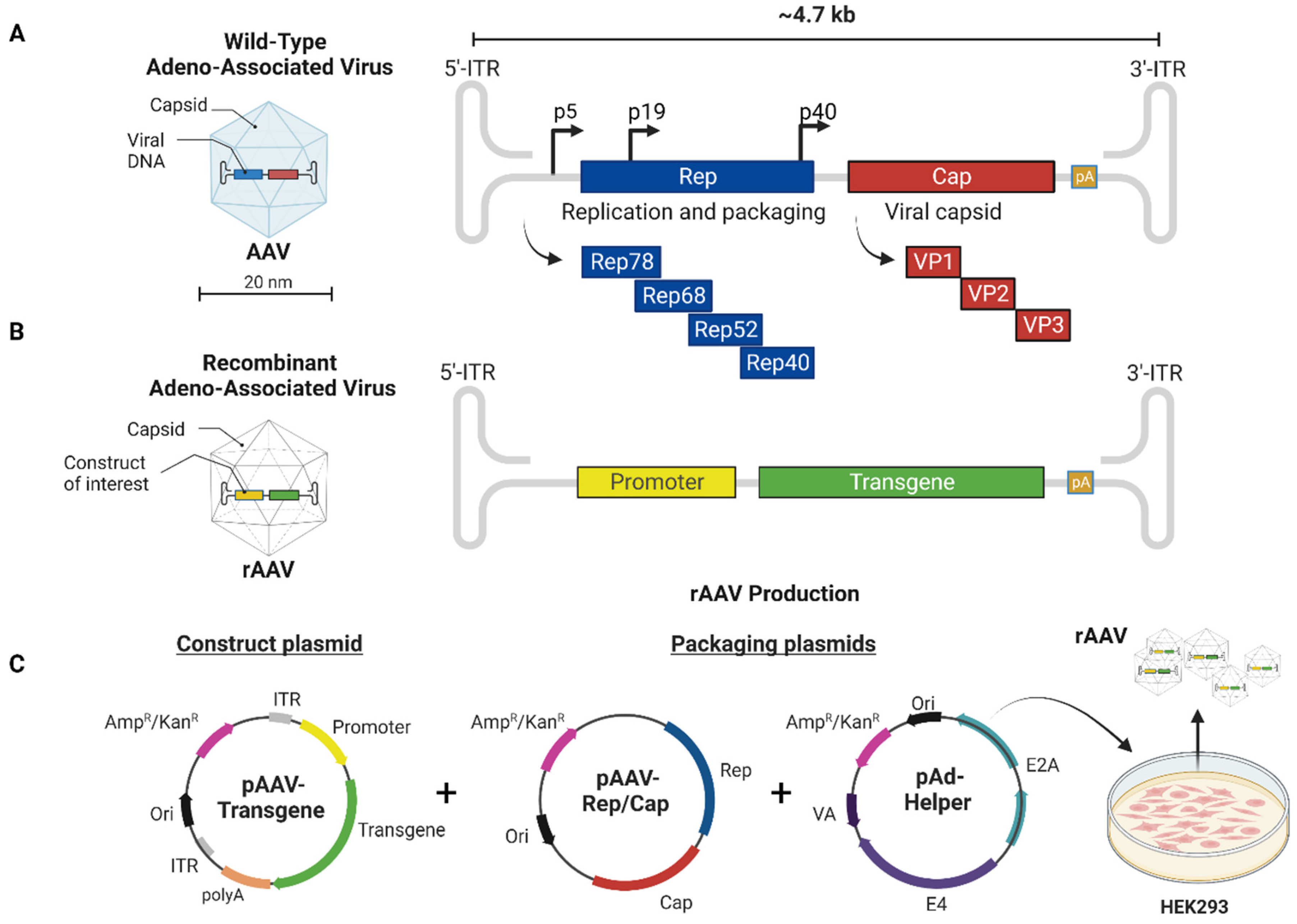
2.1. The Basics of Native AAV Vector Biology
2.2. The Basics of Recombinant AAV Vector Biology
2.3. Advantages and Disadvantages of Self-Complementary rAAV Genomes
2.4. AAV Serotype Characteristics and Tropism
2.5. Cell-Specific and Ubiquitous Promoters—Modulating Gene Expression
2.6. Engineered AAVs with Enhanced Tropism to Muscles and CNS
2.7. AAV Delivery and Immune Response
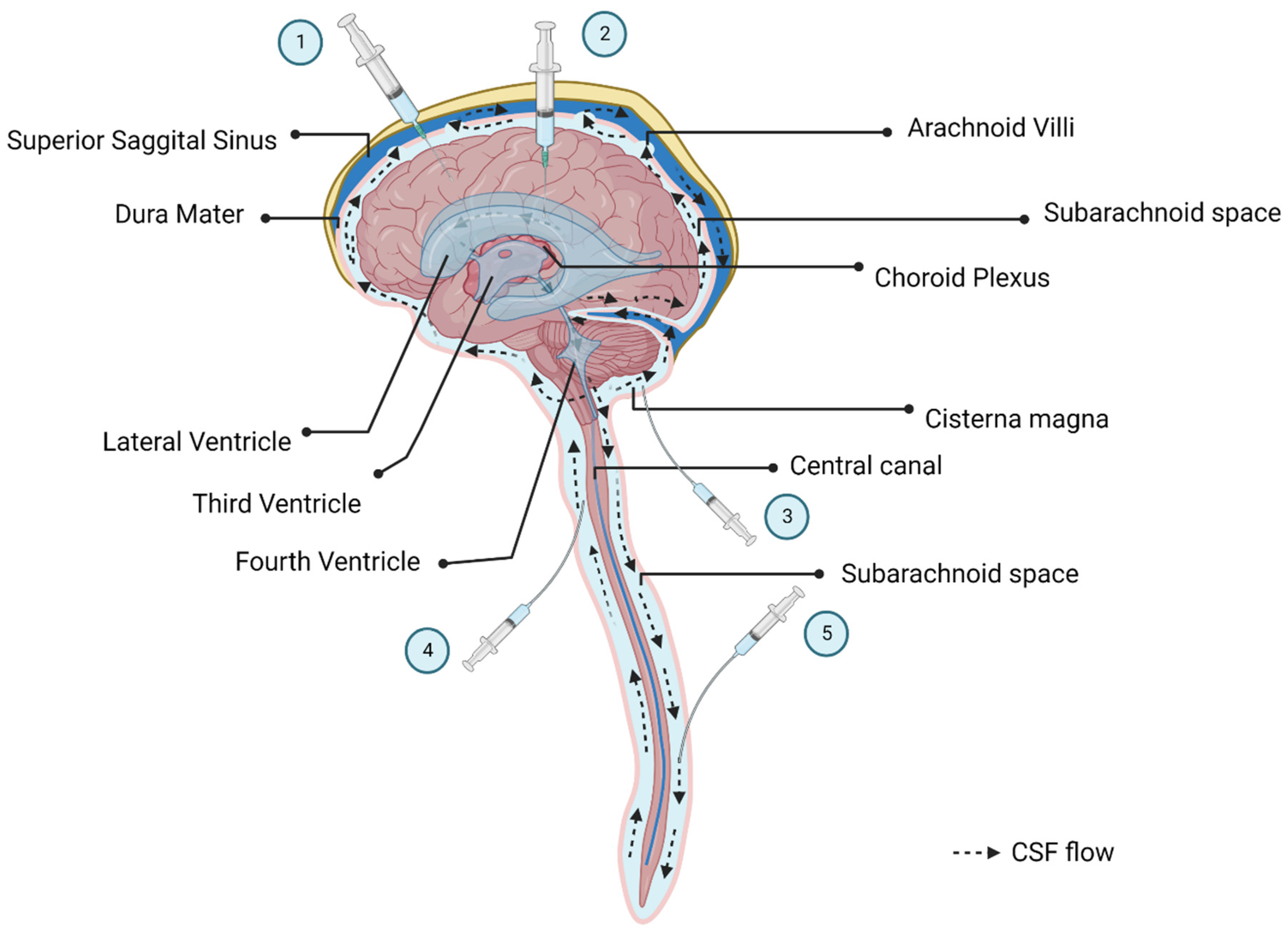
3. Routes of Administration
3.1. Local Administration
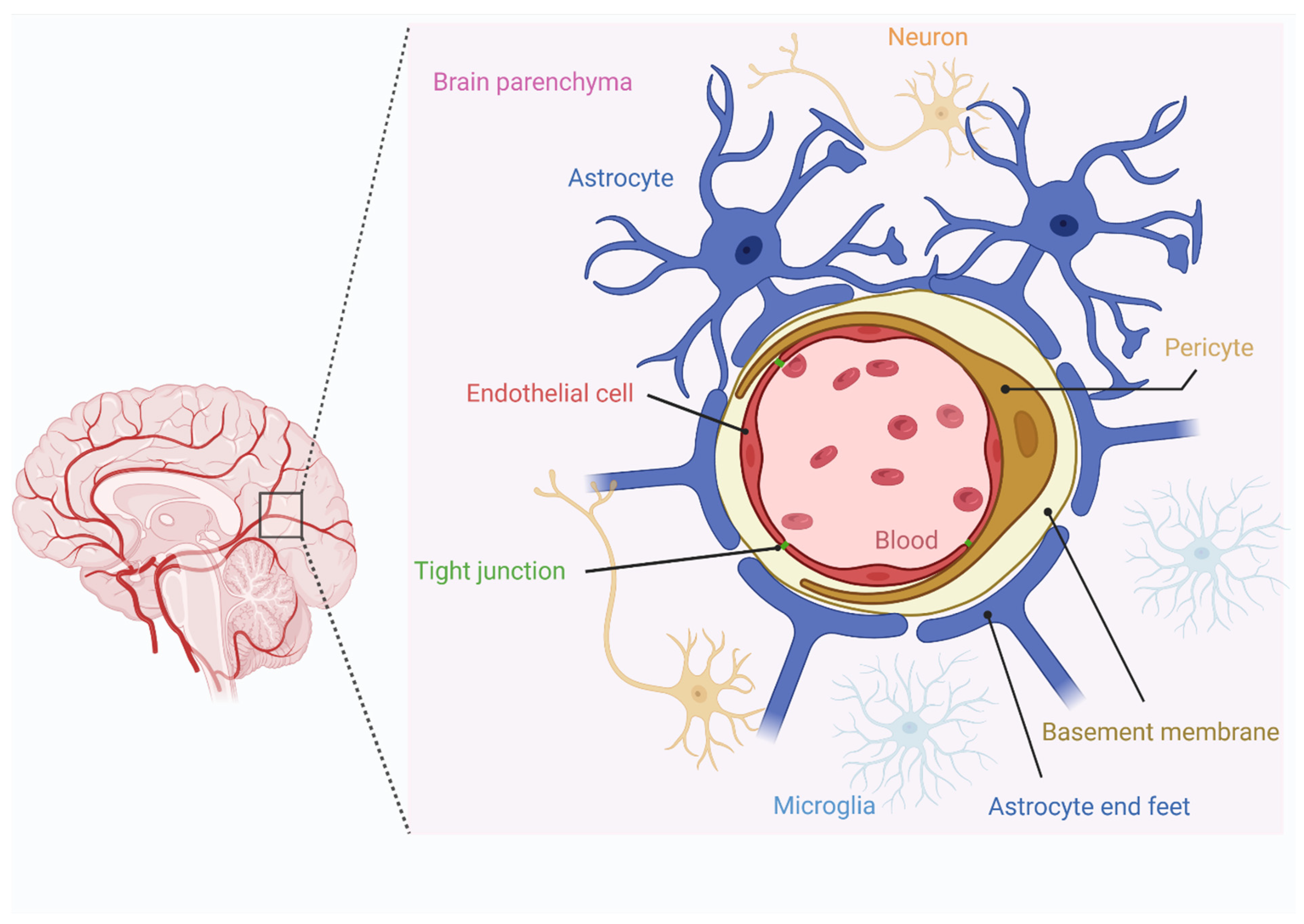
3.2. Systemic IV Delivery vs. CSF Delivery
3.3. Disruption of BBB to Improve Systemic Delivery
4. Selected Neurodegenerative and Neuromuscular Diseases and Clinical Examples for Novel Treatment Approaches Focusing on Gene Therapy
4.1. Gangliosidosis 1 Disease
4.2. Neuronal Ceroid Lipofuscinosis Type 2 and Other Forms of Batten Disease
4.3. Parkinson’s Disease
4.4. Alzheimer’s Disease
4.5. Spinal Muscular Atrophy
4.6. Duchenne Muscular Dystrophy
4.7. Pompe Disease
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardiman, O.; Doherty, C.P. (Eds.) Neurodegenerative Disorders a Clinical Guide, 2nd ed.; Infona: San Lorenzo, Paraguay, 2011. [Google Scholar]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Institute; Parsippany, N. Understanding Neuromuscular Disease Care. October 2018. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/understanding-neuromuscular-disease-care.pdf (accessed on 6 November 2022).
- Dowling, J.J.; Weihl, C.C.; Spencer, M.J. Molecular and cellular basis of genetically inherited skeletal muscle disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Rashid, I.; Srivastava, P.; Ahmad, K.; Jan, A.T.; Rabbani, G.; Choi, D.; Barreto, G.E.; Ashraf, G.; Lee, E.J.; et al. NeuroMuscleDB: A Database of Genes Associated with Muscle Development, Neuromuscular Diseases, Ageing, and Neurodegeneration. Mol. Neurobiol. 2019, 56, 5835–5843. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.D.; Chen-Plotkin, A.S. Omics in Neurodegenerative Disease: Hope or Hype? Trends Genet. 2020, 36, 152–159. [Google Scholar] [CrossRef]
- Francis, J.S.; Markov, V.; Wojtas, I.D.; Gray, S.; McCown, T.; Samulski, R.J.; Figueroa, M.; Leone, P. Preclinical biodistribution, tropism, and efficacy of oligotropic AAV/Olig001 in a mouse model of congenital white matter disease. Mol. Ther.-Methods Clin. Dev. 2021, 20, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.J.; Kalburgi, S.N.; McCown, T.J.; Samulski, R.J. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013, 20, 450–459. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Ferlini, A.; Goyenvalle, A.; Muntoni, F. RNA-targeted drugs for neuromuscular diseases. Science 2021, 371, 29–31. [Google Scholar] [CrossRef]
- Li, D.; McIntosh, C.S.; Mastaglia, F.L.; Wilton, S.D.; Aung-Htut, M.T. Correction to: Neurodegenerative diseases: A hotbed for splicing defects and the potential therapies. Transl. Neurodegener. 2021, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J. Postnatal gene therapy for neuromuscular diseases–opportunities and limitations. J. Périnat. Med. 2021, 49, 1011–1015. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Piguet, F.; Denis, T.D.S.; Audouard, E.; Beccaria, K.; André, A.; Wurtz, G.; Schatz, R.; Alves, S.; Sevin, C.; Zerah, M.; et al. The Challenge of Gene Therapy for Neurological Diseases: Strategies and Tools to Achieve Efficient Delivery to the Central Nervous System. Hum. Gene Ther. 2021, 32, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-T.; Zhao, Y.-Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.-Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef]
- Taghian, T.; Marosfoi, M.G.; Puri, A.S.; Cataltepe, O.; King, R.; Diffie, E.B.; Maguire, A.S.; Martin, D.R.; Fernau, D.; Batista, A.R.; et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol. Ther. 2019, 28, 411–421. [Google Scholar] [CrossRef]
- Martier, R.; Konstantinova, P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef]
- Fischell, J.M.; Fishman, P.S. A Multifaceted Approach to Optimizing AAV Delivery to the Brain for the Treatment of Neu-rodegenerative Diseases. Front. Neurosci. 2021, 15, 1235. [Google Scholar] [CrossRef]
- Manini, A.; Abati, E.; Nuredini, A.; Corti, S.; Comi, G.P. Adeno-Associated Virus (AAV)-Mediated Gene Therapy for Duchenne Muscular Dystrophy: The Issue of Transgene Persistence. Front. Neurol. 2022, 12, 814174. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.-I.; Li, J.; Sun, L.; Zhang, J.; Xiao, X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003, 10, 2105–2111. [Google Scholar] [CrossRef]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2008, 27, 59–65. [Google Scholar] [CrossRef]
- Gray, S.J.; Matagne, V.; Bachaboina, L.; Yadav, S.; Ojeda, S.R.; Samulski, R.J. Preclinical Differences of Intravascular AAV9 Delivery to Neurons and Glia: A Comparative Study of Adult Mice and Nonhuman Primates. Mol. Ther. 2011, 19, 1058–1069. [Google Scholar] [CrossRef]
- Samaranch, L.; Salegio, E.A.; Sebastian, W.S.; Kells, A.P.; Bringas, J.R.; Forsayeth, J.; Bankiewicz, K.S. Strong Cortical and Spinal Cord Transduction After AAV7 and AAV9 Delivery into the Cerebrospinal Fluid of Nonhuman Primates. Hum. Gene Ther. 2013, 24, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Murlidharan, G.; Samulski, R.J.; Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 2014, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Savy, A.; Dickx, Y.; Nauwynck, L.; Bonnin, D.; Merten, O.-W.; Galibert, L. Impact of Inverted Terminal Repeat Integrity on rAAV8 Production Using the Baculovirus/Sf9 Cells System. Hum. Gene Ther. Methods 2017, 28, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Merten, O.-W.; Gény-Fiamma, C.; Douar, A.M. Current issues in adeno-associated viral vector production. Gene Ther. 2005, 12, S51–S61. [Google Scholar] [CrossRef]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–755. [Google Scholar] [CrossRef]
- Hoggan, M.D.; Blacklow, N.R.; Rowe, W.P. Studies of small DNA viruses found in various adenovirus preparations: Physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. USA 1966, 55, 1467–1474. [Google Scholar] [CrossRef]
- Clément, N.; Grieger, J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther.-Methods Clin. Dev. 2016, 3, 16002. [Google Scholar] [CrossRef]
- Bennett, A.; Mietzsch, M.; Agbandje-McKenna, M. Understanding capsid assembly and genome packaging for adeno-associated viruses. Futur. Virol. 2017, 12, 283–297. [Google Scholar] [CrossRef]
- Mccarty, D.M.; Monahan, P.E.; Samulski, R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001, 8, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.I. Parvovirus replication. Microbiol. Rev. 1990, 54, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, X.; Fan, Z.; Li, C.; McCown, T.; Samulski, R.J.; Xiao, X. Adeno-associated Virus of a Single-polarity DNA Genome Is Capable of Transduction In Vivo. Mol. Ther. 2008, 16, 494–499. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.M. Self-complementary AAV Vectors; Advances and Applications. Mol. Ther. 2008, 16, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Davidoff, A.M.; Nathwani, A.C. Self-complementary adeno-associated viral vectors for gene therapy of hemophilia B: Progress and challenges. Expert Rev. Hematol. 2011, 4, 539–549. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, G.; Cao, L.; Sun, Z.; He, Y.; Cui, M.; Sun, Y.; Li, S.; Li, H.; Qin, L.; et al. Divergent engagements between adeno-associated viruses with their cellular receptor AAVR. Nat. Commun. 2019, 10, 3760. [Google Scholar] [CrossRef]
- Large, E.; Silveria, M.; Zane, G.; Weerakoon, O.; Chapman, M. Adeno-Associated Virus (AAV) Gene Delivery: Dissecting Molecular Interactions upon Cell Entry. Viruses 2021, 13, 1336. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, R.; Li, H.; Yin, K.; Ma, X.; Lou, Z. Structural basis for the neurotropic AAV9 and the engineered AAVPHP.eB recognition with cellular receptors. Mol. Ther.-Methods Clin. Dev. 2022, 26, 52–60. [Google Scholar] [CrossRef]
- Merkel, S.F.; Andrews, A.M.; Lutton, E.M.; Mu, D.; Hudry, E.; Hyman, B.T.; Maguire, C.A.; Ramirez, S.H. Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J. Neurochem. 2016, 140, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, B.; Mu, X.; Ahmed, S.S.; Su, Q.; He, R.; Wang, H.; Mueller, C.; Sena-Esteves, M.; Brown, R.; et al. Several rAAV Vectors Efficiently Cross the Blood–brain Barrier and Transduce Neurons and Astrocytes in the Neonatal Mouse Central Nervous System. Mol. Ther. 2011, 19, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Duque, S.; Joussemet, B.; Riviere, C.; Marais, T.; Dubreil, L.; Douar, A.-M.; Fyfe, J.; Moullier, P.; Colle, M.-A.; Barkats, M. Intravenous Administration of Self-complementary AAV9 Enables Transgene Delivery to Adult Motor Neurons. Mol. Ther. 2009, 17, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.J.; Katus, H.A.; Bekeredjian, R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc. Res. 2007, 73, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.J.; Leuchs, B.; Pleger, S.T.; Grimm, D.; Franz, W.-M.; Katus, H.A.; Kleinschmidt, J.A. Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc. Res. 2006, 70, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Fuess, S.; Storm, T.A.; Gibson, G.A.; Mctiernan, C.F.; Kay, M.A.; Nakai, H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006, 14, 45–53. [Google Scholar] [CrossRef]
- Yue, Y.; Ghosh, A.; Long, C.; Bostick, B.; Smith, B.F.; Kornegay, J.N.; Duan, D. A Single Intravenous Injection of Adeno-associated Virus Serotype-9 Leads to Whole Body Skeletal Muscle Transduction in Dogs. Mol. Ther. 2008, 16, 1944–1952. [Google Scholar] [CrossRef]
- Jones, D. Duchenne muscular dystrophy awaits gene therapy. Nat. Biotechnol. 2019, 37, 335–337. [Google Scholar] [CrossRef]
- Amoasii, L.; Long, C.; Li, H.; Mireault, A.A.; Shelton, J.M.; Sanchez-Ortiz, E.; McAnally, J.R.; Bhattacharyya, S.; Schmidt, F.; Grimm, D.; et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci. Transl. Med. 2017, 9, eaan8081. [Google Scholar] [CrossRef]
- Duan, D. Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther. 2018, 26, 2337–2356. [Google Scholar] [CrossRef]
- Elverman, M.; Goddard, M.A.; Mack, D.; Snyder, J.M.; Lawlor, M.W.; Meng, H.; Beggs, A.H.; Buj-Bello, A.; Poulard, K.; Marsh, A.P.; et al. Long-term effects of systemic gene therapy in a canine model of myotubular myopathy. Muscle Nerve 2017, 56, 943–953. [Google Scholar] [CrossRef]
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and Future Prospects for Gene Therapy for Rare Genetic Diseases Affecting the Brain and Spinal Cord. Front. Mol. Neurosci. 2021, 14, 695937. [Google Scholar] [CrossRef]
- Naidoo, J.; Stanek, L.M.; Ohno, K.; Trewman, S.; Samaranch, L.; Hadaczek, P.; O’Riordan, C.; Sullivan, J.; Sebastian, W.S.; Bringas, J.R.; et al. Extensive Transduction and Enhanced Spread of a Modified AAV2 Capsid in the Non-human Primate CNS. Mol. Ther. 2018, 26, 2418–2430. [Google Scholar] [CrossRef] [PubMed]
- Tervo, D.G.R.; Hwang, B.-Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Haurigot, V.A.; Marcó, S.; Ribera, A.; Garcia, M.; Ruzo, A.; Villacampa, P.; Ayuso, E.; Añor, S.; Andaluz, A.; Pineda, M.; et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J. Clin. Investig. 2013, 123, 3254–3271. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Sato-Yoshitake, R.; Yoshida, T.; Kawashima, T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J. Cell Biol. 1990, 111, 1027–1037. [Google Scholar] [CrossRef]
- Haggerty, D.; Grecco, G.; Reeves, K.C.; Atwood, B. Adeno-Associated Viral Vectors in Neuroscience Research. Mol. Ther.-Methods Clin. Dev. 2019, 17, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Wang, X.; Ma, Y.X.; Wang, S. CMV enhancer/human PDGF-β promoter for neuron-specific transgene expression. Gene Ther. 2003, 11, 52–60. [Google Scholar] [CrossRef]
- Haenraets, K.; Foster, E.; Johannssen, H.; Kandra, V.; Frezel, N.; Steffen, T.; Jaramillo, V.; Paterna, J.-C.; Zeilhofer, H.U.; Wildner, H. Spinal nociceptive circuit analysis with recombinant adeno-associated viruses: The impact of serotypes and promoters. J. Neurochem. 2017, 142, 721–733. [Google Scholar] [CrossRef]
- Sakurada, T.; Mima, K.; Kurisaki, A.; Sugino, H.; Yamauchi, T. Neuronal cell type-specific promoter of the α CaM kinase II gene is activated by Zic2, a Zic family zinc finger protein. Neurosci. Res. 2005, 53, 323–330. [Google Scholar] [CrossRef]
- Lesman, D.; Rodriguez, Y.; Rajakumar, D.; Wein, N. U7 snRNA, a Small RNA with a Big Impact in Gene Therapy. Hum. Gene Ther. 2021, 32, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Turunen, H.T.; Vandenberghe, L.H.; Wolfe, J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. In Gene Therapy for Neurological Disorders; Humana Press: New York, NY, USA, 2016; pp. 133–149. [Google Scholar] [CrossRef]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2021, 25, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Asokan, A.; Conway, J.C.; Phillips, J.L.; Li, C.; Hegge, J.; Sinnott, R.; Yadav, S.; DiPrimio, N.; Nam, H.-J.; Agbandje-McKenna, M.; et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 2010, 28, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Pulicherla, N.; Shen, S.; Yadav, S.; Debbink, K.; Govindasamy, L.; Agbandje-McKenna, M.; Asokan, A. Engineering Liver-detargeted AAV9 Vectors for Cardiac and Musculoskeletal Gene Transfer. Mol. Ther. 2011, 19, 1070–1078. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Fitzpatrick, Z.; Harris, A.F.; Maitland, S.A.; Ferreira, J.S.; Zhang, Y.; Ma, S.; Sharma, R.B.; Gray-Edwards, H.L.; Johnson, J.A.; et al. In Vivo Selection Yields AAV-B1 Capsid for Central Nervous System and Muscle Gene Therapy. Mol. Ther. 2016, 24, 1247–1257. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Harris, A.F.; Cabral, D.J.; Keeler, A.M.; Sapp, E.; Ferreira, J.S.; Gray-Edwards, H.L.; Johnson, J.A.; Johnson, A.K.; Su, Q.; et al. Widespread Central Nervous System Gene Transfer and Silencing After Systemic Delivery of Novel AAV-AS Vector. Mol. Ther. 2016, 24, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.; Drouin, L.M.; Agbandje-Mckenna, M.; Chen, C.; Qiao, C.; Pu, D.; Hu, X.; Wang, D.-Z.; Li, J.; et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl. Acad. Sci. USA 2009, 106, 3946–3951. [Google Scholar] [CrossRef]
- Weiss, A.R.; Liguore, W.A.; Domire, J.S.; Button, D.; McBride, J.L. Intra-striatal AAV2.retro administration leads to extensive retrograde transport in the rhesus macaque brain: Implications for disease modeling and therapeutic development. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.A.; Stanek, L.M.; Lukason, M.J.; Bu, J.; Osmond, S.R.; Barry, E.A.; O’Riordan, C.R.; Shihabuddin, L.S.; Cheng, S.H.; Scaria, A. Rationally designed AAV2 and AAVrh8R capsids provide improved transduction in the retina and brain. Gene Ther. 2018, 25, 205–219. [Google Scholar] [CrossRef]
- Bello, A.C.P.D.P.; Tran, K.; Chand, A.N.; Doria, M.; Allocca, M.P.; Hildinger, M.; Beniac, D.R.; Kranendonk, C.; Auricchio, A.; Kobinger, G.P. Isolation and evaluation of novel adeno-associated virus sequences from porcine tissues. Gene Ther. 2009, 16, 1320–1328. [Google Scholar] [CrossRef]
- Srivastava, A. Adeno-Associated Virus: The Naturally Occurring Virus Versus the Recombinant Vector. Hum. Gene Ther. 2016, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kruzik, A.; Fetahagic, D.; Hartlieb, B.; Dorn, S.; Koppensteiner, H.; Horling, F.M.; Scheiflinger, F.; Reipert, B.M.; de la Rosa, M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol. Ther.-Methods Clin. Dev. 2019, 14, 126–133. [Google Scholar] [CrossRef]
- Rapti, K.; Grimm, D. Adeno-Associated Viruses (AAV) and Host Immunity—A Race Between the Hare and the Hedgehog. Front. Immunol. 2021, 12, 753467. [Google Scholar] [CrossRef]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide Epidemiology of Neutralizing Antibodies to Adeno-Associated Viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhong, L.; Li, M.; Li, J.; Tran, K.; Ren, L.; He, R.; Xie, J.; Moser, R.P.; Fraser, C.; et al. Adeno-Associated Virus Neutralizing Antibodies in Large Animals and Their Impact on Brain Intraparenchymal Gene Transfer. Mol. Ther.-Methods Clin. Dev. 2018, 11, 65–72. [Google Scholar] [CrossRef]
- Tosolini, A.P.; Sleigh, J.N. Intramuscular Delivery of Gene Therapy for Targeting the Nervous System. Front. Mol. Neurosci. 2020, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Bjornsson, C.S.; Dymecki, S.M.; Gilbertson, R.J.; Holtzman, D.M.; Monuki, E.S. The Choroid Plexus and Cerebrospinal Fluid: Emerging Roles in Development, Disease, and Therapy. J. Neurosci. 2013, 33, 17553–17559. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef]
- Belur, L.R.; Romero, M.; Lee, J.; Podetz-Pedersen, K.M.; Nan, Z.; Riedl, M.S.; Vulchanova, L.; Kitto, K.F.; Fairbanks, C.A.; Kozarsky, K.F.; et al. Comparative Effectiveness of Intracerebroventricular, Intrathecal, and Intranasal Routes of AAV9 Vector Administration for Genetic Therapy of Neurologic Disease in Murine Mucopolysaccharidosis Type I. Front. Mol. Neurosci. 2021, 14, 618360. [Google Scholar] [CrossRef]
- Meyer, K.; Ferraiuolo, L.; Schmelzer, L.; Braun, L.; McGovern, V.; Likhite, S.; Michels, O.; Govoni, A.; Fitzgerald, J.; Morales, P.; et al. Improving Single Injection CSF Delivery of AAV9-mediated Gene Therapy for SMA: A Dose–response Study in Mice and Nonhuman Primates. Mol. Ther. 2015, 23, 477–487. [Google Scholar] [CrossRef]
- Duma, C.; Kopyov, O.; Kopyov, A.; Berman, M.; Lander, E.; Elam, M.; Arata, M.; Weiland, D.; Cannell, R.; Caraway, C.; et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: Results of a 3-year phase 1 study of 113 injections in 31 patients. Mol. Biol. Rep. 2019, 46, 5257–5272. [Google Scholar] [CrossRef]
- Marchi, P.M.; Marrone, L.; Azzouz, M. Delivery of therapeutic AAV9 vectors via cisterna magna to treat neurological disorders. Trends Mol. Med. 2021, 28, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Benatti, H.R.; Gray-Edwards, H.L. Adeno-Associated Virus Delivery Limitations for Neurological Indications. Hum. Gene Ther. 2022, 33, 1–7. [Google Scholar] [CrossRef]
- Castle, M.J.; Cheng, Y.; Asokan, A.; Tuszynski, M.H. Physical positioning markedly enhances brain transduction after intrathecal AAV9 infusion. Sci. Adv. 2018, 4, eaau9859. [Google Scholar] [CrossRef]
- Haché, M.; Swoboda, K.J.; Sethna, N.; Farrow-Gillespie, A.; Khandji, A.; Xia, S.; Bishop, K.M. Intrathecal Injections in Children With Spinal Muscular Atrophy. J. Child Neurol. 2016, 31, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Farrar, M.A.; Mercuri, E.; Finkel, R.S.; Foster, R.; Hughes, S.G.; Bhan, I.; Farwell, W.; Gheuens, S. An Integrated Safety Analysis of Infants and Children with Symptomatic Spinal Muscular Atrophy (SMA) Treated with Nusinersen in Seven Clinical Trials. CNS Drugs 2019, 33, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Albright, B.H.; Storey, C.M.; Murlidharan, G.; Castellanos Rivera, R.M.; Berry, G.E.; Madigan, V.J.; Asokan, A. Mapping the Structural Determinants Required for AAVrh.10 Transport across the Blood-Brain Barrier. Mol. Ther. 2018, 26, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Mastakov, M.Y.; Baer, K.; Xu, R.; Fitzsimons, H.; During, M.J. Combined Injection of rAAV with Mannitol Enhances Gene Expression in the Rat Brain. Mol. Ther. 2001, 3, 225–232. [Google Scholar] [CrossRef]
- Carty, N.; Lee, D.; Dickey, C.; Ceballos-Diaz, C.; Jansen-West, K.; Golde, T.E.; Gordon, M.N.; Morgan, D.; Nash, K. Convection-enhanced delivery and systemic mannitol increase gene product distribution of AAV vectors 5, 8, and 9 and increase gene product in the adult mouse brain. J. Neurosci. Methods 2010, 194, 144–153. [Google Scholar] [CrossRef]
- Wang, S.; Karakatsani, M.E.; Fung, C.; Sun, T.; Acosta, C.; Konofagou, E. Direct brain infusion can be enhanced with focused ultrasound and microbubbles. J. Cereb. Blood Flow Metab. 2016, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Stavarache, M.A.; Petersen, N.; Jurgens, E.M.; Milstein, E.R.; Rosenfeld, Z.B.; Ballon, D.J.; Kaplitt, M.G. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J. Neurosurg. 2019, 130, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Nicoli, E.-R.; Annunziata, I.; D’Azzo, A.; Platt, F.M.; Tifft, C.J.; Stepien, K.M. GM1 Gangliosidosis—A Mini-Review. Front. Genet. 2021, 12, 734878. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2017, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Caciotti, A.; Garman, S.C.; Rivera-Colón, Y.; Procopio, E.S.; Lorenzo Ferri, C.; Guido, C.; Martelli, P.; Parini, R.D.; Roberta Battini, A.; Sibilio, M.; et al. GM1 gangliosidosis and Morquio B disease: An update on genetic alterations and clinical find-ings. Biochim. Biophys. Acta 2011, 1812, 782–790. [Google Scholar] [CrossRef]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta 2009, 1793, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Regier, D.S.; Yoon, R.; Pan, K.S.; Johnston, J.M.; Yang, S.; Spranger, J.W.; Tifft, C.J. The skeletal phenotype of intermediate GM1 gangliosidosis: Clinical, radiographic and densitometric features, and implications for clinical monitoring and intervention. Bone 2019, 131, 115142. [Google Scholar] [CrossRef]
- Patterson, M.C. Gangliosidoses. Handb Clin. Neurol. 2013, 113, 1707–1708. [Google Scholar] [CrossRef]
- Regier, D.S.; Tifft, C.J.; Rothermel, C.E. GLB1-Related Disorders; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Singer, H.S.; Mink, J.W.; Gilbert, D.L.; Jankovic, J. Inherited Metabolic Disorders with Associated Movement Abnormalities; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Deodato, F.; Procopio, E.; Rampazzo, A.; Taurisano, R.; Donati, M.A.; Dionisi-Vici, C.; Caciotti, A.; Morrone, A.; Scarpa, M. The treatment of juvenile/adult GM1-gangliosidosis with Miglustat may reverse disease progression. Metab. Brain Dis. 2017, 32, 1529–1536. [Google Scholar] [CrossRef]
- Shield, J.P.H.; Stone, J.; Steward, C. Bone marrow transplantation correcting β-galactosidase activity does not influence neurological outcome in juvenile GM1-gangliosidosis. J. Inherit. Metab. Dis. 2005, 28, 797–798. [Google Scholar] [CrossRef]
- Condori, J.; Acosta, W.; Ayala, J.; Katta, V.; Flory, A.; Martin, R.; Radin, J.; Cramer, C.L.; Radin, D.N. Enzyme replacement for GM1-gangliosidosis: Uptake, lysosomal activation, and cellular disease correction using a novel β-galactosidase:RTB lectin fusion. Mol. Genet. Metab. 2016, 117, 199–209. [Google Scholar] [CrossRef]
- Chen, J.C.; Luu, A.R.; Wise, N.; De Angelis, R.; Agrawal, V.; Mangini, L.; Vincelette, J.; Handyside, B.; Sterling, H.; Lo, M.J.; et al. Intracerebroventricular enzyme replacement therapy with β-galactosidase reverses brain pathologies due to GM1 gangliosidosis in mice. J. Biol. Chem. 2020, 295, 13532–13555. [Google Scholar] [CrossRef]
- Latour, Y.L.; Yoon, R.; Thomas, S.E.; Grant, C.; Li, C.; Sena-Esteves, M.; Allende, M.L.; Proia, R.L.; Tifft, C.J. Human GLB1 knockout cerebral organoids: A model system for testing AAV9-mediated GLB1 gene therapy for reducing GM1 ganglioside storage in GM1 gangliosidosis. Mol. Genet. Metab. Rep. 2019, 21, 100513. [Google Scholar] [CrossRef]
- Liu, S.; Ma, W.; Feng, Y.; Zhang, Y.; Jia, X.; Tang, C.; Tang, F.; Wu, X.; Huang, Y. AAV9-coGLB1 Improves Lysosomal Storage and Rescues Central Nervous System Inflammation in a Mutant Mouse Model of GM1 Gangliosidosis. Curr. Gene Ther. 2022, 22, 352–365. [Google Scholar] [CrossRef]
- Issue: Molecular Therapy. Available online: https://www.cell.com/molecular-therapy-family/molecular-therapy/issue?pii=S1525-0016(21)X0004-4 (accessed on 28 October 2022).
- Passage Bio, Inc. Passage Bio Presents New Interim Clinical and Biomarker Data for Patients with GM1 Gangliosidosis in Imagine-1 Study at ASGCT 25th Annual Meeting. Available online: https://www.passagebio.com/investors-and-news/press-releases-and-statements/news-details/2022/Passage-Bio-Presents-New-Interim-Clinical-and-Biomarker-Data-for-Patients-with-GM1-Gangliosidosis-in-Imagine-1-Study-at-ASGCT-25th-Annual-Meeting/default.aspx (accessed on 17 October 2022).
- Mink, J.W.; Augustine, E.F.; Adams, H.R.; Marshall, F.J.; Kwon, J.M. Classification and Natural History of the Neuronal Ceroid Lipofuscinoses. J. Child Neurol. 2013, 28, 1101–1105. [Google Scholar] [CrossRef]
- Sleat, D.E.; Donnelly, R.J.; Lackland, H.; Liu, C.G.; Sohar, I.; Pullarkat, R.K.; Lobel, P. Association of Mutations in a Lysosomal Protein with Classical Late- Infantile Neuronal Ceroid Lipofuscinosis Published by: American Association for the Advancement of Science Stable. Science 1997, 277, 19–23. Available online: https://www.jstor.org/stable/2893837REFERENCES (accessed on 6 November 2022). [CrossRef] [PubMed]
- Mole, S.E.; Cotman, S.L. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 2237–2241. [Google Scholar] [CrossRef]
- Bennett, M.J.; Hofmann, S.L. The neuronal ceroid-lipofuscinoses (Batten disease): A new class of lysosomal storage diseases. J. Inherit. Metab. Dis. 1999, 22, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sleat, D.E.; Gin, R.M.; Sohar, I.; Wisniewski, K.; Sklower-Brooks, S.; Pullarkat, R.K.; Palmer, D.N.; Lerner, T.J.; Boustany, R.-M.; Uldall, P.; et al. Mutational Analysis of the Defective Protease in Classic Late-Infantile Neuronal Ceroid Lipofuscinosis, a Neurodegenerative Lysosomal Storage Disorder. Am. J. Hum. Genet. 1999, 64, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Nickel, M.; Simonati, A.; Jacoby, D.; Lezius, S.; Kilian, D.; Van de Graaf, B.; Pagovich, O.E.; Kosofsky, B.; Yohay, K.; Downs, M.; et al. Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: An observational cohort study. Lancet Child Adolesc. Health 2018, 2, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Kohlschütter, A.; Mink, J.; Simonati, A.; Williams, R. NCL diseases—Clinical perspectives. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Ajayi, T.; Specchio, N.; Reyes, E.D.L.; Gissen, P.; Ballon, D.; Dyke, J.P.; Cahan, H.; Slasor, P.; Jacoby, D.; et al. Study of Intraventricular Cerliponase Alfa for CLN2 Disease. N. Engl. J. Med. 2018, 378, 1898–1907. [Google Scholar] [CrossRef]
- Markham, A. Cerliponase Alfa: First Global Approval. Drugs 2017, 77, 1247–1249. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Fraser, J.; Arkin, L.M.; Sondhi, D.; Hackett, N.R.; Kaminsky, S.; Heier, L.; Kosofsky, B.E.; Worgall, S.; Crystal, R.G.; et al. Gene therapy for late infantile neuronal ceroid lipofuscinosis: Neurosurgical considerations. J. Neurosurg. Pediatr. 2010, 6, 115–122. [Google Scholar] [CrossRef]
- Sondhi, D.; Kaminsky, S.M.; Hackett, N.R.; Pagovich, O.E.; Rosenberg, J.B.; De, B.P.; Chen, A.; Van de Graaf, B.; Mezey, J.G.; Mammen, G.W.; et al. Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2. Sci. Transl. Med. 2020, 12, eabb5413. [Google Scholar] [CrossRef]
- 51st Annual Meeting of the Child Neurology Society. Ann. Neurol. 2022, 92, S1–S211. [CrossRef]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Palma, J.-A.; Kaufmann, H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov. Disord. 2018, 33, 372–390. [Google Scholar] [CrossRef]
- Fahn, S. A new look at levodopa based on the ELLDOPA study. J. Neural Transm. Suppl. 2006, 70, 419–426. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Anderson, R.W. Deep brain stimulation mechanisms: The control of network activity via neurochemistry modulation. J. Neurochem. 2016, 139, 338–345. [Google Scholar] [CrossRef]
- Merola, A.; Romagnolo, A.; Rizzi, L.; Rizzone, M.G.; Zibetti, M.; Lanotte, M.; Mandybur, G.; Duker, A.P.; Espay, A.J.; Lopiano, L. Impulse control behaviors and subthalamic deep brain stimulation in Parkinson disease. J. Neurol. 2016, 264, 40–48. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Park. Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef]
- Lemkau, L.R.; Comellas, G.; Lee, S.W.; Rikardsen, L.K.; Woods, W.S.; George, J.; Rienstra, C.M. Site-Specific Perturbations of Alpha-Synuclein Fibril Structure by the Parkinson’s Disease Associated Mutations A53T and E46K. PLoS ONE 2013, 8, e49750. [Google Scholar] [CrossRef]
- Zheng, J.; Zang, Q.; Hu, F.; Wei, H.; Ma, J.; Xu, Y. Alpha-synuclein gene polymorphism affects risk of dementia in Han Chinese with Parkinson’s disease. Neurosci. Lett. 2019, 706, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.A.; Zhao, H.; Collier, T.J.; Sandoval, I.; Sortwell, C.E.; Steece-Collier, K.; Daley, B.F.; Booms, A.; Lipton, J.; Welch, M.; et al. α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease. J. Clin. Investig. 2021, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.A.; Studer, L. Moving Stem Cells to the Clinic: Potential and Limitations for Brain Repair. Neuron 2015, 86, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Fričová, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. npj Regen. Med. 2020, 5, 20. [Google Scholar] [CrossRef]
- McFarthing, K.; Prakash, N.; Simuni, T. Clinical Trial Highlights: 1. Gene Therapy for Parkinson’S, 2. Phase 3 Study in Focus-Intec Pharma’S Accordion Pill, 3. Clinical Trials Resources. J. Park. Dis. 2019, 9, 251–264. [Google Scholar] [CrossRef]
- Ntetsika, T.; Papathoma, P.-E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17. [Google Scholar] [CrossRef]
- Kalia, L.V.; Kalia, S.K.; Lang, A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015, 30, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- VY-AADC01 Gene Therapy in Parkinson’s Disease: Interim Results of the On-Going Phase 1b PD-1101 Trial-MDS Abstracts. Available online: https://www.mdsabstracts.org/abstract/vy-aadc01-gene-therapy-in-parkinsons-disease-interim-results-of-the-on-going-phase-1b-pd-1101-trial (accessed on 16 October 2022).
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Feigin, A.; Kaplitt, M.G.; Tang, C.; Lin, T.; Mattis, P.; Dhawan, V.; During, M.J.; Eidelberg, D. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 19559–19564. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Baumann, T.L.; Siffert, J.; Herzog, C.D.; Alterman, R.; Boulis, N.; Turner, D.A.; Stacy, M.; Lang, A.E.; Lozano, A.M.; et al. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 2013, 80, 1698–1701. [Google Scholar] [CrossRef]
- Marks, W.J.; Bartus, R.T.; Siffert, J.; Davis, C.S.; Lozano, A.; Boulis, N.; Vitek, J.; Stacy, M.; Turner, D.; Verhagen, L.; et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial. Lancet Neurol. 2010, 9, 1164–1172. [Google Scholar] [CrossRef]
- Marks, W.J.; Baumann, T.L.; Bartus, R.T. The CERE-120 Study Group Long-Term Safety of Patients with Parkinson’s Disease Receiving rAAV2-Neurturin (CERE-120) Gene Transfer. Hum. Gene Ther. 2016, 27, 522–527. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Grabowska-Pyrzewicz, W.; Want, A.; Leszek, J.; Wojda, U. Antisense oligonucleotides for Alzheimer’s disease therapy: From the mRNA to miRNA paradigm. eBioMedicine 2021, 74, 103691. [Google Scholar] [CrossRef]
- Gauthier, S.; Zhang, H.; Ng, K.P.; Pascoal, T.; Rosa-Neto, P. Impact of the biological definition of Alzheimer’s disease using amyloid, tau and neurodegeneration (ATN): What about the role of vascular changes, inflammation, Lewy body pathology? Transl. Neurodegener. 2018, 7, 12. [Google Scholar] [CrossRef]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimer’s Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef]
- Cortes, C.J.; La Spada, A.R. TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: Molecular mechanisms, cellular processes, and emerging therapeutic opportunities. Neurobiol. Dis. 2019, 122, 83–93. [Google Scholar] [CrossRef]
- Ryman, D.C.; Acosta-Baena, N.; Aisen, P.S.; Bird, T.; Danek, A.; Fox, N.; Goate, A.; Frommelt, P.; Ghetti, B.; Langbaum, J.B.; et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef]
- Farr, S.A.; Erickson, M.A.; Niehoff, M.L.; Banks, W.A.; Morley, J.E. Central and Peripheral Administration of Antisense Oligonucleotide Targeting Amyloid-β Protein Precursor Improves Learning and Memory and Reduces Neuroinflammatory Cytokines in Tg2576 (AβPPswe) Mice. J. Alzheimer’s Dis. 2014, 40, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Hinrich, A.J.; Roman, B.; Norrbom, M.; Rigo, F.; Marr, R.A.; Norstrom, E.M.; Hastings, M.L. Targeting Amyloid-β Precursor Protein, APP, Splicing with Antisense Oligonucleotides Reduces Toxic Amyloid-β Production. Mol. Ther. 2018, 26, 1539–1551. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease Erin. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2010, 17, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Baumann, T.L.; Bakay, R.A.E.; Ostrove, J.M.; Siffert, J.; Fleisher, A.S.; Herzog, C.D.; Barba, D.; Pay, M.; Salmon, D.P.; et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Emborg, M.E. Nonhuman Primate Models of Neurodegenerative Disorders. ILAR J. 2017, 58, 190–201. [Google Scholar] [CrossRef]
- Castle, M.; Baltanás, F.C.; Kovacs, I.; Nagahara, A.H.; Barba, D.; Tuszynski, M.H. Postmortem Analysis in a Clinical Trial of AAV2-NGF Gene Therapy for Alzheimer’s Disease Identifies a Need for Improved Vector Delivery. Hum. Gene Ther. 2020, 31, 415–422. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Wilson, B.R.; Ivasyk, I.; Kovacs, I.; Rawalji, S.; Bringas, J.R.; Pivirotto, P.J.; Sebastian, W.S.; Samaranch, L.; Bankiewicz, K.S.; et al. MR-guided delivery of AAV2-BDNF into the entorhinal cortex of non-human primates. Gene Ther. 2018, 25, 104–114. [Google Scholar] [CrossRef]
- de Jesus, B.B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Park, S.B.; Vucic, S.; Carey, K.A.; Turner, B.J.; Gillingwater, T.H.; Swoboda, K.J.; Kiernan, M.C. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 2017, 81, 355–368. [Google Scholar] [CrossRef]
- NORD. Spinal Muscular Atrophy; National Organization for Rare Disorders: Qunicy, MA, USA, 2021. [Google Scholar]
- Pellizzoni, L.; Kataoka, N.; Charroux, B.; Dreyfuss, G. A Novel Function for SMN, the Spinal Muscular Atrophy Disease Gene Product, in Pre-mRNA Splicing. Cell 1998, 95, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; McGovern, V.L.; Lotti, F.; Saieva, L.; Li, D.K.; Kariya, S.; Monani, U.R.; Burghes, A.H.M.; Pellizzoni, L. A Role for SMN Exon 7 Splicing in the Selective Vulnerability of Motor Neurons in Spinal Muscular Atrophy. Mol. Cell. Biol. 2012, 32, 126–138. [Google Scholar] [CrossRef]
- Markati, T.; Fisher, G.; Ramdas, S.; Servais, L. Risdiplam: An investigational survival motor neuron 2 (SMN2) splicing modifier for spinal muscular atrophy (SMA). Expert Opin. Investig. Drugs 2022, 31, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Onasemnogene Abeparvovec: First Global Approval. Drugs 2019, 79, 1255–1262. [Google Scholar] [CrossRef]
- Schwartz, M.; Likhite, S.; Meyer, K. Onasemnogene abeparvovec-xioi: A gene replacement strategy for the treatment of infants diagnosed with spinal muscular atrophy. Drugs Today 2021, 57, 387–399. [Google Scholar] [CrossRef]
- Zolgensma® Data Shows Rapid, Significant, Clinically Meaningful Benefit in SMA including Prolonged Event-Free Survival, Motor Milestone Achievement and Durability Now Up to 5 Years Post-Dosing|Novartis. Available online: https://www.novartis.com/news/media-releases/zolgensma-data-shows-rapid-significant-clinically-meaningful-benefit-sma-including-prolonged-event-free-survival-motor-milestone-achievement-and-durability-now-5-years-post-dosing (accessed on 16 October 2022).
- Kirschner, J.; Butoianu, N.; Goemans, N.; Haberlova, J.; Kostera-Pruszczyk, A.; Mercuri, E.; van der Pol, W.L.; Quijano-Roy, S.; Sejersen, T.; Tizzano, E.F.; et al. European ad-hoc consensus statement on gene replacement therapy for spinal muscular atrophy. Eur. J. Paediatr. Neurol. 2020, 28, 38–43. [Google Scholar] [CrossRef]
- Novartis Announces Lift of Partial Clinical Trial Hold and Plans to Initiate a New, Pivotal Phase 3 Study of Intrathecal OAV-101 in Older Patients with SMA|Novartis. Available online: https://www.novartis.com/news/media-releases/novartis-announces-lift-partial-clinical-trial-hold-and-plans-initiate-new-pivotal-phase-3-study-intrathecal-oav-101-older-patients-sma (accessed on 16 October 2022).
- Emery, A.E. Population frequencies of inherited neuromuscular diseases—A world survey. Neuromuscul. Disord. 1991, 1, 19–29. [Google Scholar] [CrossRef]
- Wein, N.; Alfano, L.; Flanigan, K.M. Genetics and Emerging Treatments for Duchenne and Becker Muscular Dystrophy. Pediatr. Clin. North Am. 2015, 62, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, M.; Li, H.; He, R.; Lin, J.; Zhang, C.; Zhu, Y. Genotypes and Phenotypes of DMD Small Mutations in Chinese Patients With Dystrophinopathies. Front. Genet. 2019, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Nguyen, Q.; Yokota, T. Genotype–Phenotype Correlations in Duchenne and Becker Muscular Dystrophy Patients from the Canadian Neuromuscular Disease Registry. J. Pers. Med. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Rossi, R.; Trabanelli, C.; Mauro, A.; Selvatici, R.; Falzarano, M.S.; Spedicato, N.; Margutti, A.; Rimessi, P.; Fortunato, F.; et al. The Genetic Landscape of Dystrophin Mutations in Italy: A Nationwide Study. Front. Genet. 2020, 11, 131. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, K.M.; Waldrop, M.A.; Martin, P.T.; Dunn, D.M.; Alfano, L.N.; Simmons, T.R. A genome-wide association analysis of loss of ambulation in dystrophinopathy patients suggests multiple candidate modifiers of disease severity. medRxiv Yale 2021, 1–30. [Google Scholar] [CrossRef]
- Dumont, N.A.; Rudnicki, M.A. Targeting muscle stem cell intrinsic defects to treat Duchenne muscular dystrophy. NPJ Regen. Med. 2016, 1, 16006. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Muscle and cardiac therapeutic strategies for Duchenne muscular dystrophy: Past, present, and future. Pharmacol. Rep. 2020, 72, 1227–1263. [Google Scholar] [CrossRef]
- Vulin, A.; Wein, N.; Simmons, T.R.; Rutherford, A.M.; Findlay, A.R.; Yurkoski, J.A.; Kaminoh, Y.; Flanigan, K.M. The first exon duplication mouse model of Duchenne muscular dystrophy: A tool for therapeutic development. Neuromuscul. Disord. 2015, 25, 827–834. [Google Scholar] [CrossRef]
- Servais, L.; Mercuri, E.; Straub, V.; Guglieri, M.; Seferian, A.M.; Scoto, M.; Leone, D.; Koenig, E.; Khan, N.; Dugar, A.; et al. Long-Term Safety and Efficacy Data of Golodirsen in Ambulatory Patients with Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping: A First-in-human, Multicenter, Two-Part, Open-Label, Phase 1/2 Trial. Nucleic Acid Ther. 2022, 32, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, F.; Rossi, R.; Falzarano, M.; Ferlini, A. Innovative Therapeutic Approaches for Duchenne Muscular Dystrophy. J. Clin. Med. 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, S.; Phan, H.; Straub, V.; Muntoni, F.; Wolf, D.; Malhotra, J.; Chu, R.; Darton, E.; Mercuri, E.P. 132 Casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: Interim results from the Phase 3 ESSENCE trial. Neuromuscul. Disord. 2022, 32, S102. [Google Scholar] [CrossRef]
- Solid Biosciences Reports Efficacy and Safety Data from the Ongoing IGNITE DMD Clinical Trial and Resumption of Patient Dosing in the 2E14 vg/kg Cohort Solid Biosciences. Available online: https://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-efficacy-and-safety-data-from-the-ongoing-ignite-dmd-clinical-trial-and-resumption-of-patient-dosing-in-the-2e14-vg-kg-cohort (accessed on 16 October 2022).
- Mendell, J.R.; Sahenk, Z.; Lehman, K.; Nease, C.; Lowes, L.P.; Miller, N.F.; Iammarino, M.A.; Alfano, L.N.; Nicholl, A.; Al-Zaidy, S.; et al. Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children With Duchenne Muscular Dystrophy. JAMA Neurol. 2020, 77, 1122. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Sarepta’s DMD gene therapy falls flat. Nat. Rev. Drug Discov. 2021, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Korlimarla, A.; Lim, J.-A.; Kishnani, P.S.; Sun, B. An emerging phenotype of central nervous system involvement in Pompe disease: From bench to bedside and beyond. Ann. Transl. Med. 2019, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.; Puertollano, R.; Raben, N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018, 15, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Hers, H.G. α-Glucosidase deficiency in generalized glycogen-storage disease (Pompe’s disease). Biochem. J. 1963, 86, 11–16. [Google Scholar] [CrossRef]
- Fukuda, T.; Ewan, L.; Bauer, M.; Mattaliano, R.J.; Zaal, K.; Ralston, E.; Plotz, P.H.; Raben, N. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann. Neurol. 2006, 59, 700–708. [Google Scholar] [CrossRef]
- Hahn, A.; Schänzer, A. Long-term outcome and unmet needs in infantile-onset Pompe disease. Ann. Transl. Med. 2019, 7, 283. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Hwu, W.-L.; Mandel, H.; Nicolino, M.; Yong, F.; Corzo, D.; Infantile-Onset Pompe Disease Natural History Study Group. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006, 148, 671–676.e2. [Google Scholar] [CrossRef]
- Hagemans, M.L.C.; Winkel, L.P.F.; Van Doorn, P.A.; Hop, W.J.C.; Loonen, M.C.B.; Reuser, A.J.J.; Van Der Ploeg, A.T. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain 2005, 128, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, L.; Hogrel, J.-Y.; Perniconi, B.; Kruijshaar, M.E.; Rizopoulos, D.; Taouagh, N.; Canal, A.; Brusse, E.; van Doorn, P.A.; van der Ploeg, A.T.; et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology 2019, 93, e1756–e1767. [Google Scholar] [CrossRef] [PubMed]
- Banugaria, S.G.; Prater, S.N.; Ng, Y.-K.; Kobori, J.A.; Finkel, R.S.; Ladda, R.L.; Chen, Y.-T.; Rosenberg, A.S.; Kishnani, P.S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: Lessons learned from infantile Pompe disease. Anesthesia Analg. 2011, 13, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; McVie-Wylie, A.; Jiang, C.; Thurberg, B.L.; Raben, N.; Mattaliano, R.J.; Cheng, S.H. Carbohydrate-remodelled acid α-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem. J. 2005, 389, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Yang, H.W.; Pennybacker, M.; Ichihara, N.; Mizutani, M.; Van Hove, J.L.; Chen, Y.T. Clinical and metabolic correction of pompe disease by enzyme therapy in acid maltase-deficient quail. J. Clin. Investig. 1998, 101, 827–833. [Google Scholar] [CrossRef]
- Pena, L.D.; Barohn, R.J.; Byrne, B.J.; Desnuelle, C.; Goker-Alpan, O.; Ladha, S.; Laforêt, P.; Mengel, K.E.; Pestronk, A.; Pouget, J.; et al. Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalglucosidase alfa (neoGAA) in treatment-naïve and alglucosidase alfa-treated patients with late-onset Pompe disease: A phase 1, open-label, multicenter, multinational, ascending dose study. Neuromuscul. Disord. 2019, 29, 167–186. [Google Scholar] [CrossRef]
- D’Alonzo, D.; De Fenza, M.; Porto, C.; Iacono, R.; Huebecker, M.; Cobucci-Ponzano, B.; Priestman, D.A.; Platt, F.; Parenti, G.; Moracci, M.; et al. N-Butyl-l-deoxynojirimycin (l-NBDNJ): Synthesis of an Allosteric Enhancer of α-Glucosidase Activity for the Treatment of Pompe Disease. J. Med. Chem. 2017, 60, 9462–9469. [Google Scholar] [CrossRef]
- Hordeaux, J.; Dubreil, L.; Robveille, C.; Deniaud, J.; Pascal, Q.; Dequéant, B.; Pailloux, J.; Lagalice, L.; Ledevin, M.; Babarit, C.; et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathol. Commun. 2017, 5, 66. [Google Scholar] [CrossRef]
- MDA2022—Pompe Gene Therapy ACT-101 Boosts GAA Levels in Trial. Available online: https://pompediseasenews.com/news/mda-2022-gene-therapy-act-101-well-tolerated-boosts-gaa-levels-early-trial/ (accessed on 28 October 2022).
| Gene Therapy | Delivery Route | Sponsor | Phase | Identifier | Participants Estimated Enrollment | Duration |
|---|---|---|---|---|---|---|
| GM1 | ||||||
| AAV9–GLB1 | IV | NHGRI | Phase I/II | NCT03952637 | 45 | 2019–2027 |
| AAVhu68-GLB1 | ICM | Passage Bio, Inc. | Phase I/II | NCT04713475 | 20 | 2021–2029 |
| AAVrh.10–GLB1 | ICM | Lysogene | PhaseI/II | NCT04273269 | 16 | 2021–2030 |
| Batten disease | ||||||
| AAV9-CLN3 | IT | Amicus Therapeutics | Phase I/II | NCT03770572 | 7 | 2018–2023 |
| AAV9–CLN5 | ICV, IVT | Neurogene Inc | Phase I/II | NCT05228145 | 3 | 2022–2028 |
| AAV9–CLN7 | IT | Benjamin Greenberg | Phase I | NCT04737460 | 4 | 2021–2029 |
| PD | ||||||
| AAV2–AADC | IP (striatum) | Neurocrine Biosciences | Phase II | NCT03562494 | 85 | 2018–2023 |
| AAV2–GDNF | IP (putamen) | Brain Neurotherapy | Phase I | NCT04167540 | 12 | 2020–2027 |
| AAV9–GCase | ICM | Prevail Therapeutics | Phase I/IIa | NCT04127578 | 24 | 2020–2028 |
| AD | ||||||
| AONS–MAPT | IT | Ionis Pharmaceuticals, Inc. | Phase I/II | NCT03186989 | 44 | 2017–2022 |
| AAV2-BDNF | stereotactic administration-brain | Mark Tuszynski | Phase I | NCT05040217 | 12 | 2021–2025 |
| AAVrh.10-APOE2 | IT | Lexeo Therapeutics | Phase I | NCT03634007 | 15 | 2019–2024 |
| AAVrh.10-APOE2 | IT | Lexeo Therapeutics | Phase I | NCT05400330 | 15 | 2022–2027 |
| SMA | ||||||
| AONS–SMN2 | IT | Biogen | Phase II | NCT02386553 | 25 | 2015–2025 |
| AONS–SMN2 | IT | Biogen | Phase III | NCT02594124 | 292 | 2015–2023 |
| AONS–SMN2 | IT | NYU Langone Health | Early Phase I | NCT04050852 | 0 | 2019–2022 |
| AONS–SMN2 | IT | Biogen | Phase II/III | NCT04089566 | 145 | 2020–2023 |
| AONS–SMN2 | IT | Biogen | Phase III | NCT04729907 | 172 | 2021–2026 |
| DMD | ||||||
| AAV9–DMD | IV | Pfizer | Phase III | NCT04281485 | 99 | 2020–2029 |
| AAVrh74.MHCK7. micro-dystrophin | IV | Serapta Therapeuitics, Inc. | Phase III | NCT05096221 | 120 | 2021–2024 |
| AAV9-CK8.micro-dystrophin | IV | Solid Biosciencese, LLC | Phase I/II | NCT03368742 | 16 | 2017–2028 |
| AAVrh74.MHCK7. micro-dystrophin | IV | Serapta Therapeuitics, Inc. | Phase I/II | NCT03375164 | 4 | 2018–2023 |
| AAVrh74.MHCK7. micro-dystrophin | IV | Serapta Therapeuitics, Inc. | Phase II | NCT03769116 | 41 | 2018–2026 |
| AAV9-micro-dystrophin | IV | Pfizer | Phase I | NCT03362502 | 23 | 2018–2026 |
| scAAV9.U7.ACCA | IV | Megan Waldrop | Phase I/II | NCT04240314 | 3 | 2020–2025 |
| Pompe Disease | ||||||
| AAV2/8–GAA | IV | Asklepois Biopharmaceutical | Phase I/II | NCT03533673 | 13 | 2018–2028 |
| AAV–GAA | IV | Spark Therapeutics | Phase I/II | NCT04093349 | 30 | 2020–2027 |
| AAV8–GAA | IV | Audentes Therapeutics | Phase I/II | NCT04174105 | 12 | 2020–2027 |
| Serotype | Host | Receptor | Co-Receptor | AAVR (Recognition PKD Receptors) | Tropism | Organ Tropism | Type of Transport |
|---|---|---|---|---|---|---|---|
| AAV1 | Human/ Monkey | 2,3N/2,6N-sialic Acid | Unknown | PKD1/2 or PKD2 [40] | medium | SM, CNS, liver, retina, pancreas, heart, airways, CM | retrograde anterograde |
| AAV2 | Human | HSPG | FGFR-1, integrin, HGFR, LamR | PKD2 | low | SM, CNS, liver, kidney, retina | retrograde anterograde |
| AAV3 | Human | HSPG | FGFR-1, HGFR, LamR, | PKD, AAV3B [41] | low | SM, HCC, liver | N/A |
| AAV4 | Monkey | 2,3O-sialic acid | Unknown | N/A | low | CNS, retina, lung, kidney | N/A |
| AAV5 | Human | 2,3N-sialic acid | PDGFR | PKD1 [40] | medium | SM, CNS, airway, retina | retrograde anterograde |
| AAV6 | AAV1/ AAV2 hybrid | 2,3N/2,6N-sialicAcid, HSPG | EGFR | PKD | medium | SM, heart, airway | retrograde |
| AAV7 | Monkey | N-sialic acid | PDGF | N/A | high | SM, retina, CNS, liver | |
| AAV8 | Monkey | Unknown | LamR | PKD1/2 or PKD2 | high | SM, CNS, liver, retina, pancreas, heart, and kidney | retrograde anterograde |
| AAV9 | Human | N-galactose | LamR | PKD2 [42] | high | SM, CNS; liver, heart, lung, pancreas, retina, testes, kidney | retrograde anterograde |
| AAVrh.10 | Monkey | sulfated N-acetyl-lactosamine | Unknown | N/A | high | SM, CNS; liver, heart, lung, pancreas, retina, kidney | retrograde anterograde |
| AAV10 | Monkey | Unknown | Unknown | N/A | high | SM, CNS; liver | N/A |
| AAV11 | Monkey | Unknown | Unknown | N/A | medium | spleen | N/A |
| AAV12 | Monkey | Unknown | Unknown | N/A | medium | Submandibular glands, liver | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez Limia, C.; Baird, M.; Schwartz, M.; Saxena, S.; Meyer, K.; Wein, N. Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders. J. Pers. Med. 2022, 12, 1979. https://doi.org/10.3390/jpm12121979
Gomez Limia C, Baird M, Schwartz M, Saxena S, Meyer K, Wein N. Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders. Journal of Personalized Medicine. 2022; 12(12):1979. https://doi.org/10.3390/jpm12121979
Chicago/Turabian StyleGomez Limia, Cintia, Megan Baird, Maura Schwartz, Smita Saxena, Kathrin Meyer, and Nicolas Wein. 2022. "Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders" Journal of Personalized Medicine 12, no. 12: 1979. https://doi.org/10.3390/jpm12121979
APA StyleGomez Limia, C., Baird, M., Schwartz, M., Saxena, S., Meyer, K., & Wein, N. (2022). Emerging Perspectives on Gene Therapy Delivery for Neurodegenerative and Neuromuscular Disorders. Journal of Personalized Medicine, 12(12), 1979. https://doi.org/10.3390/jpm12121979







