Feasibility of Precision Medicine in Hypertension Management—Scope and Technological Aspects
Abstract
1. Introduction
2. Scope of Personalized Hypertension Management
3. Technological Aspects of Personalized Hypertension Management
3.1. Selection and Evaluation of Biomarkers
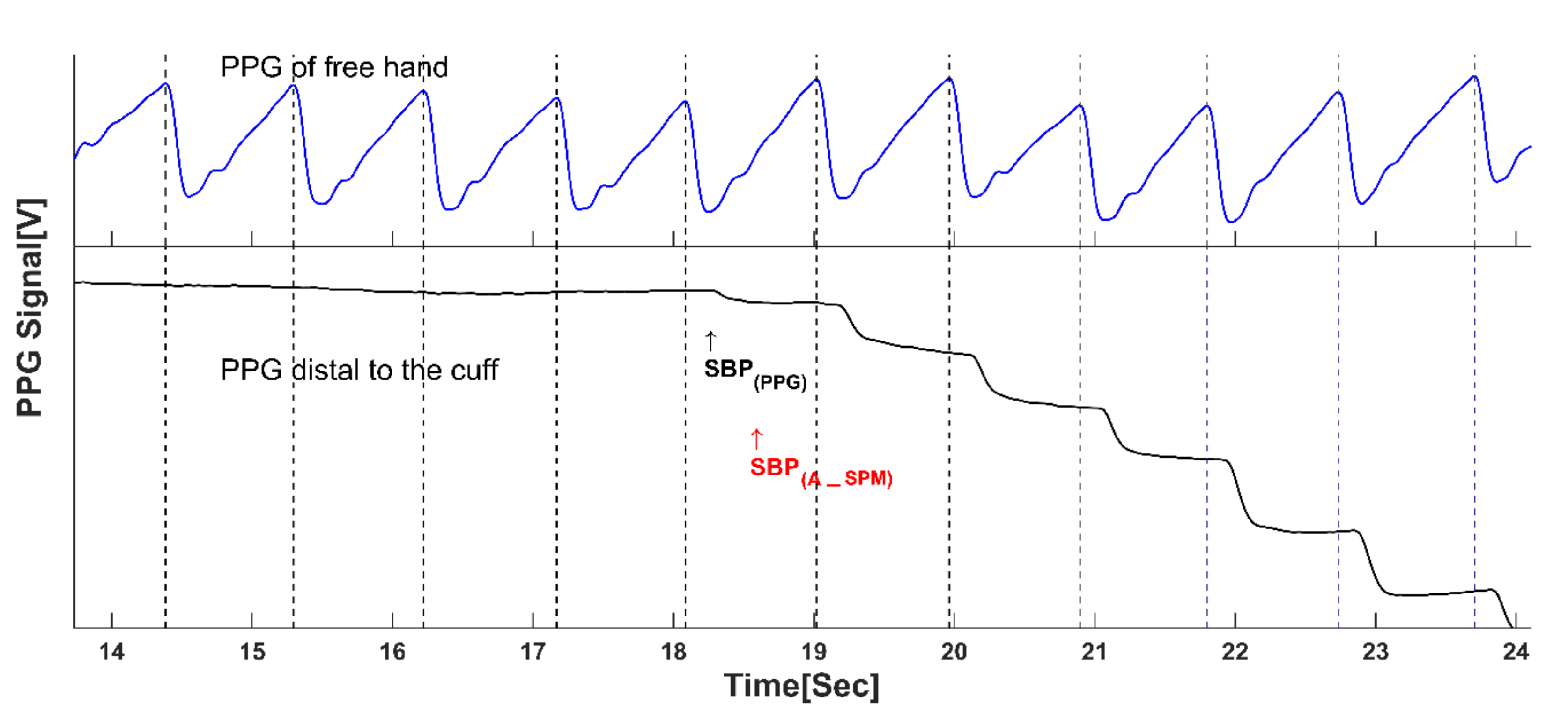
3.2. Accurate Measurement of Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glaab, E.; Rauschenberger, A.; Banzi, R.; Gerardi, C.; Garcia, P.; Demotes, J. Biomarker discovery studies for patient stratification using machine learning analysis of omics data: A scoping review. BMJ Open 2021, 11, e053674. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Constantin, C.; Neagu, M.; Tampa, M. Apprising Diagnostic and Prognostic Biomarkers in Cutaneous Melanoma-Persistent Updating. J. Pers. Med. 2022, 12, 1506. [Google Scholar] [CrossRef]
- Hodson, R. Precision medicine. Nature 2016, 537, S49. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Zhao, Z.; Zhang, Q.; Shao, J.; Wang, C.; Qiu, Z.; Li, W. Artificial intelligence-assisted decision making for prognosis and drug efficacy prediction in lung cancer patients: A narrative review. J. Thorac. Dis. 2021, 13, 7021–7033. [Google Scholar] [CrossRef]
- Dugger, S.A.; Platt, A.; Goldstein, D.B. Drug development in the era of precision medicine. Nat. Rev. Drug Discov. 2018, 17, 183–196. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Kaduthanam, S.; Jackson, D.B. Precision Oncology-The Quest for Evidence. J. Pers. Med. 2019, 9, 43. [Google Scholar] [CrossRef]
- Morieri, M.L.; Pipino, C.; Doria, A. Pharmacogenetics of Cardiovascular Prevention in Diabetes: From Precision Medicine to Identification of Novel Targets. J. Pers. Med. 2022, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Eadon, M.T.; Maddatu, J.; Moe, S.M.; Sinha, A.D.; Ferreira, R.M.; Miller, B.W.; Sher, S.J.; Su, J.; Pratt, V.M.; Chapman, A.B.; et al. Pharmacogenomics of Hypertension in CKD: The CKD-PGX Study. Kidney360 2022, 3, 307–316. [Google Scholar] [CrossRef]
- Geng, T.T.; Jafar, T.H. Hypertension Pharmacogenomics in CKD: The Clinical Relevance and Public Health Implications. Kidney360 2022, 3, 204–207. [Google Scholar] [CrossRef]
- Reitz, C. Toward precision medicine in Alzheimer’s disease. Ann. Transl. Med. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kanayama, N.; Nakayama, Y.; Matsushima, N. Current Status, Issues and Future Prospects of Personalized Medicine for Each Disease. J. Pers. Med. 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Echols, M.R.; Taylor, H. Heart Failure, Precision Medicine, and Incremental Equity: The Case of Hereditary Amyloid Cardiomyopathy. JAMA 2022, 327, 1341–1343. [Google Scholar] [CrossRef]
- Jo, Y.J.; Kim, D.H.; Sohn, M.K.; Lee, J.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Lee, S.Y.; Song, M.K.; et al. Clinical Characteristics and Risk Factors of First-Ever Stroke in Young Adults: A Multicenter, Prospective Cohort Study. J. Pers. Med. 2022, 12, 1505. [Google Scholar] [CrossRef]
- Siao, W.; Chen, Y.; Tsai, C.; Lee, C.; Jong, G. Diabetes Mellitus and Heart Failure. J. Pers. Med. 2022, 12, 1698. [Google Scholar] [CrossRef]
- Tanaka, S. Current and Future Perspectives in Cardiac Rehabilitation. J. Pers. Med. 2022, 12, 1510. [Google Scholar] [CrossRef]
- Khamaysa, M.; Pradat, P.-F. Status of ALS Treatment, Insights into Therapeutic Challenges and Dilemmas. J. Pers. Med. 2022, 12, 1601. [Google Scholar] [CrossRef]
- Evangelou, E.; Warren, H.R. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Giri, A.; Hellwege, J.N.; Keaton, J.M.; Park, J.; Qiu, C.X.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 2019, 51, 51–62. [Google Scholar] [CrossRef]
- McDonough, C.W.; Warren, H.R.; Jack, J.R.; Motsinger-Reif, A.A.; Armstrong, N.D.; Bis, J.C.; House, J.S.; Singh, S.; El Rouby, N.M.; Gong, Y.; et al. Adverse Cardiovascular Outcomes and Antihypertensive Treatment: A Genome-Wide Interaction Meta-Analysis in the International Consortium for Antihypertensive Pharmacogenomics Studies. Clin. Pharmacol. Ther. 2021, 110, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Bis, J.C.; Sitlani, C.; Irvin, R.; Avery, C.L.; Smith, A.V.; Sun, F.; Evans, D.S.; Musani, S.K.; Li, X.; Trompet, S.; et al. Drug-gene interactions of antihypertensive medications and risk of incident cardiovascular disease: A pharmacogenomics study from the CHARGE Consortium. PLoS ONE 2015, 10, e0140496. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Diagnosis and Treatment of Hypertension in the 2017 ACC/AHA Guidelines and in the Real World. JAMA 2018, 319, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Oh, S.H.; Lee, S.J.; Park, J. Precision Medicine for Hypertension Patients with Type 2 Diabetes via Reinforcement Learning. J. Pers. Med. 2022, 12, 87. [Google Scholar] [CrossRef]
- Briant, L.J.; Charkoudian, N.; Hart, E.C. Sympathetic regulation of blood pressure in normotension and hypertension: When sex matters. Exp. Physiol. 2016, 101, 219–229. [Google Scholar] [CrossRef]
- Gerdts, E.; de Simone, G. Hypertension in Women: Should There be a Sex-specific Threshold? Eur. Cardiol. 2021, 16, e38. [Google Scholar] [CrossRef]
- Pyle, W.G. Sex, cardiovascular disease, and the inequities of COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H535–H537. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 2—Genetic and Imaging Markers. Heart Lung Circ. 2019, 28, 678–689. [Google Scholar] [CrossRef]
- Mozos, I.; Jianu, D.; Gug, C.; Stoian, D. Links between High-Sensitivity C-Reactive Protein and Pulse Wave Analysis in Middle-Aged Patients with Hypertension and High Normal Blood Pressure. Dis. Markers 2019, 2019, 2568069. [Google Scholar] [CrossRef]
- Rothwell, P.M. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010, 375, 938–948. [Google Scholar] [CrossRef]
- Karmali, K.N.; Lloyd-Jones, D.M.; van der Leeuw, J.; Goff, D.C., Jr.; Yusuf, S.; Zanchetti, A.; Glasziou, P.; Jackson, R.; Woodward, M.; Rodgers, A.; et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data. PLoS Med. 2018, 15, e1002538. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J. Small artery remodelling in hypertension. Basic Clin. Pharmacol. Toxicol. 2012, 110, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; Jiménez-Altayó, F. Small Resistance Artery Disease and ACE2 in Hypertension: A New Paradigm in the Context of COVID-19. Front. Cardiovasc. Med. 2020, 7, 588692. [Google Scholar] [CrossRef]
- Messerli, F.H. Individualization of antihypertensive therapy: An approach based on hemodynamics and age. J. Clin. Pharmacol. 1981, 21, 517–528. [Google Scholar] [CrossRef]
- Kaplan, N.M. Kaplan’s Clinical hypertension, Lippincott, 8th ed.; Chapter 3; Williams & Wilkins: Philadephia, PA, USA, 2002. [Google Scholar]
- Safar, M.E.; Asmar, R.; Benetos, A.; Blacher, J.; Boutouyrie, P.; Lacolley, P.; Laurent, S.; London, G.; Pannier, B.; Protogerou, A.; et al. French Study Group on Arterial Stiffness. Interaction Between Hypertension and Arterial Stiffness. Hypertension 2018, 72, 796–805. [Google Scholar]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- Cucci, M.D.; Benken, S.T. Blood pressure variability in the management of hypertensive emergency: A narrative review. J. Clin. Hypertens. 2019, 21, 1684–1692. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Salvi, P.; Bilo, G. Assessment and interpretation of blood pressure variability in a clinical setting. Blood Press. 2013, 22, 345–354. [Google Scholar] [CrossRef]
- Mancia, G.; Bombelli, M.; Brambilla, G.; Facchetti, R.; Sega, R.; Toso, E.; Grassi, G. Long-term prognostic value of white coat hypertension: An insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension 2013, 62, 168–174. [Google Scholar] [CrossRef]
- Pierdomenico, S.D.; Pierdomenico, A.M.; Coccina, F.; Porreca, E. Prognosis of Masked and White Coat Uncontrolled Hypertension Detected by Ambulatory Blood Pressure Monitoring in Elderly Treated Hypertensive Patients. Am. J. Hypertens. 2017, 30, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Dinstag, G.; Amar, D.; Ingelsson, E.; Ashley, E.; Shamir, R. Personalized prediction of adverse heart and kidney events using baseline and longitudinal data from SPRINT and ACCORD. PLoS ONE 2019, 14, e0219728. [Google Scholar] [CrossRef] [PubMed]
- SPRINT Research Group; Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar]
- ACCORD Study Group; Cushman, W.C.; Evans, G.W.; Byington, R.P.; Goff, D.C., Jr.; Grimm, R.H., Jr.; Cutler, J.A.; Simons-Morton, D.G.; Basile, J.N.; Corson, M.A.; et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1575–1585. [Google Scholar]
- Wu, C.; Smit, E.; Peralta, C.A.; Sarathy, H.; Odden, M.C. Functional Status Modifies the Association of Blood Pressure with Death in Elders: Health and Retirement Study. J. Am. Geriatr. Soc. 2017, 65, 1482–1489. [Google Scholar] [CrossRef]
- van Dalen, J.W.; Brayne, C.; Crane, P.K.; Fratiglioni, L.; Larson, E.B.; Lobo, A.; Lobo, E.; Marcum, Z.A.; Moll van Charante, E.P.; Qiu, C.; et al. Association of Systolic Blood Pressure with Dementia Risk and the Role of Age, U-Shaped Associations, and Mortality. JAMA Intern. Med. 2022, 182, 142–152. [Google Scholar] [CrossRef]
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Bahrainwala, J.; Patel, A.; Diaz, K.M.; Veerabhadrappa, P.; Cohen, D.L.; Cucchiara, A.; Townsend, R.R. Ambulatory Arterial Stiffness Index and circadian blood pressure variability. J. Am. Soc. Hypertens. 2015, 9, 705–710. [Google Scholar] [CrossRef]
- Willemet, M.; Chowienczyk, P.; Alastruey, J. A database of virtual healthy subjects to assess the accuracy of foot-to-foot pulse wave velocities for estimation of aortic stiffness. Am. J. Physiol Heart Circ. Physiol. 2015, 309, H663–H675. [Google Scholar] [CrossRef]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Lassnig, E.; Berent, R.; Eber, B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004, 109, 184–189. [Google Scholar] [CrossRef]
- Lieber, A.; Millasseau, S.; Bourhis, L.; Blacher, J.; Protogerou, A.; Levy, B.I.; Safar, M.E. Aortic wave reflection in women and men. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H236–H242. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Correia, C.; Cardoso, J. Novel Methods for Pulse Wave Velocity Measurement. J. Med. Biol. Eng. 2015, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H.; Paliakaitė, B.; Pilt, K.; Bachler, M.; Zanelli, S.; Kulin, D.; Allen, J.; Hallab, M.; Bianchini, E.; Mayer, C.C.; et al. Assessing hemodynamics from the photoplethysmogram to gain insights into vascular age: A review from VascAgeNet. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H493–H522. [Google Scholar] [CrossRef] [PubMed]
- Hockin, B.C.D.; Tang, E.Z.; Lloyd, M.G.; Claydon, V.E. Forearm vascular resistance responses to the Valsalva maneuver in healthy young and older adults. Clin. Auton. Res. 2021, 31, 737–753. [Google Scholar] [CrossRef]
- Humeau, A.; Steenbergen, W.; Nilsson, H.; Strömberg, T. Laser Doppler perfusion monitoring and imaging: Novel approaches. Med. Biol. Eng. Comput. 2007, 45, 421–435. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Shelley, K.H. Photoplethysmography: Beyond the calculation of arterial oxygen saturation and heart rate. Anesth. Analg. 2007, 105 (Suppl. 6), S31–S36. [Google Scholar] [CrossRef]
- He, H.W.; Liu, D.W.; Long, Y.; Wang, X.T. The peripheral perfusion index and transcutaneous oxygen challenge test are predictive of mortality in septic patients after resuscitation. Crit. Care 2013, 17, R116. [Google Scholar] [CrossRef]
- Abay, T.Y.; Kyriacou, P.A. Photoplethysmography in oxygenation and blood volume measurement. In Photoplethysmography; Chapter 5; Kyriacou, P.A., Allen, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Camm, A.J.; Malik, M. Co-chairmen: Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- de Souza Filho, L.F.M.; de Oliveira, J.C.M.; Ribeiro, M.K.A.; Moura, M.C.; Fernandes, N.D.; de Sousa, R.D.; Pedrino, G.R.; Rebelo, A.C.S. Evaluation of the autonomic nervous system by analysis of heart rate variability in the preterm infants. BMC Cardiovasc. Disord. 2019, 19, 198. [Google Scholar] [CrossRef] [PubMed]
- Weimer, L.H. Autonomic testing: Common techniques and clinical applications. Neurologist 2010, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Tomalia, V.A.; Park, K.J. Autonomic function tests: Some clinical applications. J. Clin. Neurol. 2013, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.J.; Cheshire, W.P.; Claydon, V.E.; Norcliffe-Kaufmann, L.; Peltier, A.; Singer, W.; Snapper, H.; Vernino, S.; Raj, S.R.; American Autonomic Society. Autonomic function testing in the COVID-19 pandemic: An American Autonomic Society position statement. Clin. Auton. Res. 2020, 30, 295–297. [Google Scholar] [CrossRef]
- Nitzan, M.; Babchenko, A.; Khanokh, B. Very low frequency variability in arterial blood pressure and blood volume pulse. Med. Biol. Eng. Comput. 1999, 37, 54–58. [Google Scholar] [CrossRef]
- Viera, A.J.; Yano, Y.; Lin, F.C.; Simel, D.L.; Yun, J.; Dave, G.; Von Holle, A.; Viera, L.A.; Shimbo, D.; Hardy, S.T.; et al. Does This Adult Patient Have Hypertension?: The Rational Clinical Examination Systematic Review. JAMA 2021, 326, 339–347. [Google Scholar] [CrossRef]
- Ng, K.G. Small CF: Survey of automated noninvasive blood pressure monitors. J. Clin. Eng. 1994, 19, 452–475. [Google Scholar] [CrossRef]
- Forouzanfar, M.; Dajani, H.R.; Groza, V.Z.; Bolic, M.; Rajan, S.; Batkin, I. Oscillometric Blood Pressure Estimation: Past, Present, and Future. IEEE Rev. Biomed. Eng. 2015, 8, 44–63. [Google Scholar] [CrossRef]
- Ursino, M.; Cristalli, C. A mathematical study of some biomechanical factors affecting the oscillometric blood pressure measurement. IEEE Trans. Biomed. Eng. 1996, 43, 761–778. [Google Scholar] [CrossRef]
- van Popele, N.M.; Bos, W.J.; de Beer, N.A.; van Der Kuip, D.A.; Hofman, A.; Grobbee, D.E.; Witteman, J.C. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension 2000, 36, 484–488. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Lourida, P.; Tzamouranis, D.; Baibas, N.M. Unreliable oscillometric blood pressure measurement: Prevalence, repeatability and characteristics of the phenomenon. J. Hum. Hypertens. 2009, 23, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.E.; Marwick, T.H. Accuracy of blood pressure monitoring devices: A critical need for improvement that could resolve discrepancy in hypertension guidelines. J. Hum. Hypertens. 2019, 33, 89–93. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Asmar, R.; Ioannidis, J.P.; Kollias, A.; Lacy, P.; McManus, R.J.; Myers, M.G.; Parati, G.; Shennan, A.; et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J. Hypertens. 2019, 37, 459–466. [Google Scholar] [PubMed]
- Nitzan, M.; Slotki, I.; Shavit, L. More accurate systolic blood pressure measurement is required for improved hypertension management: A perspective. Med. Devices 2017, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Shalom, E.; Hirshtal, E.; Slotki, I.; Shavit, L.; Yitzhaky, Y.; Engelberg, S.; Nitzan, M. Systolic blood pressure measurement by detecting the photoplethysmographic pulses and electronic Korotkoff-sounds during cuff deflation. Physiol. Meas. 2020, 41, 034001. [Google Scholar] [CrossRef]
- Holland, W.W.; Humerfelt, S. Measurement of blood-pressure: Comparison of intra-arterial and cuff values. Br. Med. J. 1964, 2, 1241–1243. [Google Scholar] [CrossRef]
- Fagher, B.; Magnússon, J.; Thulin, T. Direct and indirect blood pressure in normotensive and hypertensive subjects. J. Intern. Med. 1994, 236, 85–90. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Larsen, B.; Holstein, P.; Poulsen, H.L. Accuracy of auscultatory blood pressure measurements in hypertensive and obese subjects. Hypertension 1983, 5, 122–127. [Google Scholar] [CrossRef]
- Siennicki-Lantz, A.; Elmståhl, S. Phenomenon of declining blood pressure in elderly--high systolic levels are undervalued with Korotkoff method. BMC Geriatr. 2011, 11, 57. [Google Scholar] [CrossRef]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Adar, Y.; Hoffman, E.; Shalom, E.; Engelberg, S.; Ben-Dov, I.Z.; Bursztyn, M. Comparison of systolic blood pressure values obtained by photoplethysmography and by Korotkoff sounds. Sensors 2013, 13, 14797–14812. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R. Clinical classification of syncope. Prog. Cardiovasc. Dis. 2013, 55, 339–344. [Google Scholar] [CrossRef]
- Sim, J.J.; Shi, J.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Jacobsen, S.J. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J. Am. Coll. Cardiol. 2014, 64, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lu, F.; Wang, Z.; Zhong, M.; Sun, L.; Hu, N.; Liu, Z.; Zhang, W. Excessive lowering of blood pressure is not beneficial for progression of brain white matter hyperintensive and cognitive impairment in elderly hypertensive patients: 4-year follow-up study. J. Am. Med. Dir. Assoc. 2014, 15, 904–910. [Google Scholar] [CrossRef]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; van der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Heart-Brain Connection Collaborative Research Group. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef]
- Moretti, R.; Torre, P.; Antonello, R.M.; Manganaro, D.; Vilotti, C.; Pizzolato, G. Risk factors for vascular dementia: Hypotension as a key point. Vasc. Health Risk Manag. 2008, 4, 395–402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitzan, M.; Nitzan, I. Feasibility of Precision Medicine in Hypertension Management—Scope and Technological Aspects. J. Pers. Med. 2022, 12, 1861. https://doi.org/10.3390/jpm12111861
Nitzan M, Nitzan I. Feasibility of Precision Medicine in Hypertension Management—Scope and Technological Aspects. Journal of Personalized Medicine. 2022; 12(11):1861. https://doi.org/10.3390/jpm12111861
Chicago/Turabian StyleNitzan, Meir, and Itamar Nitzan. 2022. "Feasibility of Precision Medicine in Hypertension Management—Scope and Technological Aspects" Journal of Personalized Medicine 12, no. 11: 1861. https://doi.org/10.3390/jpm12111861
APA StyleNitzan, M., & Nitzan, I. (2022). Feasibility of Precision Medicine in Hypertension Management—Scope and Technological Aspects. Journal of Personalized Medicine, 12(11), 1861. https://doi.org/10.3390/jpm12111861





