A Radiomics Approach on Chest CT Distinguishes Primary Lung Cancer from Solitary Lung Metastasis in Colorectal Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
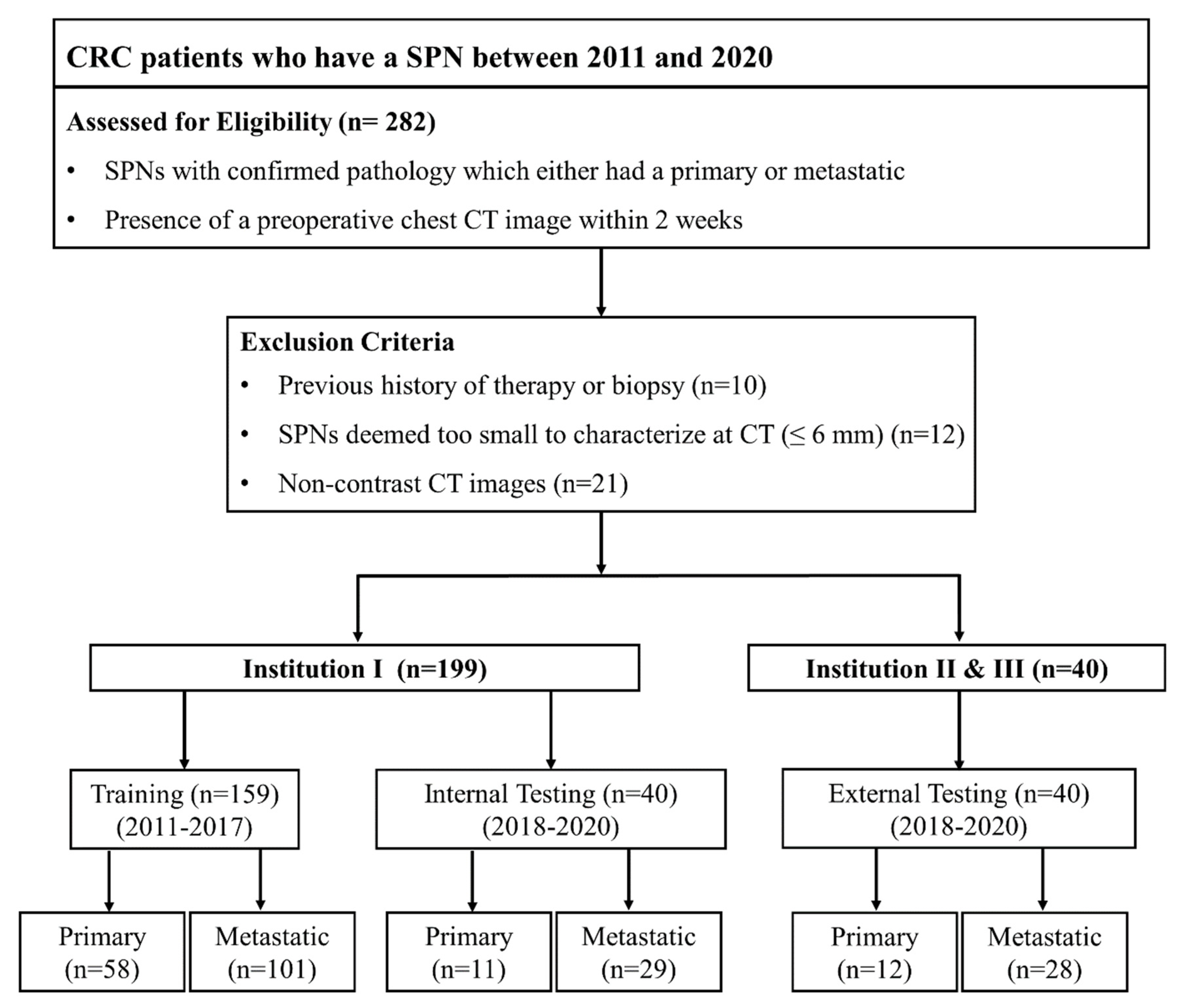
2.1. Patient Selection
2.2. CT Data Acquisition
2.3. Visual Analysis of CT Images
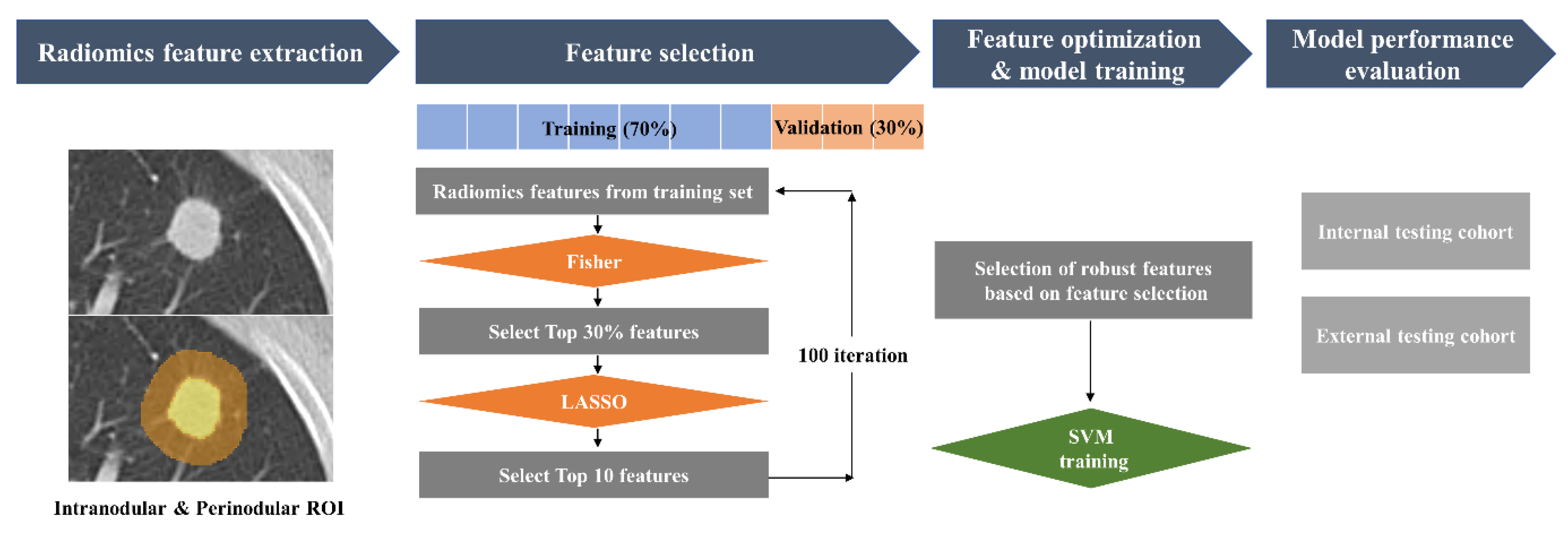
2.4. Region of Interest (ROI) Segmentation and Radiomics Features Extraction
2.5. Feature Selection and Model Training
2.6. Model Performance Evaluation and Reader Test
2.7. Comparison between the Radiomics and Semantic CT Imaging Features
2.8. Statistical Analysis
3. Results
3.1. Analysis of Semantic CT Imaging Features
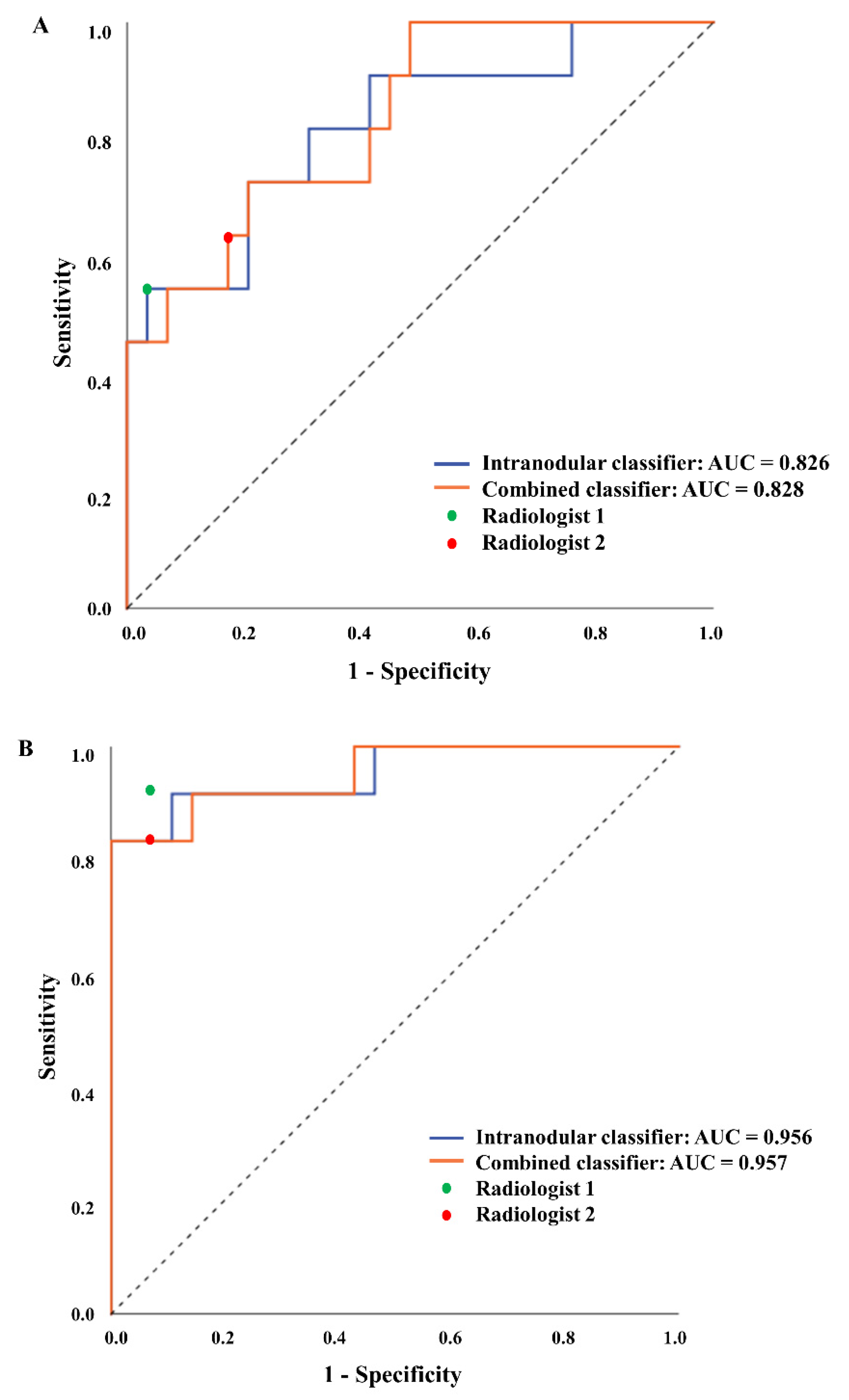
3.2. Radiomics Model Performance and Comparison with Radiologists
3.3. Associations between the Radiomics and Semantic CT Imaging Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.H.; Huh, J.W.; Kim, H.R.; Kim, Y.J. Indeterminate pulmonary nodules in colorectal cancer: Follow-up guidelines based on a risk predictive model. Ann. Surg. 2015, 261, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Schmidt, L.; Greenson, J.K.; Chang, A.C.; Myers, J.L. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: Case report and review of literature. Am. J. Clin. Pathol. 2009, 131, 129–133. [Google Scholar] [CrossRef]
- Peng, Y.-F.; Gu, J. Synchronous colorectal and lung cancer: Report of three cases. World J. Gastroenterol. 2008, 14, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Rena, O.; Davoli, F.; Boldorini, R.; Roncon, A.; Baietto, G.; Papalia, E.; Turello, D.; Massera, F.; Casadio, C. The solitary pulmonary nodule in patients with previous cancer history: Results of surgical treatment. Eur. J. Surg. Oncol. 2013, 3, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Varoli, F.; Vergani, C.; Caminiti, R.; Francese, M.; Gerosa, C.; Bongini, M.; Roviaro, G. Management of solitary pulmonary nodule. Eur. J. Cardiothorac. Surg. 2008, 33, 461–465. [Google Scholar] [CrossRef]
- Lee, W.-S.; Yun, S.H.; Chun, H.-K.; Lee, W.-Y.; Yun, H.-R.; Kim, J.; Kim, K.; Shim, Y.M. Pulmonary resection for metastases from colorectal cancer: Prognostic factors and survival. Int. J. Colorectal Dis. 2007, 22, 699–704. [Google Scholar] [CrossRef]
- Snoeckx, A.; Reyntiens, P.; Desbuquoit, D.; Spinhoven, M.J.; Van Schil, P.E.; van Meerbeeck, J.P.; Parizel, P.M. Evaluation of the solitary pulmonary nodule: Size matters, but do not ignore the power of morphology. Insights Imaging 2018, 9, 73–86. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, H.-J.; Sung, P.; Yoen, H.; Kim, S.; Han, S.; Park, S.; Hong, J.H.; Kim, H.; Lim, J. A novel algorithm to differentiate between multiple primary lung cancers and intrapulmonary metastasis in multiple lung cancers with multiple pulmonary sites of involvement. J. Thorac. Oncol. 2020, 15, 203–215. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Choy, G.; Khalilzadeh, O.; Michalski, M.; Do, S.; Samir, A.E.; Pianykh, O.S.; Geis, J.R.; Pandharipande, P.V.; Brink, J.A.; Dreyer, K.J. Current applications and future impact of machine learning in radiology. Radiology 2018, 288, 318–328. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.-X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Park, I.; Chang, S.E.; Lim, W.; Kim, M.S.; Park, G.H.; Chae, J.B.; Huh, C.H.; Na, J.-I. Augmented intelligence dermatology: Deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J. Investig. Dermatol. 2020, 140, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Beig, N.; Khorrami, M.; Alilou, M.; Prasanna, P.; Braman, N.; Orooji, M.; Rakshit, S.; Bera, K.; Rajiah, P.; Ginsberg, J. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology 2019, 290, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Devaraj, A. Radiomics of pulmonary nodules and lung cancer. Transl. Lung Cancer Res. 2017, 6, 86–91. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Hu, T.; Wang, S.; Huang, L.; Wang, J.; Shi, D.; Li, Y.; Tong, T.; Peng, W. A clinical-radiomics nomogram for the preoperative prediction of lung metastasis in colorectal cancer patients with indeterminate pulmonary nodules. Eur. Radiol. 2019, 29, 439–449. [Google Scholar] [CrossRef]
- Inamura, K.; Satoh, Y.; Okumura, S.; Nakagawa, K.; Tsuchiya, E.; Fukayama, M.; Ishikawa, Y. Pulmonary adenocarcinomas with enteric differentiation: Histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am. J. Surg. Pathol. 2005, 29, 660–665. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeong, W.G.; Kim, Y.-H. Differentiation of primary lung cancer from solitary lung metastasis in patients with colorectal cancer: A retrospective cohort study. World J. Surg. Oncol. 2021, 19, 28. [Google Scholar] [CrossRef]
- Limkin, E.J.; Reuzé, S.; Carré, A.; Sun, R.; Schernberg, A.; Alexis, A.; Deutsch, E.; Ferté, C.; Robert, C. The complexity of tumor shape, spiculatedness, correlates with tumor radiomic shape features. Sci. Rep. 2019, 9, 4329. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Paris, M.T.; Mourtzakis, M. Muscle composition analysis of ultrasound images: A narrative review of texture analysis. Ultrasound Med. Biol. 2021, 47, 880–895. [Google Scholar] [CrossRef]
- Feng, Q.; Ding, Z. MRI radiomics classification and prediction in Alzheimer’s disease and mild cognitive impairment: A review. Curr. Alzheimer Res. 2020, 17, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Lee, N.; Ahn, S.S.; Chang, J.H.; Lee, S.-K. Radiomics and Deep Learning in Brain Metastases: Current Trends and Roadmap to Future Applications. Investig. Magn. Reson. Imaging 2021, 25, 266–280. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wee, W.G. Neighboring gray level dependence matrix for texture classification. Comput. Vis. Graph. Image Process. 1983, 23, 341–352. [Google Scholar] [CrossRef]
- Chu, Z.-G.; Zhang, Y.; Li, W.-J.; Li, Q.; Zheng, Y.-N.; Lv, F.-J. Primary solid lung cancerous nodules with different sizes: Computed tomography features and their variations. BMC Cancer 2019, 19, 1060. [Google Scholar] [CrossRef] [PubMed]
- Debray, T.P.; Vergouwe, Y.; Koffijberg, H.; Nieboer, D.; Steyerberg, E.W.; Moons, K.G. A new framework to enhance the interpretation of external validation studies of clinical prediction models. J. Clin. Epidemiol. 2015, 68, 279–289. [Google Scholar] [CrossRef]

| Training (n = 159) | Internal Testing (n = 40) | External Testing (n = 40) | |
|---|---|---|---|
| Age (years) | 65.9 ± 9.96 | 65.8 ± 10.68 | 66.1 ± 8.37 |
| Sex | |||
| Male | 107 (67.3) | 24 (60.0) | 26 (65.0) |
| Female | 52 (32.7) | 16 (40.0) | 14 (35.0) |
| History of smoking | |||
| Never | 88 (55.3) | 25 (62.5) | 21 (52.5) |
| Yes | 71 (44.7) | 15 (37.5) | 19 (47.5) |
| Index tumor location | |||
| Colon | 63 (39.6) | 25 (62.5) | 20 (50.0 |
| Rectum | 96 (60.4) | 15 (37.5) | 20 (50.0) |
| Index tumor T stage | |||
| T1-2 | 34 (21.4) | 10 (25.0) | 6 (15.0) |
| T3-4 | 126 (78.6) | 30 (75.0) | 34 (85.0) |
| Index tumor N stage | |||
| N0 | 66 (41.5) | 17 (42.5) | 12 (30.0) |
| N1-2 | 93 (58.5) | 23 (57.5) | 28 (70.0) |
| Extrathoracic metastasis | |||
| No | 139 (87.4) | 37 (92.5) | 38 (95.0) |
| Yes | 20 (12.6) | 3 (7.5) | 2 (5.0) |
| Histopathology of SPN | |||
| Metastatic | 101 (63.5) | 29 (72.5) | 28 (70.0) |
| Primary | 58 (36.5) | 11 (27.5) | 12 (30.0) |
| Primary LC (n = 81) | Solitary LM (n = 158) | p Value | |
|---|---|---|---|
| Size (mm) | 21.4 ± 7.7 | 14.7 ± 6.2 | <0.001 |
| Margin | <0.001 | ||
| Smooth | 9 (11.1) | 78 (49.4) | |
| Lobulated | 34 (42.0) | 70 (44.3) | |
| Spiculated | 38 (46.9) | 10 (6.3) | |
| Density | <0.001 | ||
| Solid | 54 (66.7) | 157 (99.4) | |
| Subsolid | 27 (33.3) | 1 (0.6) | |
| Air-bronchogram | <0.001 | ||
| Absent | 48 (59.3) | 151 (95.6) | |
| Present | 33 (40.7) | 7 (4.4) | |
| Cavitation | 0.459 | ||
| Absent | 72 (88.9) | 135 (85.4) | |
| Present | 9 (11.1) | 23 (14.6) | |
| Pleural tag | <0.001 | ||
| Absent | 34 (42.0) | 126 (79.7) | |
| Present | 47 (58.0) | 32 (20.3) |
| Internal Test | External Test | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | Sensitivity | Specificity | AUC | |
| SVM models | ||||||
| Intranodular | 0.545 | 0.828 | 0.826 | 0.833 | 0.964 | 0.956 |
| Combined | 0.545 | 0.966 | 0.828 | 0.833 | 1.000 | 0.957 |
| Radiologists | ||||||
| Reader 1 | 0.545 | 0.966 | - | 0.917 | 0.929 | - |
| Reader 2 | 0.636 | 0.828 | - | 0.833 | 0.929 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Do, L.N.; Jeong, W.G.; Lee, H.J.; Chae, K.J.; Kim, Y.H.; Park, I. A Radiomics Approach on Chest CT Distinguishes Primary Lung Cancer from Solitary Lung Metastasis in Colorectal Cancer Patients. J. Pers. Med. 2022, 12, 1859. https://doi.org/10.3390/jpm12111859
Lee JE, Do LN, Jeong WG, Lee HJ, Chae KJ, Kim YH, Park I. A Radiomics Approach on Chest CT Distinguishes Primary Lung Cancer from Solitary Lung Metastasis in Colorectal Cancer Patients. Journal of Personalized Medicine. 2022; 12(11):1859. https://doi.org/10.3390/jpm12111859
Chicago/Turabian StyleLee, Jong Eun, Luu Ngoc Do, Won Gi Jeong, Hyo Jae Lee, Kum Ju Chae, Yun Hyeon Kim, and Ilwoo Park. 2022. "A Radiomics Approach on Chest CT Distinguishes Primary Lung Cancer from Solitary Lung Metastasis in Colorectal Cancer Patients" Journal of Personalized Medicine 12, no. 11: 1859. https://doi.org/10.3390/jpm12111859
APA StyleLee, J. E., Do, L. N., Jeong, W. G., Lee, H. J., Chae, K. J., Kim, Y. H., & Park, I. (2022). A Radiomics Approach on Chest CT Distinguishes Primary Lung Cancer from Solitary Lung Metastasis in Colorectal Cancer Patients. Journal of Personalized Medicine, 12(11), 1859. https://doi.org/10.3390/jpm12111859







