Vitamin D May Be Connected with Health-Related Quality of Life in Psoriasis Patients Treated with Biologics
Abstract
1. Introduction
2. Materials and Methods
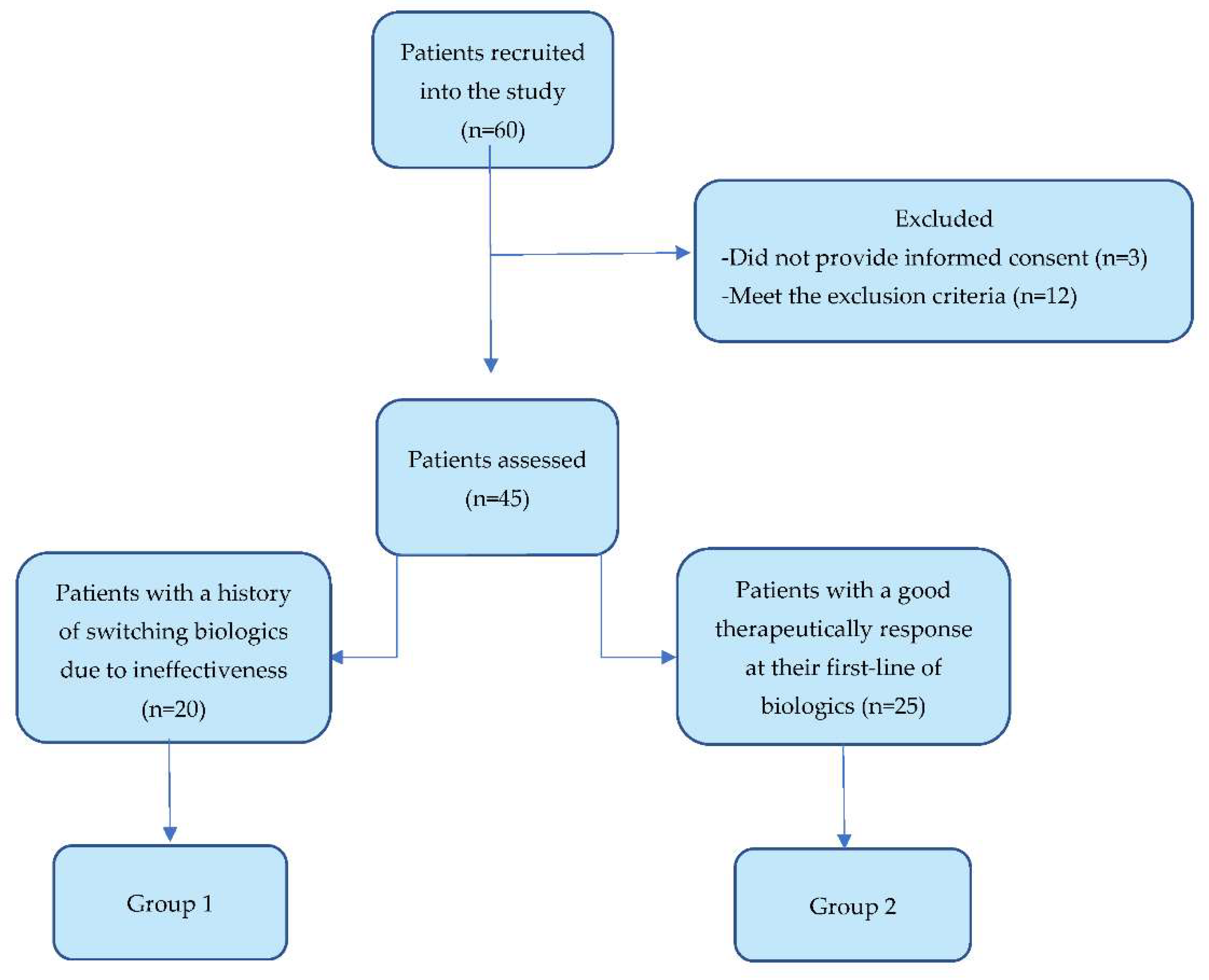
2.1. Selection and Distribution of Patients into Groups
2.2. Measurement of Vitamin D and Other Variables
2.3. Statistical Analysis
3. Results
3.1. The Analysis of Demographic Characteristics and Disease-Related Aspects
3.2. The Analysis of Biological Treatment Evolution Regarding Vitamin D Aspects
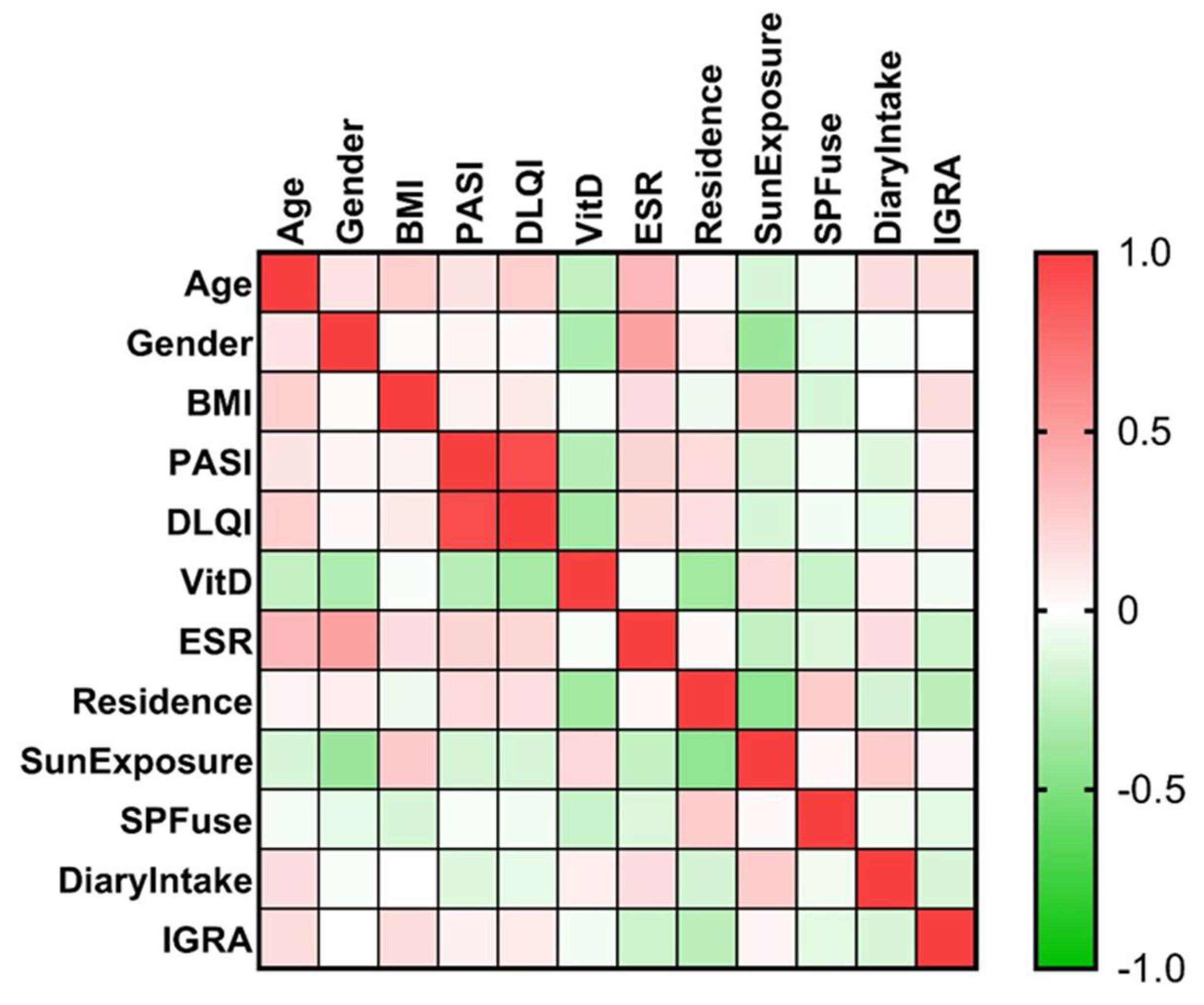
3.3. Factors That Might Influence the Vitamin D Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hugh, J.M.; Weinberg, J.M. Update on the pathophysiology of psoriasis. Cutis 2018, 102, 6–12. [Google Scholar] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA-J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Pitukweerakul, S.; Thavaraputta, S.; Prachuapthunyachart, S.; Karnchanasorn, R. Hypovitaminosis D is Associated with Psoriasis: A Systematic Review and Meta- Analysis. Kansas J. Med. 2019, 12, 103–108. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin d on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Di Liberto, D.; Scazzone, C.; La Rocca, G.; Cipriani, P.; Lo Pizzo, M.; Ruscitti, P.; Agnello, L.; Ciaccio, M.; Dieli, F.; Giacomelli, R.; et al. Erratum: Vitamin D increases the production of IL-10 by regulatory T cells in patients with systemic sclerosis (Clinical and experimental rheumatology (2019) 37 Suppl 119 4 (76-81)). Clin. Exp. Rheumatol. 2020, 38, 1276. [Google Scholar]
- Lee, Y.H.; Song, G.G. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: A meta-analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef]
- Roche, H.; Bouiller, K.; Puzenat, E.; Deveza, E.; Roche, B.; Pelletier, F.; van de Laak, A.; Dupond, A.S.; Nardin, C.; Aubin, F. Efficacy and survival of biologic agents in psoriasis: A practical real-life 12-year experience in a French dermatology department. J. Dermatol. Treat. 2019, 30, 540–544. [Google Scholar] [CrossRef]
- Ruggiero, A.; Fabbrocini, G.; Cinelli, E.; Ocampo Garza, S.S.; Camela, E.; Megna, M. Anti-interleukin-23 for psoriasis in elderly patients: Guselkumab, risankizumab and tildrakizumab in real-world practice. Clin. Exp. Dermatol. 2022, 47, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; O’Toole, A.; Gooderham, M. Real-world experience with risankizumab in patients with plaque psoriasis: A retrospective study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e685–e688. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Picone, V.; Martora, F.; Fabbrocini, G.; Megna, M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Psoriasis: A Review of the Real-World Evidence. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.C.; Gupta, R.; Brown, G.; Malakouti, M.; Koo, J. Biologic fatigue in psoriasis. J. Dermatol. Treat. 2014, 25, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Polesie, S.; Gillstedt, M.; Osmancevic, A. Impact of Etanercept on Vitamin D Status and Vitamin D-binding Protein in Bio-naïve Patients with Psoriasis. Acta Derm. Venereol. 2021, 101, adv00604. [Google Scholar] [CrossRef]
- Filoni, A.; Congedo, M.; Lobreglio, D.; Caldarola, G.; Lobreglio, G.; De Simone, C.; Bonamonte, D. Free and total vitamin D in psoriatic patients treated with biological drugs. Exp. Dermatol. 2021, 30, 995–996. [Google Scholar] [CrossRef]
- Grassi, T.; Panico, A.; Bagordo, F.; Imbriani, G.; Gambino, I.; Lobreglio, D.; Lobreglio, G.; Congedo, M.; De Donno, A. Direct detection of free vitamin D as a tool to assess risk conditions associated with chronic plaque psoriasis. J. Prev. Med. Hyg. 2020, 61, E489–E495. [Google Scholar] [CrossRef]
- Ganzetti, G.; Campanati, A.; Scocco, V.; Brugia, M.; Tocchini, M.; Liberati, G.; Giuliodori, K.; Brisigotti, V.; Offidani, A. The potential effect of the tumour necrosis factor-α inhibitors on vitamin D status in psoriatic patients. Acta Derm. Venereol. 2014, 94, 715–717. [Google Scholar] [CrossRef]
- Zator, Z.A.; Cantu, S.M.; Konijeti, G.G.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Ananthakrishnan, A.N. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. J. Parenter. Enter. Nutr. 2014, 38, 385–391. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Valle, G.A.; Bata-Csörgö, Z.; Boonen, H.; de Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Meca, A.D.; Subțirelu, M.S.; Turcu-știolica, A.; Nițu, F.M.; Bogdan, M.; Matei, M.; Cioboată, R.; Stănoiu, B.P.; Pisoschi, C.G. Prevalence of tuberculosis in south-west romania and the direct costs burden. Farmacia 2021, 69, 174–181. [Google Scholar] [CrossRef]
- Megna, M.; Ferrillo, M.; Ruggiero, A.; Cinelli, E.; Gallo, L.; Fabbrocini, G. QuantiFERON TB-gold conversion rate among psoriasis patients under biologics: A 9-year retrospective study. Int. J. Dermatol. 2021, 60, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hu, Y.L.; Wang, Y.H.; Chen, C.H.; Lai, C.C.; Huang, K.L. Association between vitamin D and latent tuberculosis infection in the United States: NHANES, 2011–2012. Infect. Drug Resist. 2019, 12, 2251–2257. [Google Scholar] [CrossRef]
- Meca, A.-D.; Ștefănescu, S.; Bogdan, M.; Turcu-stiolică, A.; Nițu, F.; Matei, M.; Cioboată, R.; Bugă, A.; Pisoschi, C.-G. Crosstalk between vitamin D axis, inflammation and host immunity mechanisms: A prospective study. Exp. Ther. Med. 2021, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Annex I List of the Names, Pharmaceutical Form(s), Strength(s) of the Medicinal Product(s), Route(s) of Administration, Applicant(s) Marketing Authorisation Holder(s) in the Member States. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/daivobet (accessed on 27 October 2022).
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef]
- Niculescu, D.A.; Capatina, C.A.M.; Dusceac, R.; Caragheorgheopol, A.; Ghemigian, A.; Poiana, C. Seasonal variation of serum vitamin D levels in Romania. Arch. Osteoporos. 2017, 12, 113. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on Clinial Investigation of Medicinal Products Indicated for the Treatment of Psoriasis; European Medicines Agency: London, UK, 2004; pp. 1–18.
- Lewis, V.; Finlay, A.Y. 10 Years experience of the Dermatology Life Quality Index (DLQI). J. Investig. Dermatol. Symp. Proc. 2004, 9, 169–180. [Google Scholar] [CrossRef]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Salek, M.S.; Finlay, A.Y. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J. Investig. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Valvano, M.; Magistroni, M.; Mancusi, A.; D’ascenzo, D.; Longo, S.; Stefanelli, G.; Vernia, F.; Viscido, A.; Necozione, S.; Latella, G. The usefulness of serum vitamin D levels in the assessment of IBD activity and response to biologics. Nutrients 2021, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Patruno, C.; Bongiorno, M.R.; Gambardella, A.; Guarneri, C.; Foti, C.; Lembo, S.; Loconsole, F.; Fabbrocini, G. Lack of reactivation of tuberculosis in patients with psoriasis treated with secukinumab in a real-world setting of latent tuberculosis infection. J. Dermatol. Treat. 2022, 33, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Warren, R.B.; Torres, T. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL-23 inhibitors in psoriasis–time for a paradigm change. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral vitamin D therapy in patients with psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral vitamin D3 supplementation for chronic plaque psoriasis: A randomized, double-blind, placebo-controlled trial. J. Dermatol. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.R.; Audish, D.; Kotol, P.; Coda, A.; Kabigting, F.; Miller, J.; Alexandrescu, D.; Boguniewicz, M.; Taylor, P.; Aertker, L.; et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 781–789. [Google Scholar] [CrossRef]
- Jarrett, P.; Camargo, C.A.; Coomarasamy, C.; Scragg, R. A randomized, double-blind, placebo-controlled trial of the effect of monthly vitamin D supplementation in mild psoriasis. J. Dermatol. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef]
- Rapp, S.R.; Feldman, S.R.; Exum, M.L.; Fleischer, A.B.; Reboussin, D.M. Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol. 1999, 41, 401–407. [Google Scholar] [CrossRef]
- Ju, S.Y.; Lee, Y.J.; Jeong, S.N. Serum 25-hydroxyvitamin D levels and the risk of depression: A systematic review and meta-analysis. J. Nutr. Health Aging 2013, 17, 447–455. [Google Scholar] [CrossRef]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; Sarah, D.M. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Ford, D.E. Role of depression in quality of life for patients with psoriasis. Dermatology 2007, 215, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A.; Nedoszytko, B.; Żmijewski, M.A.; Cubała, W.J.; Slominski, A.T. The Brain–Skin Axis in Psoriasis—Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int. J. Mol. Sci. 2022, 23, 669. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, E2133–E2137. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Harinarayan, C.V.; Ramalakshmi, T.; Prasad, U.V.; Sudhakar, D.; Srinivasarao, P.V.L.N.; Sarma, K.V.S.; Kumar, E.G.T. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am. J. Clin. Nutr. 2007, 85, 1062–1067. [Google Scholar] [CrossRef]
- Holick, M.F. McCollum award lecture, 1994: Vitamin D-New horizons for the 21st century. Am. J. Clin. Nutr. 1994, 60, 619–630. [Google Scholar] [CrossRef]
- Nurbazlin, M.; Chee, W.S.S.; Rokiah, P.; Tan, A.T.B.; Chew, Y.Y.; Nusaibah, A.R.S.; Chan, S.P. Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 391–399. [Google Scholar] [CrossRef]
- Wakayo, T.; Belachew, T.; Vatanparast, H.; Whiting, S.J. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: The case of school children in Central Ethiopia. PLoS ONE 2015, 10, e0120963. [Google Scholar] [CrossRef]
- Chailurkit, L.O.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef]
- Koon Poh, B.; Rojroongwasinkul, N.; Khanh Le Nguyen, B.; Sandjaja, S.; Ruzita, A.T.; Yamborisut, U.; Hong, T.N.; Ernawati, F.; Deurenberg, P.; Parikh, P. 25-Hydroxy-Vitamin D Demography and the Risk of Vitamin D insufficiency in the South East Asian Nutrition Surveys (SEANUTS). Asia Pac. J. Clin. Nutr. 2016, 25, 538–548. [Google Scholar] [CrossRef]
- Grigorie, D.; Sucaliuc, A.; Ivan, M.; Neacsu, E.; Popa, O.; Diaconescu, A. High prevalence of vitamin D deficiency in 1048 Romanian women with postmenopausal osteoporosis. Acta Endocrinol. 2008, 4, 33–45. [Google Scholar] [CrossRef]
- O’Mahony, L.; Stepien, M.; Gibney, M.J.; Nugent, A.P.; Brennan, L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef]
- Cashman, K.D.; Ritz, C.; Kiely, M. Improved dietary guidelines for vitamin D: Application of individual participant data (IPD)-level meta-regression analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef]
| Mean ± SD Median (Interquartile Range) Range | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Vitamin D serum concentration | 0.443 | ||
| Hypovitaminosis | 3 (15%) | 4 (16%) | |
| Insufficiency | 10 (50%) | 8 (32%) | |
| Normal | 7 (35%) | 13 (52%) | |
| Age category | 58.90 ± 11.0 | 54.88 ± 10.59 | |
| 64.50 (48.75–68.50) | 52 (49–65) | 0.262 | |
| 39–75 | 29–71 | ||
| <60 years | 9 (45%) | 16 (55%) | 0.821 |
| Hypovitaminosis | 1 (5%) | 1 (4%) | |
| Insufficiency | 4 (20%) | 6 (24%) | |
| Normal | 4 (20%) | 9 (36%) | |
| >60 years | 11 (51%) | 9 (36%) | 0.603 |
| Hypovitaminosis | 2 (10%) | 3 (12%) | |
| Insufficiency | 6 (24%) | 3 (12%) | |
| Normal | 3 (15%) | 3 (12%) | |
| Psoriasis onset < 40 years (type I) | 10 (50%) | 12 (48%) | 0.438 |
| Hypovitaminosis | 1 (5%) | 0 (0%) | |
| Insufficiency | 5 (25%) | 5 (20%) | |
| Normal | 4 (20%) | 7 (28%) | |
| Psoriasis onset > 40 years (type II) | 10 (50%) | 13 (42%) | 0.637 |
| Hypovitaminosis | 2 (10%) | 4 (16%) | |
| Insufficiency | 5 (25%) | 4 (16%) | |
| Normal | 3 (15%) | 5 (20%) | |
| Gender | |||
| Female | 7 (35%) | 11 (44%) | 0.557 |
| Hypovitaminosis | 1 (5%) | 3 (12%) | |
| Insufficiency | 5 (25%) | 5 (20%) | |
| Normal | 1 (5%) | 3 (12%) | |
| Male | 13 (65%) | 14 (56%) | 0.603 |
| Hypovitaminosis | 2 (10%) | 1 (4%) | |
| Insufficiency | 5 (25%) | 4 (14%) | |
| Normal | 6 (24%) | 9 (36%) | |
| Residence | |||
| Urban | 13 (65%) | 12 (48%) | 0.368 |
| Hypovitaminosis | 2 (10%) | 3 (12%) | |
| Insufficiency | 8 (40%) | 4 (16%) | |
| Normal | 3 (15%) | 5 (20%) | |
| Rural | 7 (35%) | 13 (52%) | 0.846 |
| Hypovitaminosis | 1 (5%) | 1 (4%) | |
| Insufficiency | 2 (10%) | 5 (20%) | |
| Normal | 4 (20%) | 7 (28%) | |
| BMI (kg/m2) | 31.05 ± 6.77 | 30.48 ± 5.2 | |
| 32.7 (25.25–35.25) | 30.86 (26.62–34.69) | 0.775 | |
| 19.05–43.25 | 19.84–38.48 | ||
| >30 kg/m2 | 12 | 16 | 0.343 |
| Hypovitaminosis | 1 (5%) | 3 (12%) | |
| Insufficiency | 7 (35%) | 5 (20%) | |
| Normal | 4 (20%) | 8 (32%) | |
| <30 kg/m2 | 8 | 9 | 0.755 |
| Hypovitaminosis | 2 (10%) | 1 (4%) | |
| Insufficiency | 3 (15%) | 4 (16%) | |
| Normal | 3 (15%) | 4 (16%) | |
| ESR | 0.472 | ||
| Hypovitaminosis | 3 (15%) | 5 (20%) | |
| Insufficiency | 10 (50%) | 8 (32%) | |
| Normal | 7 (35%) | 12 (48%) | |
| IGRA result | |||
| Positive | 9 (45%) | 16 (64%) | 0.788 |
| Hypovitaminosis | 2 (10%) | 3 (12%) | |
| Insufficiency | 4 (20%) | 6 (24%) | |
| Normal | 3 (15%) | 7 (28%) | |
| Negative | 11 (55%) | 9 (36%) | 0.353 |
| Hypovitaminosis | 4 (20%) | 5 (20%) | |
| Insufficiency | 6 (30%) | 2 (8%) | |
| Normal | 4 (20%) | 5 (20%) |
| Mean ± SD Median (Interquartile Range) Range | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Anti-TNFα agent | 2 (10%) | 16 (64%) | <0.0001 **** |
| Anti-IL agent | 18 (90%) | 9 (36%) | |
| Etanercept | 2 (10%) | 9 (36%) | 0.01 * |
| Adalimumab | 0 | 6 (24%) | |
| Infliximab | 0 | 1 (4%) | |
| Secukinumab | 7 (35%) | 2 (8%) | |
| Risankizumab | 5 (25%) | 1 (4%) | |
| Ustekinumab | 2 (10%) | 3 (12%) | |
| Ixekizumab | 4 (20%) | 3 (12%) | |
| Number of patients with hypovitaminosis | 3 (15%) | 4 (16%) | |
| Treated with TNF-α inhibitors | 1 (5%) | 2 (8%) | |
| Treated with IL-inhibitors | 2 (10%) | 2 (8%) | |
| Vitamin D (ng/mL) | 18.09 ± 0.75 (17.44–19.15) | 15.22 ± 2.67 (11.68–19.92) | |
| PASI score | 5.83 ± 4.69 0–11.50 | 2.62 ± 2.82 1–11 | |
| DLQI score | 6.33 ± 4.10 0.80–8.00 | 2.20 ± 1.60 1–4 | |
| Number of patients with insufficiency | 10 (50%) | 9 (36%) | |
| Treated with TNF-α inhibitors | 0% | 6 (24%) | |
| Treated with IL-inhibitors | 10 (50%) | 3 (12%) | |
| Vitamin D (ng/mL) | 24.29 ± 3.12 (20.04–29.58) | 24.21 ± 3.10 (20.58–28.61) | |
| PASI score | 3.51 ± 3.34 0–11.50 | 3.15 ± 3.89 0–10.20 | |
| DLQI score | 3.7 ± 4.05 0–12 | 2.62 ± 3.56 0–10 | |
| Number of patients with a normal concentration of vitamin D | 7 (35%) | 12 (48%) | |
| Treated with TNF-α inhibitors | 1 (5%) | 8 (32%) | |
| Treated with IL-inhibitors | 6 (30%) | 4 (16%) | |
| Vitamin D (ng/mL) | 43.82 ± 6.63 (33.96–50.84) | 42.38 ± 10.30 (31.57–63.62) | |
| PASI score | 3.84 ± 4.62 0.50–14.40 | 0.68 ±1.26 0–4.20 | |
| DLQI score | 3 ± 3.85 0–12 | 0.75 ± 1.36 0–4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paliu, I.-A.; Ianosi, S.-L.; Turcu-Stiolica, A.; Pisoschi, C.-G.; Predoi, L.-G.; Tica, A.-A. Vitamin D May Be Connected with Health-Related Quality of Life in Psoriasis Patients Treated with Biologics. J. Pers. Med. 2022, 12, 1857. https://doi.org/10.3390/jpm12111857
Paliu I-A, Ianosi S-L, Turcu-Stiolica A, Pisoschi C-G, Predoi L-G, Tica A-A. Vitamin D May Be Connected with Health-Related Quality of Life in Psoriasis Patients Treated with Biologics. Journal of Personalized Medicine. 2022; 12(11):1857. https://doi.org/10.3390/jpm12111857
Chicago/Turabian StylePaliu, Iulia-Alexandra, Simona-Laura Ianosi, Adina Turcu-Stiolica, Catalina-Gabriela Pisoschi, Luminita-Georgeta Predoi, and Andrei-Adrian Tica. 2022. "Vitamin D May Be Connected with Health-Related Quality of Life in Psoriasis Patients Treated with Biologics" Journal of Personalized Medicine 12, no. 11: 1857. https://doi.org/10.3390/jpm12111857
APA StylePaliu, I.-A., Ianosi, S.-L., Turcu-Stiolica, A., Pisoschi, C.-G., Predoi, L.-G., & Tica, A.-A. (2022). Vitamin D May Be Connected with Health-Related Quality of Life in Psoriasis Patients Treated with Biologics. Journal of Personalized Medicine, 12(11), 1857. https://doi.org/10.3390/jpm12111857








