Optimal Nozzle Position and Patient’s Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model
Abstract
1. Introduction
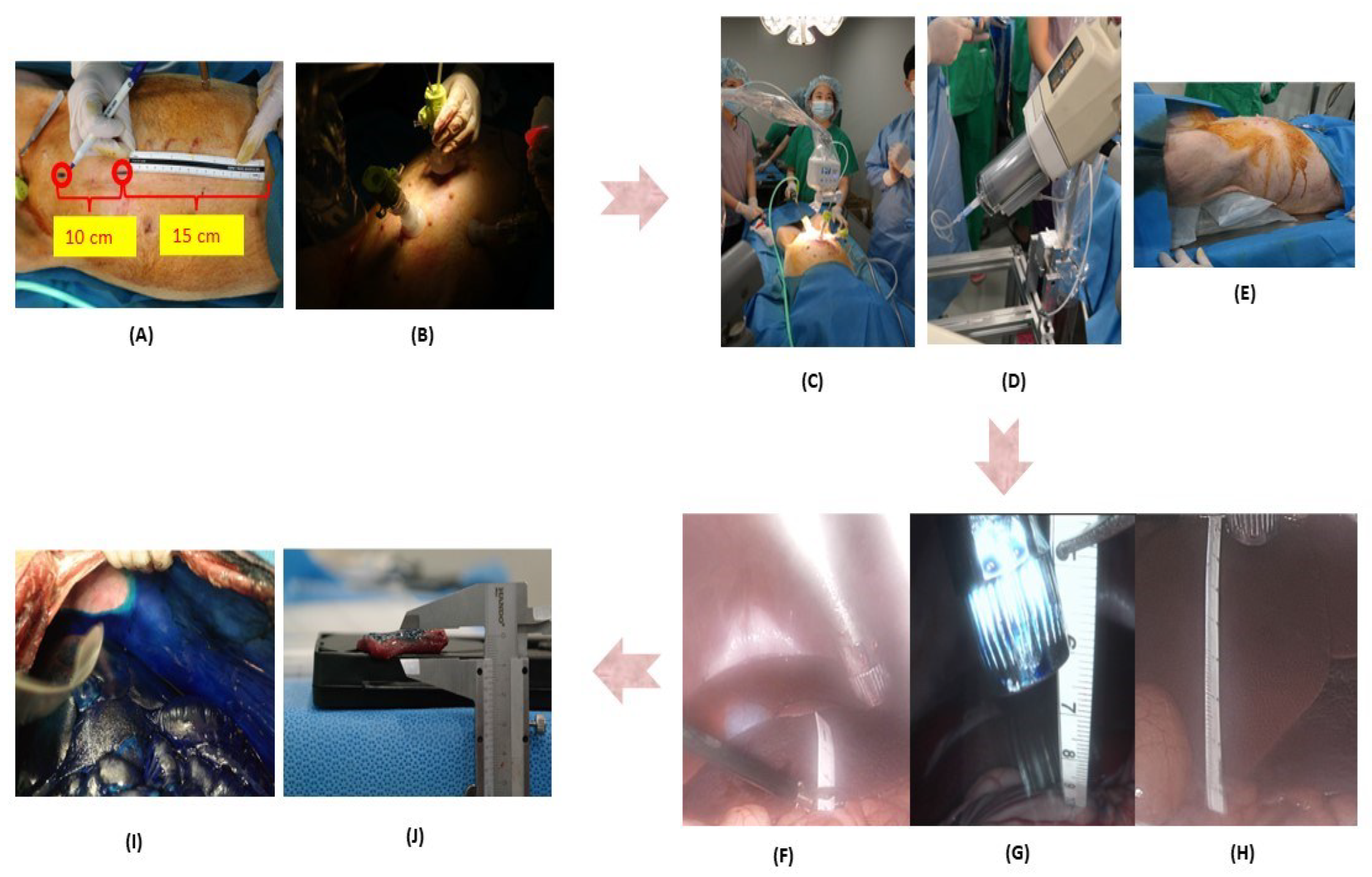
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzi, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef]
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J. Clin. 2015, 65, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Van Oudheusden, T.R.; Grull, H.; Dankers, P.Y.W.; De Hingh, I. Targeting the peritoneum with novel drug delivery systems in peritoneal carcinomatosis: A review of the literature. Anticancer Res. 2015, 35, 627–634. [Google Scholar] [PubMed]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A.; et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Odendahl, K.; Solass, W.; Demtröder, C.; Giger-Pabst, U.; Zieren, J.; Tempfer, C.; Reymond, M. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur. J. Surg. Oncol. (EJSO) 2015, 41, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Hendrix, R.J. Palliative Management of Advanced Peritoneal Carcinomatosis. Surg. Oncol. Clin. North Am. 2018, 27, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Paik, H.; Park, S.J.; Lee, E.J.; Kim, H.S. Pressurized intraperitoneal aerosol chemotherapy for recurrent ovarian, fallopian or primary peritoneal cancer with peritoneal carcinomatosis: A narrative review. Gland Surg. 2021, 10, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Cho, J.; Lee, E.J.; Park, S.; Park, S.J.; Seol, A.; Lee, N.; Yim, G.W.; Lee, M.; Lim, W.; et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: A systematic review and meta-analysis. Medicine 2019, 98, e18355. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Kurtz, F.; Struller, F.; Horvath, P.; Solass, W.; Bösmüller, H.; Königsrainer, A.; Reymond, M.A. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterol. Res. Pract. 2018, 2018, 2743985. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Khosrawipour, T.; Kern, A.J.P.; Osma, A.; Kabakci, B.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Fakhrian, K. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J. Cancer Res. Clin. Oncol. 2016, 142, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Giger-Pabst, U. Exploring the Spatial Drug Distribution Pattern of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Ann. Surg. Oncol. 2015, 23, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Park, S.J.; Kim, H.S. Rotational intraperitoneal pressurized aerosol chemotherapy in a porcine model. Gland Surg. 2021, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, E.J.; Lee, H.S.; Kim, J.; Park, S.; Ham, J.; Mun, J.; Paik, H.; Lim, H.; Seol, A.; et al. Development of rotational intraperitoneal pressurized aerosol chemotherapy to enhance drug delivery into the peritoneum. Drug Deliv. 2021, 28, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, J.; Lee, E.J.; Park, S.J.; Mun, J.; Paik, H.; Oh, S.H.; Park, S.; Ryu, S.; Lim, W.; et al. Evaluation of a Novel Prototype for Pressurized Intraperitoneal Aerosol Chemotherapy. Cancers 2020, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Park, S.J.; Lee, H.; Kim, J.; Park, S.; Lee, N.; Kim, S.I.; Lee, M.; Song, G.; Lee, J.C.; et al. Ideal Nozzle Position During Pressurized Intraperitoneal Aerosol Chemotherapy in an Ex Vivo Model. Anticancer Res. 2021, 41, 5489–5498. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, E.J.; Seol, A.; Park, S.; Ham, J.; Yim, G.W.; Shim, S.-H.; Lim, W.; Chang, S.-J.; Song, G.; et al. Rotational intraperitoneal pressurized aerosol chemotherapy with paclitaxel and cisplatin: Pharmacokinetics, tissue concentrations, and toxicities in a pig model. J. Gynecol. Oncol. 2022, 33, e56. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Khosrawipour, T.; Falkenstein, T.A.; Diaz-Carballo, D.; Förster, E.; Osma, A.; Adamietz, I.A.; Zieren, J.; Fakhrian, K. Evaluating the Effect of Micropump© Position, Internal Pressure and Doxorubicin Dosage on Efficacy of Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) in an Ex Vivo Model. Anticancer Res. 2016, 36, 4595–4600. [Google Scholar] [CrossRef] [PubMed][Green Version]

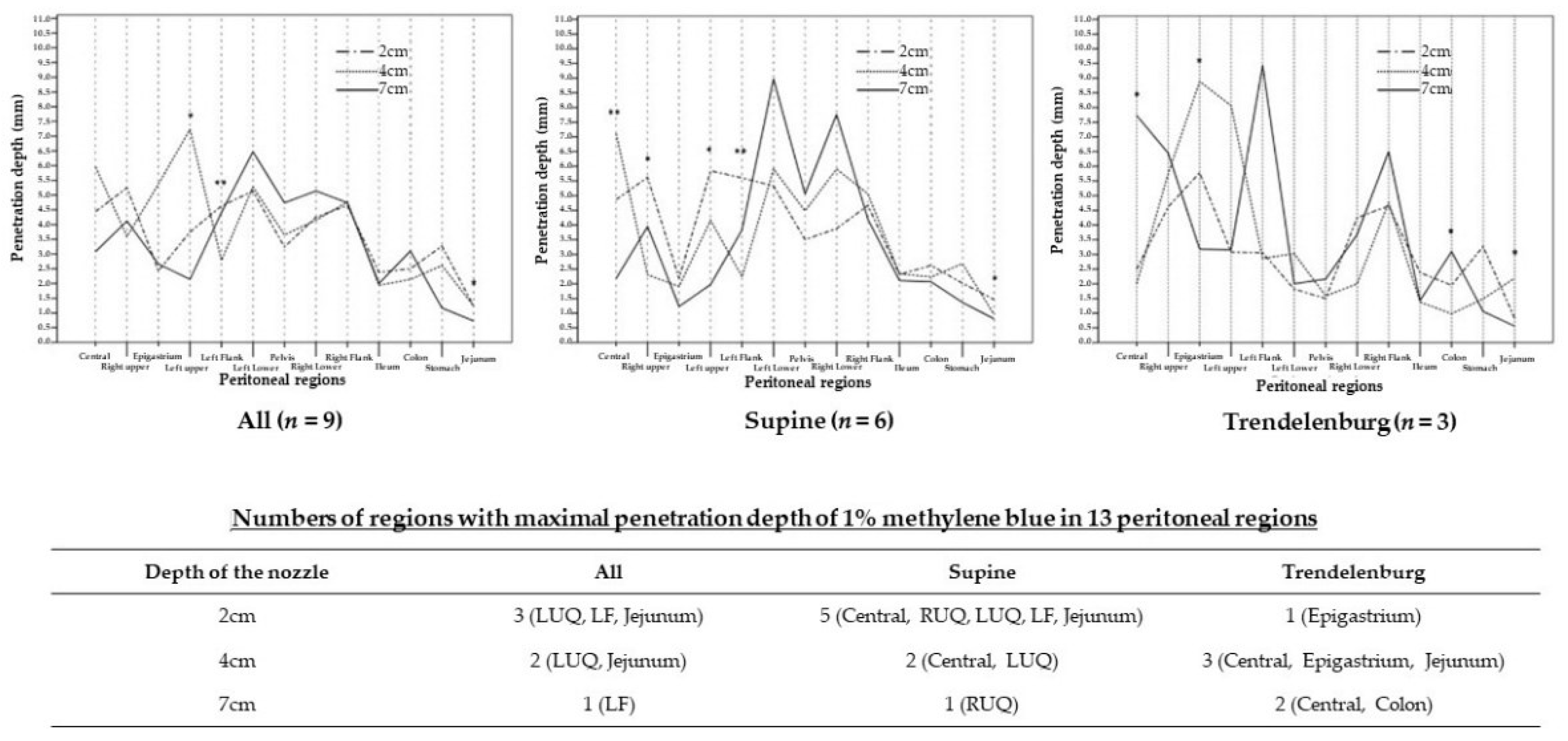
| Regions | The Depth of the Nozzle | p Value | ||
|---|---|---|---|---|
| 2 cm | 4 cm | 7 cm | ||
| Central | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| RUQ | 12 (9, 12) | 12 (6, 12) | 12 (6, 12) | 0.953 |
| Epigastrium | 6 (6, 12) | 6 (6, 12) | 9 (4, 12) | 1.000 |
| LUQ | 9 (6, 12) | 6 (6, 12) | 8 (4, 12) | 0.867 |
| LF | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| LLQ | 12 (8, 12) | 12 (4, 12) | 12 (12, 12) | 0.558 |
| Pelvis | 12 (8, 12) | 12 (1, 12) | 12 (12, 12) | 0.558 |
| RLQ | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| RF | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| Ileum | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| Colon | 12 (12, 12) | 12 (12, 12) | 12 (12, 12) | 1.000 |
| Stomach | 4 (3, 12) | 6 (3, 6) | 8 (4, 12) | 0.569 |
| Jejunum | 8 (6, 12) | 8 (1, 8) | 8 (6, 12) | 0.717 |
| Regions | The Depth of the Nozzle | p Value | ||
|---|---|---|---|---|
| 2 cm | 4 cm | 7 cm | ||
| All | ||||
| Central | 4.44 (2.00, 6.67) | 5.96 (1.75, 9.03) | 3.08 (1.28, 9.09) | 0.591 |
| RUQ | 5.25 (3.08, 7.14) | 3.59 (0.63, 6.00) | 4.12 (3.55, 6.45) | 0.143 |
| Epigastrium | 2.43 (1.29, 6.67) | 5.38 (0.88, 11.84) | 2.6 (0.65, 4.09) | 0.270 |
| LUQ | 3.75 a (2.31, 11.39) | 7.23 a,c (0.33, 10.28) | 2.14 c (1.33, 7.11) | 0.042 |
| LF | 4.63 b (1.94, 8.37) | 2.78 (1.43, 3.61) | 4.41 b (1.75, 9.44) | 0.006 |
| LLQ | 5.14 (0.91, 6.38) | 5.28 (1.21, 7.73) | 6.47 (1.43, 12.00) | 0.426 |
| Pelvis | 3.24 (1.00, 6.73) | 3.65 (1.36, 7.11) | 4.74 (1.89, 6.84) | 0.365 |
| RLQ | 4.25 (2.40, 6.36) | 4.15 (1.60, 10.77) | 5.14 (2.63, 10.53) | 0.454 |
| RF | 4.65 (1.86, 6.00) | 4.77 (1.36, 5.95) | 4.74 (2.25, 7.25) | 0.686 |
| Ileum | 2.38 (1.56, 3.30) | 1.94 (1.03, 2.89) | 2.00 (1.43, 2.31) | 0.129 |
| Colon | 2.50 (1.71, 4.08) | 2.14 (0.59, 2.82) | 3.10 (1.09, 4.85) | 0.102 |
| Stomach | 3.27 (0, 5.74) | 2.61 (1.06, 2.86) | 1.17 (0.64, 5.12) | 0.313 |
| Jejunum | 1.25 a (0.83, 2.45) | 1.25 a,c (0.63, 2.19) | 0.73 c (0.56, 1.32) | 0.048 |
| Supine position | ||||
| Central | 4.87 a (4.44, 6.67) | 7.12 a (3.94, 9.03) | 2.18 (1.28, 3.08) | 0.002 |
| RUQ | 5.62 b (3.59, 7.14) | 2.31 (0.63, 5.22) | 3.95 b (3.68, 6.29) | 0.019 |
| Epigastrium | 2.19 (1.29, 2.94) | 1.91 (0.88, 8.04) | 1.23 (0.65, 2.93) | 0.420 |
| LUQ | 5.84 a (3.33, 11.39) | 4.17 a,c (0.33, 7.44) | 1.98 c (1.33, 2.89) | 0.024 |
| LF | 5.61 (4.15, 8.37) | 2.24 c (1.43, 3.61) | 3.84 c (1.75, 5.14) | 0.005 |
| LLQ | 5.33 (5.00. 6.38) | 5.91 (4.09. 7.73) | 8.97 (4.71, 12.00) | 0.090 |
| Pelvis | 3.51 (3.16, 6.73) | 4.48 (2.69, 7.11) | 5.06 (2.37, 6.84) | 0.587 |
| RLQ | 3.88 (2.40, 6.36) | 5.91 (2.93, 10.77) | 7.77 (4.05, 10.53) | 0.115 |
| RF | 4.67 (3.81, 6.00) | 5.06 (1.88, 5.95) | 4.19 (2.25, 7.25) | 0.630 |
| Ileum | 2.32 (1.56, 3.39) | 2.34 (1.88, 2.89) | 2.11 (1.78, 2.31) | 0.584 |
| Colon | 2.63 (2.36, 4.08) | 2.24 (1.92, 2.82) | 2.07 (1.09, 4.85) | 0.413 |
| Stomach | 2.02 (0, 5.74) | 2.69 (1.90, 2.86) | 1.37 (0.67, 5.12) | 0.674 |
| Jejunum | 1.47 (1.11, 2.45) | 0.96 c (0.63, 1.54) | 0.81 c (0.67, 1.32) | 0.028 |
| Trendelenburg position | ||||
| Central | 2.50 a,b (2.00, 3.00) | 2.00 a,c (1.75, 2.25) | 7.73 b,c (6.59, 9.09) | 0.044 |
| Right upper | 4.62 (3.08, 5.64) | 5.75 (4.00, 6.00) | 6.45 (3.55, 6.45) | 0.390 |
| Epigastrium | 5.78 a (5.33, 6.67) | 8.89 a (7.22, 11.94) | 3.18 (3.18, 4.09) | 0.027 |
| Left upper | 3.08 (2.31, 5.13) | 8.06 (7.78, 10.28) | 3.16 (2.63, 7.11) | 0.061 |
| Left flank | 3.06 (1.94, 4.44) | 2.86 (1.71, 3.14) | 9.44 (7.78, 9.44) | 0.059 |
| Left lower | 1.82 (0.91, 2.27) | 3.03 (1.21, 3.33) | 2.00 (1.43, 2.29) | 0.491 |
| Pelvis | 1.50 (1.00, 1.50) | 1.59 (1.35, 3.41) | 2.16 (1.89, 6.49) | 0.111 |
| Right lower | 4.25 (3.00, 5.50) | 2.00 (1.60, 3.20) | 3.68 (2.63, 3.68) | 0.146 |
| Right flank | 4.65 (1.86, 4.88) | 4.77 (1.36, 5.23) | 6.50 (6.00, 7.00) | 0.066 |
| Ileum | 2.38 (2.14, 2.86) | 1.38 (1.03, 1.72) | 1.43 (1.43, 1.90) | 0.050 |
| Colon | 1.95 (1.71, 2.44) | 0.98 (0.59, 0.98) | 3.10 (3.10, 3.79) | 0.026 |
| Stomach | 3.27 (2.65, 3.67) | 1.49 (1.06, 2.77) | 1.06 (0.64, 1.28) | 0.067 |
| Jejunum | 0.83 b (0.83, 1.25) | 2.19 (1.88, 2.19) | 0.56 b (0.56, 1.11) | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.W.; Lee, E.J.; Chung, J.Y.; Lee, E.J.; Kim, D.; Oh, S.H.; Lee, S.; Yim, G.W.; Shim, S.-H.; Lee, S.J.; et al. Optimal Nozzle Position and Patient’s Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model. J. Pers. Med. 2022, 12, 1799. https://doi.org/10.3390/jpm12111799
Hwang DW, Lee EJ, Chung JY, Lee EJ, Kim D, Oh SH, Lee S, Yim GW, Shim S-H, Lee SJ, et al. Optimal Nozzle Position and Patient’s Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model. Journal of Personalized Medicine. 2022; 12(11):1799. https://doi.org/10.3390/jpm12111799
Chicago/Turabian StyleHwang, Dong Won, Eun Ji Lee, Joo Yeon Chung, Eun Joo Lee, Dayoung Kim, Soo Hyun Oh, Seungmee Lee, Ga Won Yim, Seung-Hyuk Shim, Sung Jong Lee, and et al. 2022. "Optimal Nozzle Position and Patient’s Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model" Journal of Personalized Medicine 12, no. 11: 1799. https://doi.org/10.3390/jpm12111799
APA StyleHwang, D. W., Lee, E. J., Chung, J. Y., Lee, E. J., Kim, D., Oh, S. H., Lee, S., Yim, G. W., Shim, S.-H., Lee, S. J., Lee, S.-H., Park, J. W., Chang, S.-J., Pak, K. A., Park, S. J., Kim, H. S., & on behalf of the KoRIA Trial Group. (2022). Optimal Nozzle Position and Patient’s Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model. Journal of Personalized Medicine, 12(11), 1799. https://doi.org/10.3390/jpm12111799







